Wheat Varietal Response to Tilletia controversa J. G. Kühn Using qRT-PCR and Laser Confocal Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Fungal Inoculation
2.2. RNA Extraction and cDNA Synthesis
2.3. Quantitative Real-Time PCR Analysis
2.4. Observation by Laser Confocal Microscopy
2.5. Effects of T. controversa on Pollen Germination
2.6. Assessment of Wheat Cultivars Against T. controversa
2.7. Statistical Analysis
3. Results
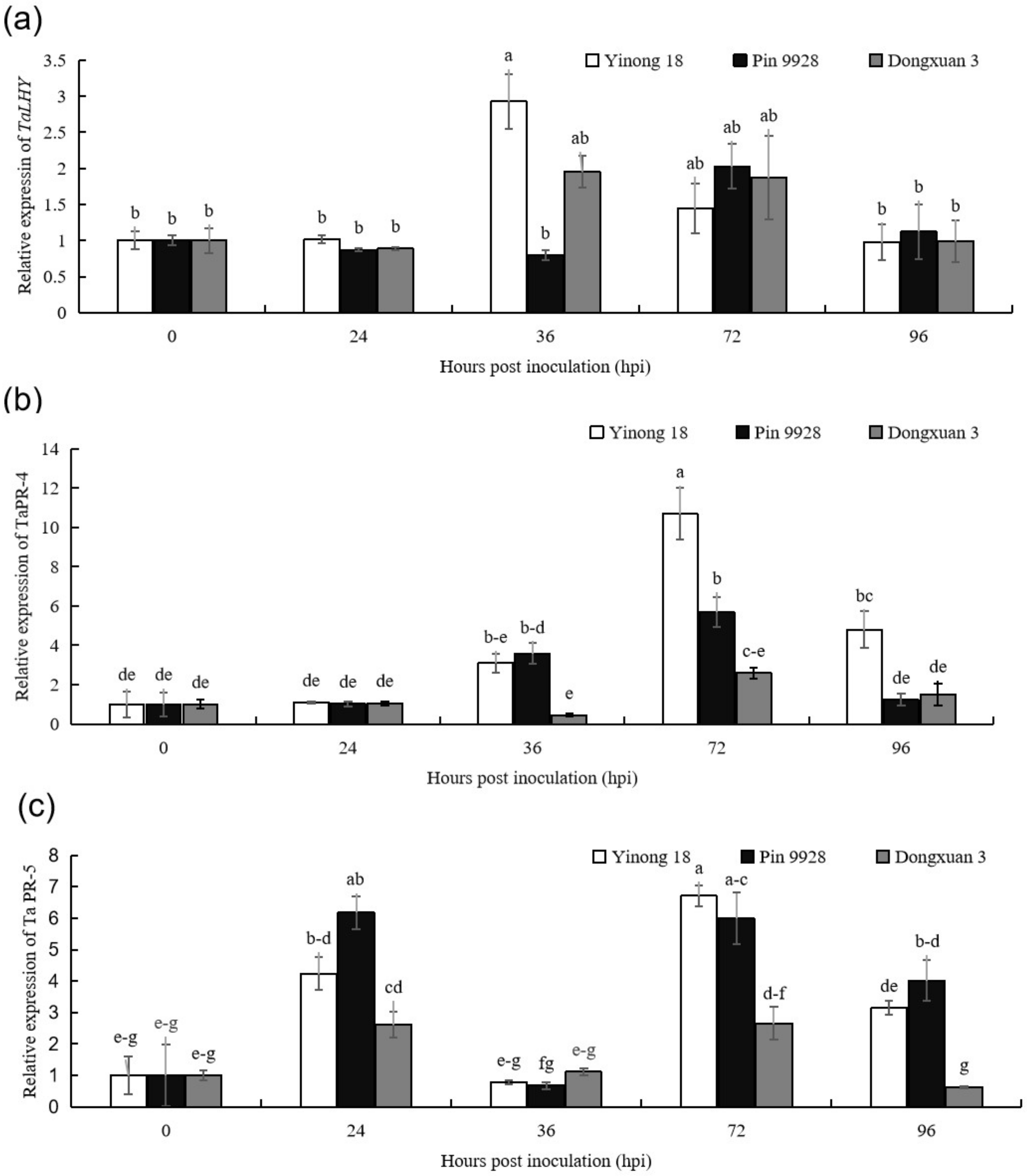
3.1. The Expression Patterns of TaLHY, TaPR-4, and TaPR-5 in Response to T. controversa Infection
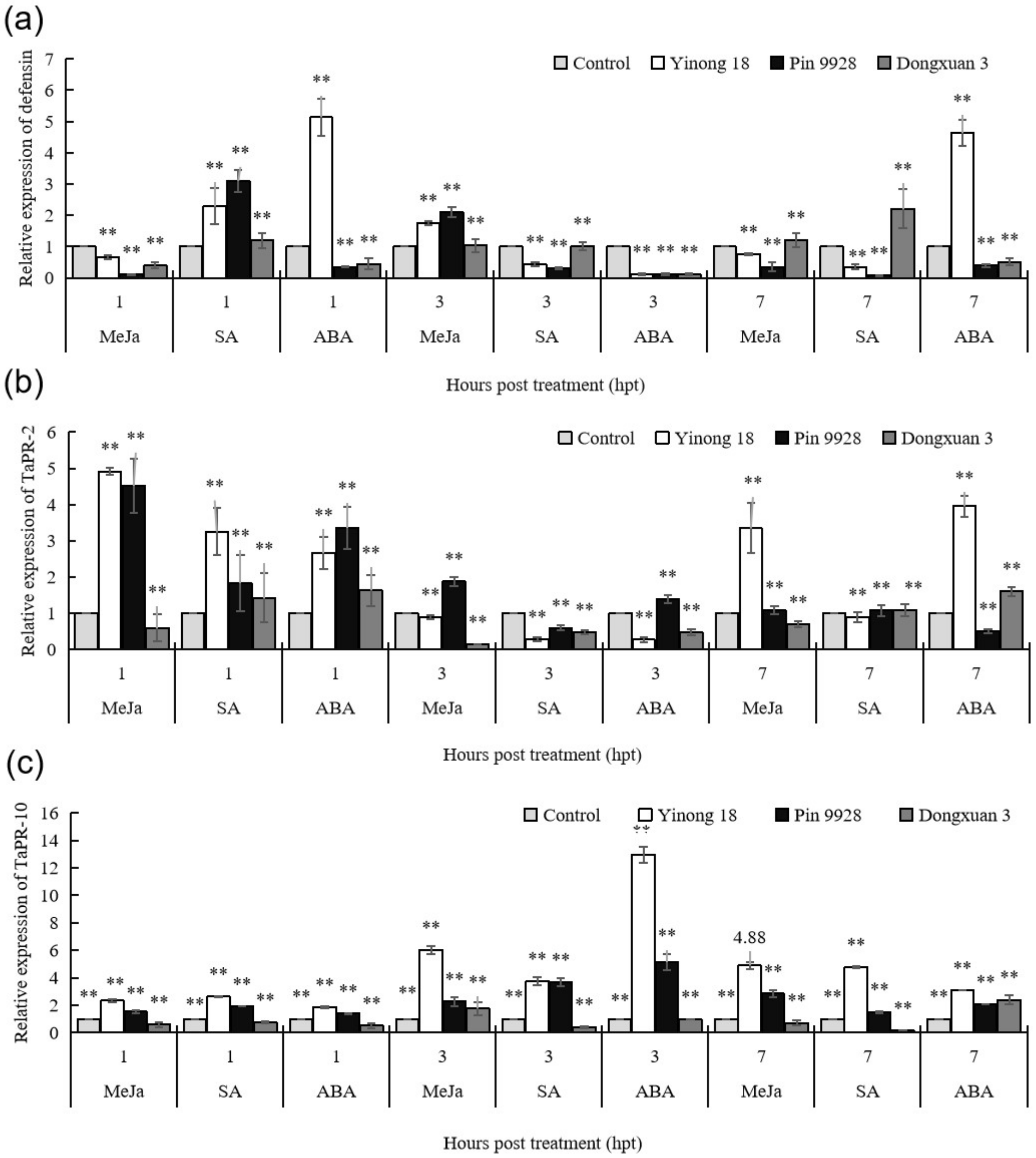
3.2. Response of Pathogenesis Related Proteins against to Exogenous Hormones in Different Wheat Cultivars
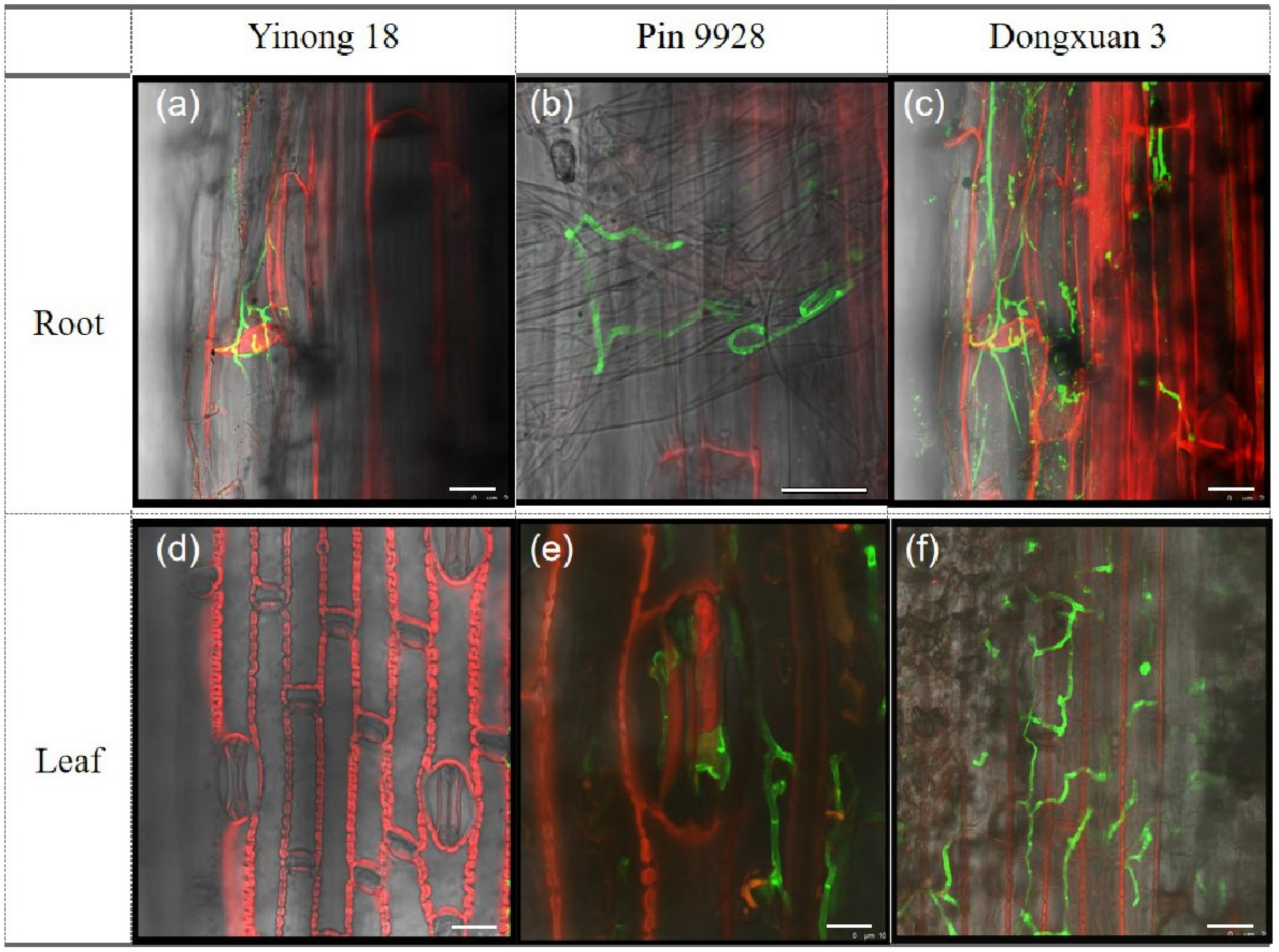
3.3. Proliferation of Fungal Hyphae in Root and Leaf Cells
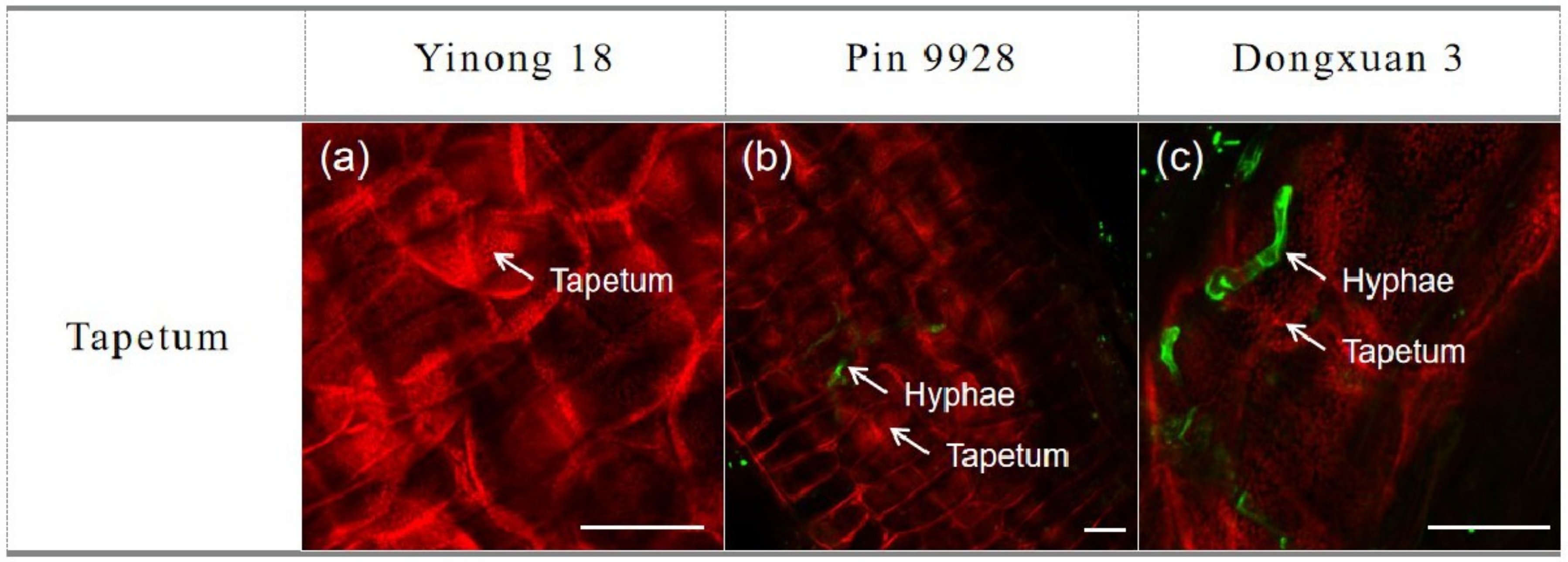
3.4. Proliferation of Fungal Hyphae in Anther Cells
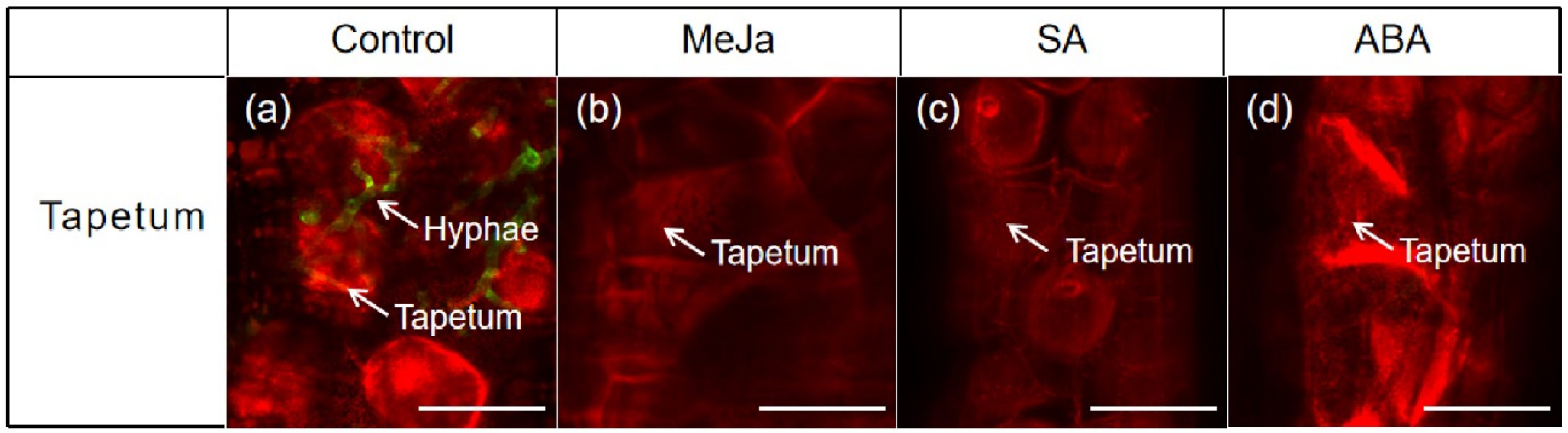
3.5. Effects of Exogenous Hormones on Tapetum Cells of Anther
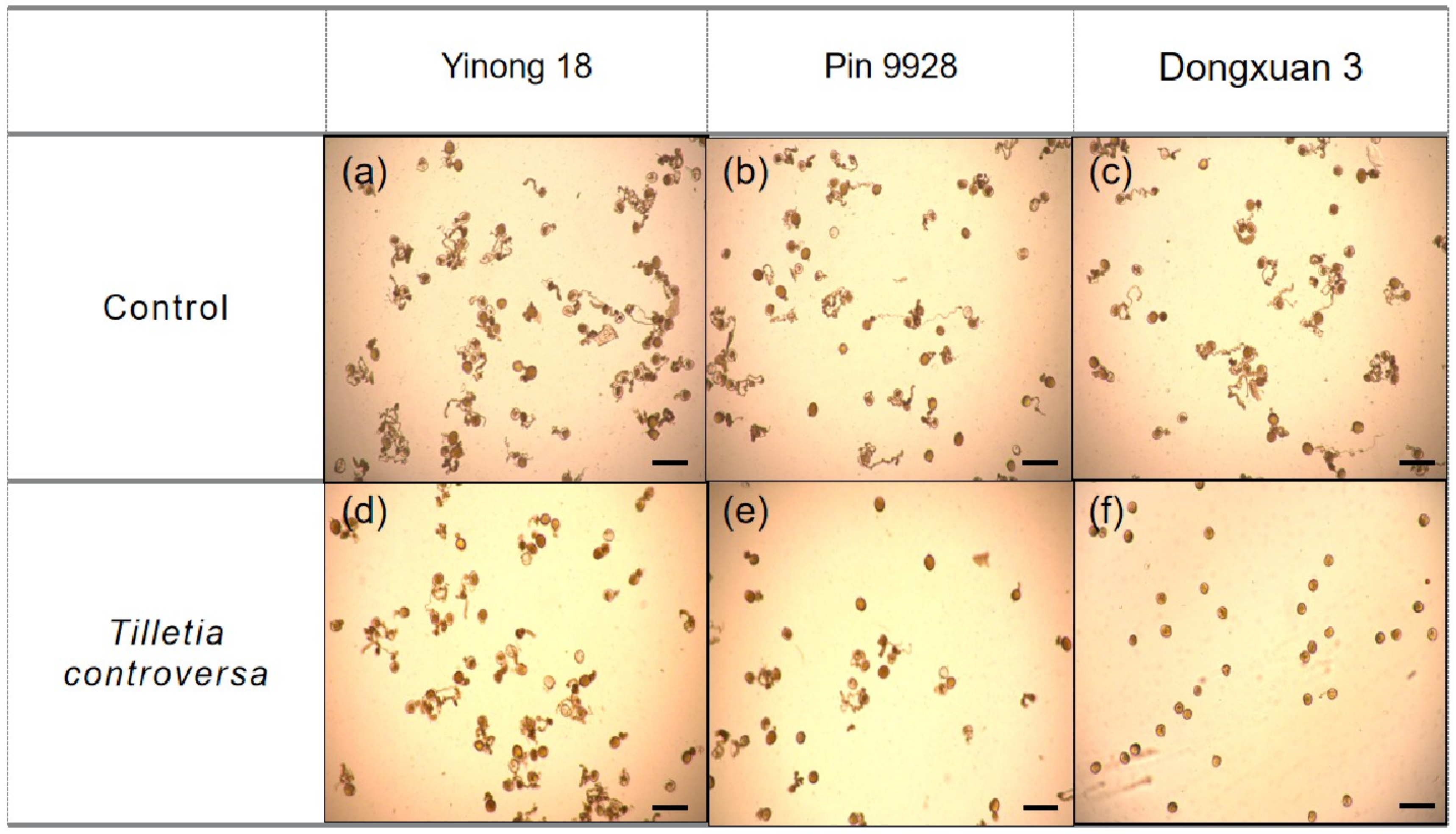
3.6. Effects of T. controversa on Pollen Grain Germination
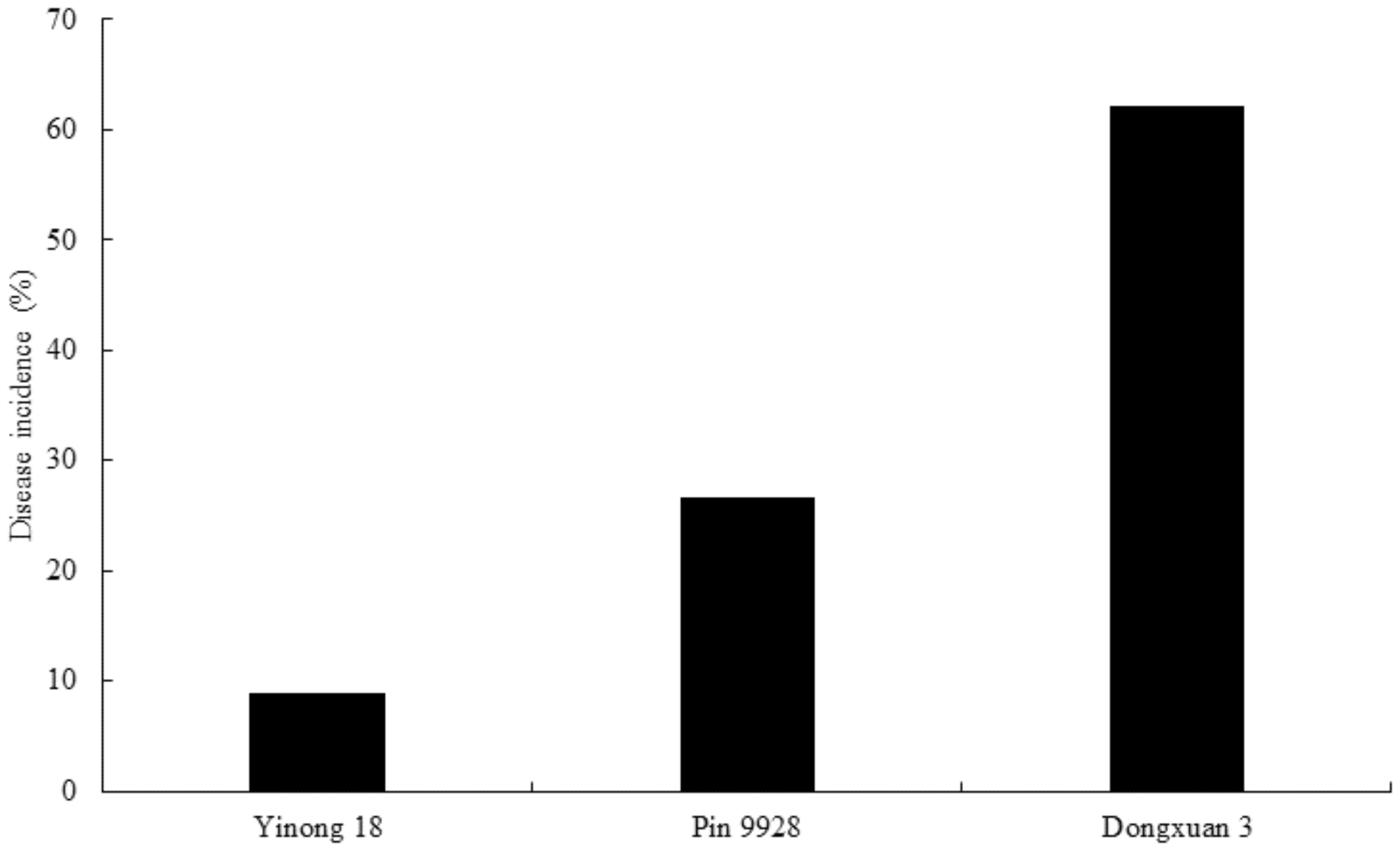
3.7. Evaluation of Dwarf Bunt Resistance in Wheat Cultivars
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ghosh, T.; Pradhan, C.; Das, A.B. Control of stem-rot disease of rice caused by Sclerotium oryzae catt and its cellular defense mechanism—A review. Physiol. Mol. Plant Pathol. 2020, 112, 101536. [Google Scholar] [CrossRef]
- Muhae-Ud-Din, G.; Chen, D.; Liu, T.; Chen, W.; Gao, L. Methyljasmonate and salicylic acid contribute to the control of Tilletia controversa Kühn, causal agent of wheat dwarf bunt. Sci. Rep. 2020, 10, 19175. [Google Scholar] [CrossRef] [PubMed]
- Trione, E.J. Dwarf bunt of wheat and its importance in international wheat trade. Plant Dis. 1982, 66, 1083. [Google Scholar] [CrossRef]
- Goates, B.J.; Peterson, G.L. Relationship between soilborne and seedborne inoculum density and the incidence of dwarf bunt of wheat. Plant Dis. 1999, 83, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Mathre, D.E. Dwarf bunt: Politics, identification, and biology. Annu. Rev. Phytopathol. 1996, 34, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yu, H.; Han, W.; Gao, F.; Liu, T.; Liu, B.; Kang, X.; Gao, J.; Chen, W. Development of a SCAR marker for molecular detection and diagnosis of Tilletia controversa Kühn, the causal fungus of wheat dwarf bunt. World J. Microbiol. Biotechnol. 2014, 30, 3185–3195. [Google Scholar] [CrossRef]
- Collins, C.M.; Cunningham, C.O. Characterization of the Gyrodactylus salaris Malmberg, 1957 (Platyhelminthes: Monogenea) ribosomal intergenic spacer (IGS) DNA. Parasitology 2000, 121, 555–563. [Google Scholar] [CrossRef]
- Pimentel, G.; Peever, T.L.; Carris, L.M. Genetic variation among natural populations of Tilletia controversa and T. bromi. Phytopathology 2000, 90, 376–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muhae-Ud-Din, G.; Chen, D.; Liu, T.; Chen, W.; Gao, L. Characterization of the wheat cultivars against Tilletia controversa Kühn, causal agent of wheat dwarf bunt. Sci. Rep. 2020, 10, 9029. [Google Scholar] [CrossRef] [PubMed]
- Wilson, Z.A.; Zhang, D.-B. From Arabidopsis to rice: Pathways in pollen development. J. Exp. Bot. 2009, 60, 1479–1492. [Google Scholar] [CrossRef]
- Chang, F.; Wang, Y.; Wang, S.; Ma, H. Molecular control of microsporogenesis in Arabidopsis. Curr. Opin. Plant Biol. 2011, 14, 66–73. [Google Scholar] [CrossRef]
- Walbot, V.; Skibbe, D.S. Maize host requirements for Ustilago maydis tumor induction. Sex. Plant Reprod. 2009, 23, 1–13. [Google Scholar] [CrossRef][Green Version]
- Goates, B.J. Identification of new pathogenic races of common bunt and dwarf bunt fungi, and evaluation of known races using an expanded set of differential wheat lines. Plant Dis. 2012, 96, 361–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mengiste, T.; Chen, X.; Salmeron, J.; Dietrich, R. The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 2003, 15, 2551–2565. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.Y.; Kim, J.-H.; Joung, Y.-H.; Lee, S.; Kim, W.-T.; Yu, S.H.; Choi, D. the pepper transcription factor capf1 confers pathogen and freezing tolerance in Arabidopsis. Plant Physiol. 2004, 136, 2862–2874. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van Der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Hill, L.; Crooks, C.; Doerner, P.; Lamb, C. Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant Physiol. 2009, 150, 1750–1761. [Google Scholar] [CrossRef]
- Lee, S.C.; Luan, S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 2012, 35, 53–60. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Wang, X.; Zhou, M.; Zhou, X.; Ye, X.; Wei, X. An R2R3 MYB transcription factor in wheat, Ta PIMP 1, mediates host resistance to Bipolaris sorokiniana and drought stresses through regulation of defense-and stress-related genes. New Phytol. 2012, 196, 1155–1170. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Linthorst, H.J.; Van Loon, L. Pathogenesis-related proteins of plants. Crit. Rev. Plant Sci. 1991, 10, 123–150. [Google Scholar] [CrossRef]
- Edreva, A. Pathogenesis-related proteins: Research progress in the last 15 years. Gen. Appl. Plant Physiol. 2005, 31, 105–124. [Google Scholar]
- Zhang, Y.-L.; Zhang, C.-L.; Wang, G.-L.; Wang, Y.-X.; Qi, C.-H.; Zhao, Q.; You, C.-X.; Li, Y.-Y.; Hao, Y.-J. The R2R3 MYB transcription factor MdMYB30 modulates plant resistance against pathogens by regulating cuticular wax biosynthesis. BMC Plant Biol. 2019, 19, 362. [Google Scholar] [CrossRef] [PubMed]
- Camargo-Ramírez, R.; Val-Torregrosa, B.; Segundo, B.S. MiR858-Mediated regulation of flavonoid-specific MYB transcription factor genes controls resistance to pathogen infection in Arabidopsis. Plant Cell Physiol. 2017, 59, 190–204. [Google Scholar] [CrossRef]
- Lee, M.-W.; Qi, M.; Yang, Y. A novel jasmonic acid-inducible rice myb gene associates with fungal infection and host cell death. Mol. Plant Microbe Interact. 2001, 14, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Sels, J.; Mathys, J.; De Coninck, B.M.; Cammue, B.P.; De Bolle, M.F. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Ekramoddoullah, A.K. The family 10 of plant pathogenesis-related proteins: Their structure, regulation, and function in response to biotic and abiotic stresses. Physiol. Mol. Plant Pathol. 2006, 68, 3–13. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Su, Y.; Liu, H.; Chen, Y.; Luo, P.; Du, X.; Wang, D.; Zhang, H. TaLHY, a 1R-myb transcription factor, plays an important role in disease resistance against stripe rust fungus and ear heading in wheat. PLoS ONE 2015, 10, e0127723. [Google Scholar] [CrossRef] [PubMed]
- Manghwar, H.; Hussain, A.; Ullah, A.; Gul, S.; Shaban, M.; Khan, A.H.; Ali, M.; Sani, S.G.A.S.; Chaudhary, H.J.; Munis, M.F.H. Expression analysis of defense related genes in wheat and maize against Bipolaris sorokiniana. Physiol. Mol. Plant Pathol. 2018, 103, 36–46. [Google Scholar] [CrossRef]
- Chen, W.J.; Zhu, T. Networks of transcription factors with roles in environmental stress response. Trends Plant Sci. 2004, 9, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.; Feuillet, C.; Messmer, M. Genetics of Disease Resistance. In Mechanisms of Resistance to Plant Diseases, 1st ed.; Slusarenko, A.J., Fraser, R.S., van Loon, L.C., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 101–160. [Google Scholar]
- Kitajima, S.; Sato, F. Plant pathogenesis-related proteins: Molecular mechanisms of gene expression and protein function. J. Biochem. 1999, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Caporale, C.; Di Berardino, I.; Leonardi, L.; Bertini, L.; Cascone, A.; Buonocore, V.; Caruso, C. Wheat pathogenesis-related proteins of class 4 have ribonuclease activity. FEBS Lett. 2004, 575, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Anguelova-Merhar, V.S.; VanDer Westhuizen, A.J.; Pretorius, Z.A. β-1, 3-glucanase and chitinase activities and the resistance response of wheat to leaf rust. J. Phytopathol. 2001, 149, 381–384. [Google Scholar] [CrossRef]
- Bufe, A.; Spangfort, M.; Kahlert, H.; Schlaak, M.; Becker, W.-M. The major birch pollen allergen, Bet v 1, shows ribonuclease activity. Planta 1996, 199, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Rivière, M.-P.; Marais, A.; Ponchet, M.; Willats, W.; Galiana, E. Silencing of acidic pathogenesis-related PR-1 genes increases extracellular β-(1→3)-glucanase activity at the onset of tobacco defence reactions. J. Exp. Bot. 2008, 59, 1225–1239. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Velazhahan, R.; Oliva, N.; Ona, I.; Mew, T.; Khush, G.S.; Muthukrishnan, S.; Datta, S.K. Over-expression of the cloned rice thaumatin-like protein (PR-5) gene in transgenic rice plants enhances environmental friendly resistance to Rhizoctonia solani causing sheath blight disease. Theor. Appl. Genet. 1999, 98, 1138–1145. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef]
- McGee, J.D.; Hamer, J.E.; Hodges, T.K. Characterization of a PR-10 pathogenesis-related gene family induced in rice during infection with Magnaporthe grisea. Mol. Plant Microbe Interact. 2001, 14, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Gao, L.; Zhang, W.-H.; Liu, J.-K.; Zhang, Y.-J.; Wang, H.-Y.; Liu, D.-Q. Characteristic expression of wheat PR5 gene in response to infection by the leaf rust pathogen, Puccinia triticina. J. Plant Interact. 2015, 10, 132–141. [Google Scholar] [CrossRef]
- Oide, S.; Bejai, S.; Staal, J.; Guan, N.; Kaliff, M.; Dixelius, C. A novel role of PR2 in abscisic acid (ABA) mediated, pathogen-induced callose deposition in Arabidopsis thaliana. New Phytol. 2013, 200, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Kachroo, P.; Klessig, D.F. The Arabidopsis ssi1 mutation restores pathogenesis-related gene expression in npr1 plants and renders defensin gene expression salicylic acid dependent. Plant Cell 1999, 11, 191. [Google Scholar] [CrossRef]
- Cullen, D.W.; Lees, A.K.; Toth, I.K.; Duncan, J.M. Detection of Colletotrichum coccodes from soil and potato tubers by conventional and quantitative real-time PCR. Plant Pathol. 2002, 51, 281–292. [Google Scholar] [CrossRef]
- Zouhar, M.; MaZákoVá, J.; ProkiNoVá, E.; VáňoVá, M.; Ryšánek, P. Quantification of Tilletia caries and Tilletia controversa mycelium in wheat apical meristem by real-time PCR. Plant Prot. Sci. 2010, 46, 107–115. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Garcia, D.F.; Zhou, Y.; Appels, R.; Li, A.; Mao, L. The battle to sequence the bread wheat genome: A tale of the three kingdoms. Genom. Proteom. Bioinform. 2020, 18, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Dubcovsky, J.; Dvorak, J. Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 2007, 316, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Kelliher, T.; Nguyen, L.; Walbot, V. Ustilago maydis reprograms cell proliferation in maize anthers. Plant J. 2013, 75, 903–914. [Google Scholar] [CrossRef]
- Hou, L.J.; Song, Y.L.; Niu, N.; Ma, S.C.; Song, Y.Z.; Wang, Q.; Zhang, G.S.; Junwei, W. Screening of pollen germination in vitro cultivation system for wheat (Triticum aestivum L.). Chin. Agric. Sci. Bull. 2015, 31, 111–114. (In Chinese) [Google Scholar]
- Goates, J. Common bunt. In Bunt and Smut Diseases of Wheat: Concept and Methods of Disease Management; Wilcoxson, R.D., Saari, E.E., Eds.; CMMYT: El Batán, Mexico, 1998; pp. 12–25. [Google Scholar]
- Truernit, E.; Haseloff, J. A simple way to identify non-viable cells within living plant tissue using confocal microscopy. Plant Methods 2008, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.-X.; Gaudet, D.; Puchalski, B.; Despins, T.; Frick, M.; Laroche, A. Inducers of resistance reduce common bunt infection in wheat seedlings while differentially regulating defence-gene expression. Physiol. Mol. Plant Pathol. 2005, 67, 138–148. [Google Scholar] [CrossRef]
- Lay, F.T.; Anderson, M. Defensins-components of the innate immune system in plants. Curr. Protein Pept. Sci. 2005, 6, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1, 3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Park, C.-M. MYB96-mediated abscisic acid signals induce pathogen resistance response by promoting salicylic acid biosynthesis in Arabidopsis. New Phytol. 2010, 186, 471–483. [Google Scholar] [CrossRef]
- Moosa, A.; Farzand, A.; Sahi, S.T.; Khan, S.A. Transgenic expression of antifungal pathogenesis-related proteins against phytopathogenic fungi—15 years of success. Isr. J. Plant Sci. 2017, 32, 1–17. [Google Scholar] [CrossRef]
- Rivero, M.; Furman, N.; Mencacci, N.; Picca, P.; Toum, L.; Lentz, E.; Bravo-Almonacid, F.; Mentaberry, A. Stacking of antimicrobial genes in potato transgenic plants confers increased resistance to bacterial and fungal pathogens. J. Biotechnol. 2012, 157, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Rong, W.; Xu, H.; Du, L.; Liu, X.; Zhang, Z. The wheat R2R3-MYB transcription factor TaRIM1 participates in resistance response against the pathogen Rhizoctonia cerealis infection through regulating defense genes. Sci. Rep. 2016, 6, 28777. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Su, Z.; Lv, L.; Zhang, Z. Silencing of the wheat protein phosphatase 2A catalytic subunit TaPP2Ac enhances host resistance to the necrotrophic pathogen Rhizoctonia cerealis. Front. Plant Sci. 2018, 9, 1437. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Liang, F.; Zhang, Y.; Ma, L.; Wang, H.; Liu, D. Functional analysis of a pathogenesis-related thaumatin-like protein gene TaLr35PR5 from wheat induced by leaf rust fungus. BMC Plant Biol. 2018, 18, 76. [Google Scholar] [CrossRef]
- Persson, M.; Falk, A.; Dixelius, C. Studies on the mechanism of resistance to Bipolaris sorokiniana in the barley lesion mimic mutantbst1. Mol. Plant Pathol. 2009, 10, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.H.; Duran, R.; Schafer, J.F. Histological aspects of dwarf bunt resistance in wheat. Phytopathology 1978, 68, 1417–1421. [Google Scholar] [CrossRef]
- Ghareeb, H.; Becker, A.; Iven, T.; Feussner, I.; Schirawski, J. Sporisorium reilianum infection changes inflorescence and branching architectures of maize. Plant Physiol. 2011, 156, 2037–2052. [Google Scholar] [CrossRef] [PubMed]
- Heslop-Harrison, J. Pollen Wall Development. Science 1968, 161, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Jacobowitz, J.R.; Doyle, W.C.; Weng, J.-K. PRX9 and PRX40 are extensin peroxidases essential for maintaining tapetum and microspore cell wall integrity during Arabidopsis anther development. Plant Cell 2019, 31, 848–861. [Google Scholar] [CrossRef] [PubMed]
| Yinong 18 | Pin 9928 | Dongxuan 3 | ||||
|---|---|---|---|---|---|---|
| Germination (%) | Control | T. controversa | Control | T. controversa | Control | T. controversa |
| (a) | 87.14 ± 2.16 | 80.06 ± 3.09 | 88.39 ± 1.57 | 58.73 ± 2.56 | 86.95 ± 3.60 | 0.67 ± 0.38 |
| (b) | 0.0003 * | 0.0004 * | 0.0005 * | 0.0004 * | 0.0003 * | 0.0003 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Muhae-Ud-Din, G.; Liu, T.; Chen, W.; Liu, C.; Gao, L. Wheat Varietal Response to Tilletia controversa J. G. Kühn Using qRT-PCR and Laser Confocal Microscopy. Genes 2021, 12, 425. https://doi.org/10.3390/genes12030425
Chen D, Muhae-Ud-Din G, Liu T, Chen W, Liu C, Gao L. Wheat Varietal Response to Tilletia controversa J. G. Kühn Using qRT-PCR and Laser Confocal Microscopy. Genes. 2021; 12(3):425. https://doi.org/10.3390/genes12030425
Chicago/Turabian StyleChen, Delai, Ghulam Muhae-Ud-Din, Taiguo Liu, Wanquan Chen, Changzhong Liu, and Li Gao. 2021. "Wheat Varietal Response to Tilletia controversa J. G. Kühn Using qRT-PCR and Laser Confocal Microscopy" Genes 12, no. 3: 425. https://doi.org/10.3390/genes12030425
APA StyleChen, D., Muhae-Ud-Din, G., Liu, T., Chen, W., Liu, C., & Gao, L. (2021). Wheat Varietal Response to Tilletia controversa J. G. Kühn Using qRT-PCR and Laser Confocal Microscopy. Genes, 12(3), 425. https://doi.org/10.3390/genes12030425







