Retinal Genomic Fabric Remodeling after Optic Nerve Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Handling
2.2. Optic Nerve Crush
2.3. Quantification of Retinal Ganglion Cell Survival
2.4. Microarray Gene Expression
2.5. Data Processing
- condition = ONC, CTR
- µik = average expression level of gene I probed by spot k (=1, …, Ri) in the 4 biological replicas
- sik = standard deviation of the expression level of gene I probed by spot k.
- ri = 4Ri − 1 = number of degrees of freedom
- Ri = number of microarray spots probing redundantly gene i
2.6. Analysis of Predicted Protein–Protein Interactions
2.7. Enrichment Analyses
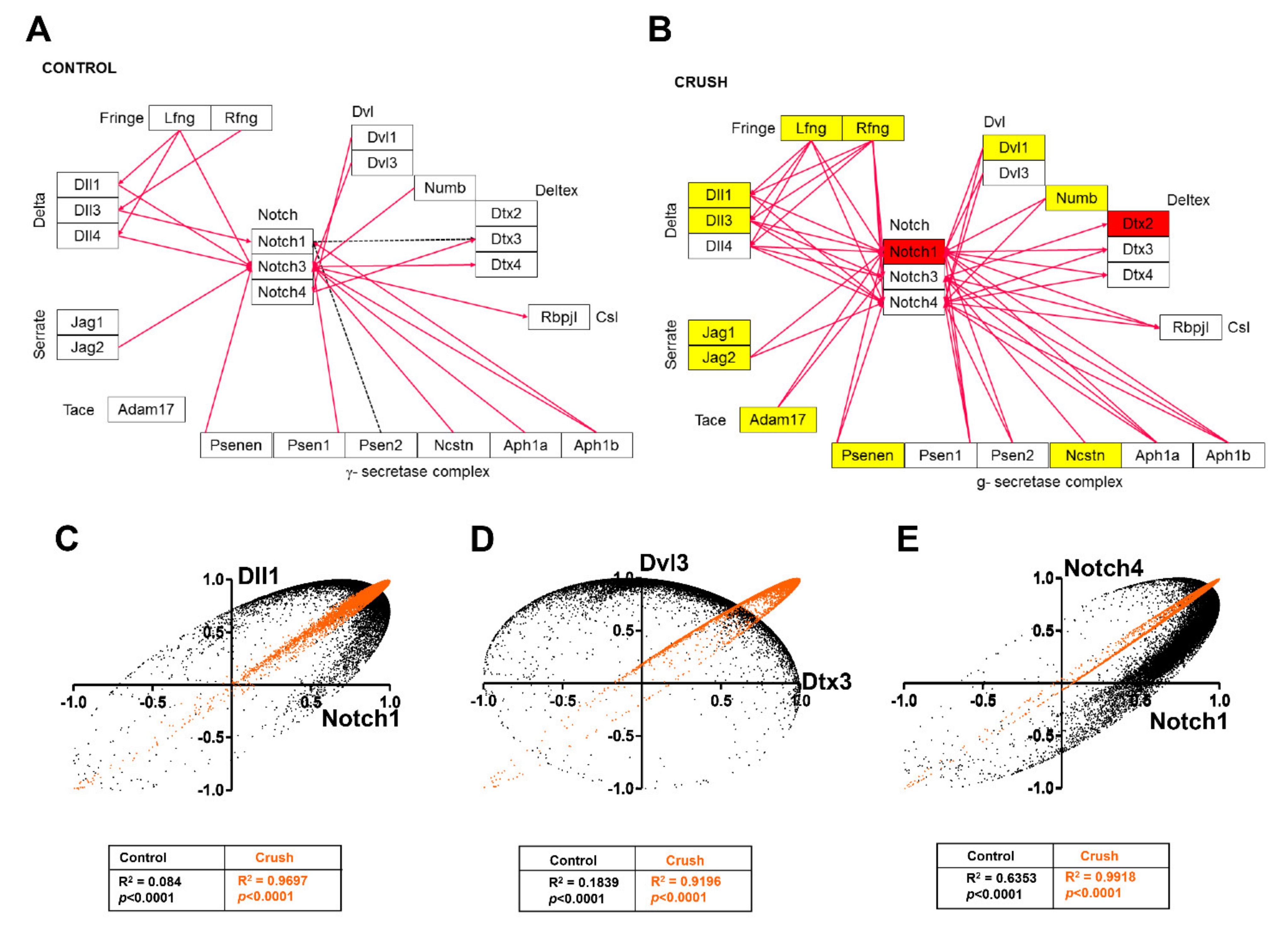
2.8. Coordination of Expression
2.9. Quantitative Real-Time PCR
3. Results
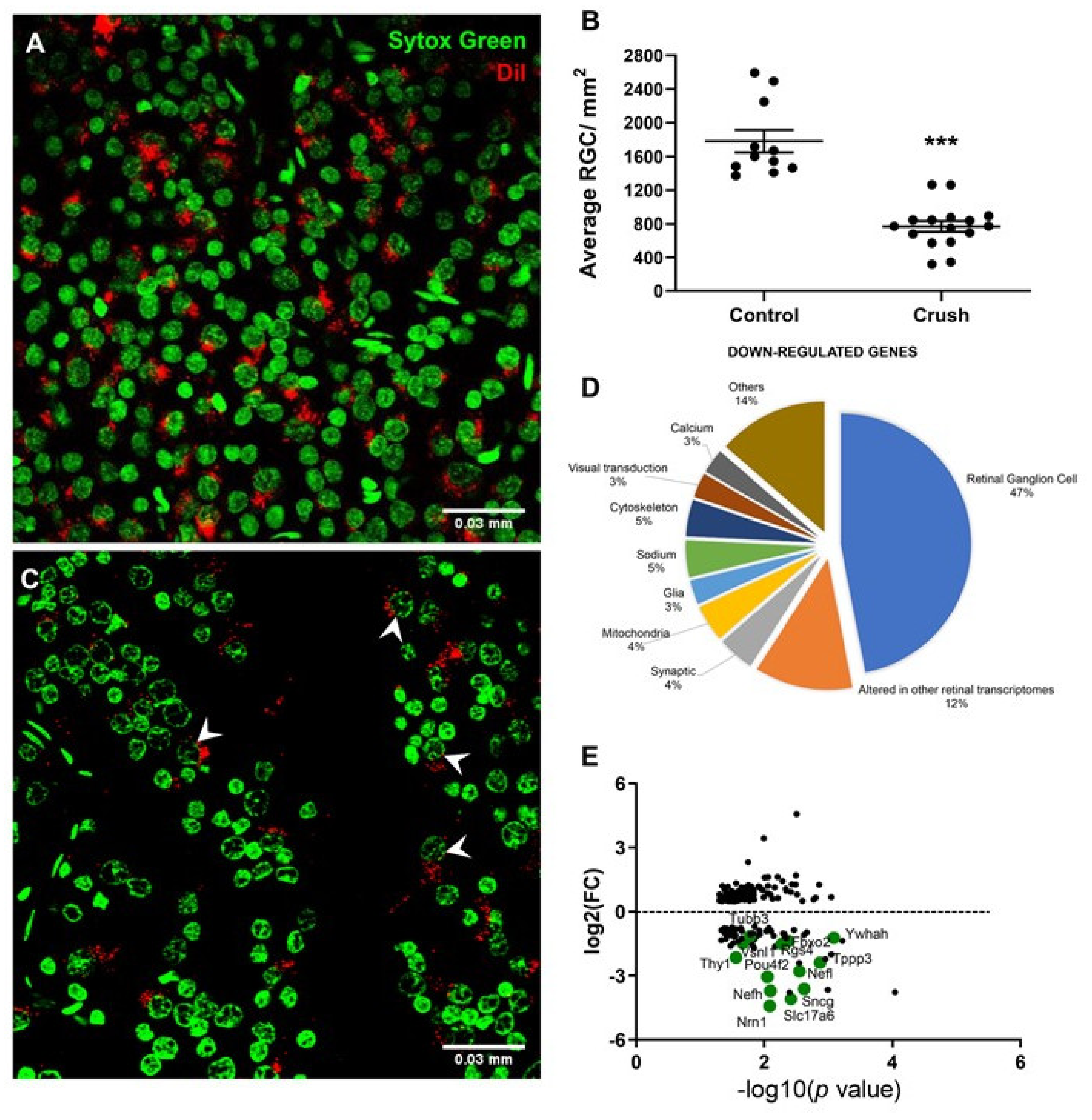
3.1. Histopathological Observations
3.2. Transcriptomic Alterations in Rat Retina after ONC
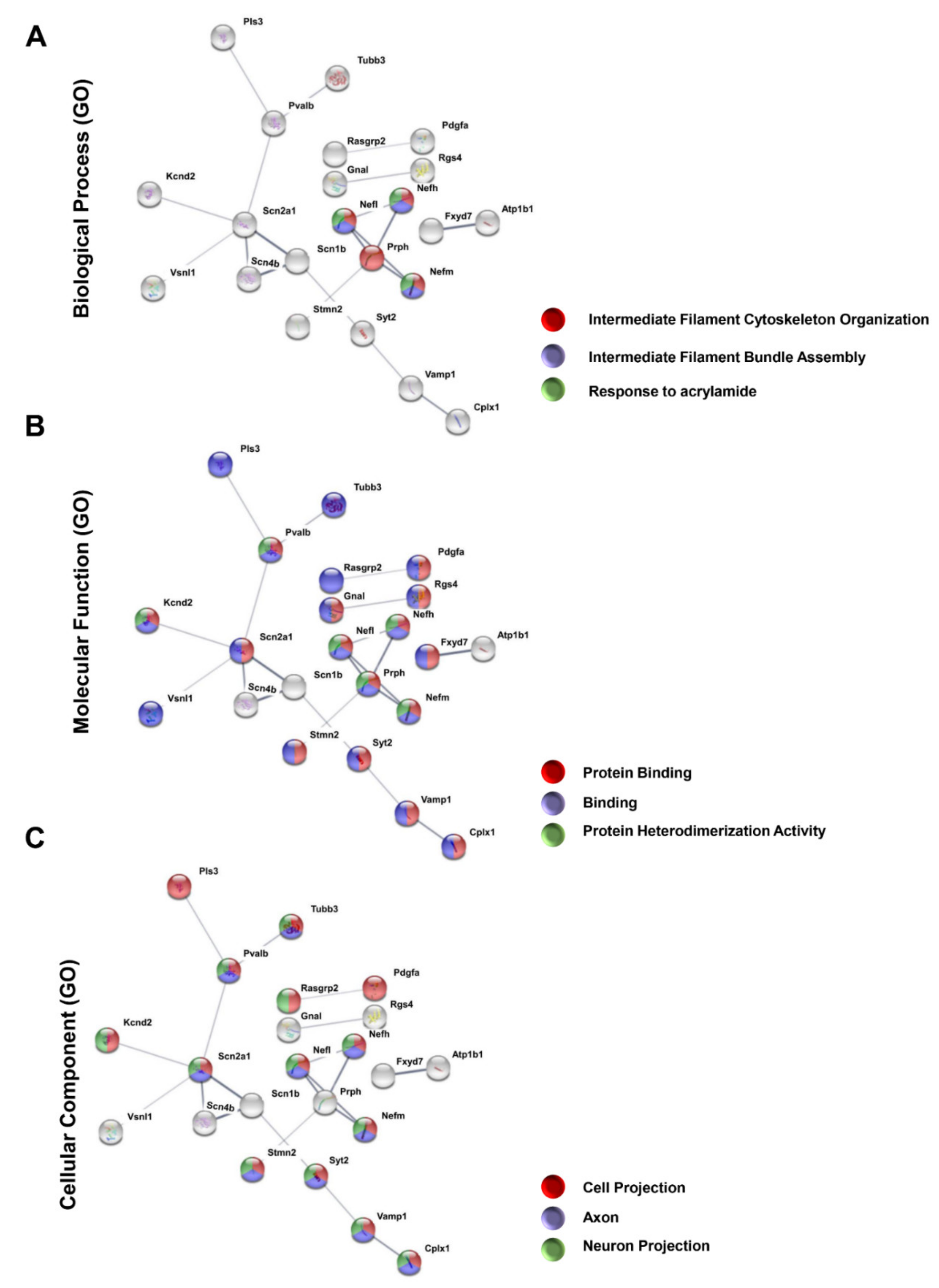
3.3. Protein–Protein Interaction Encoded by the Most Downregulated Genes
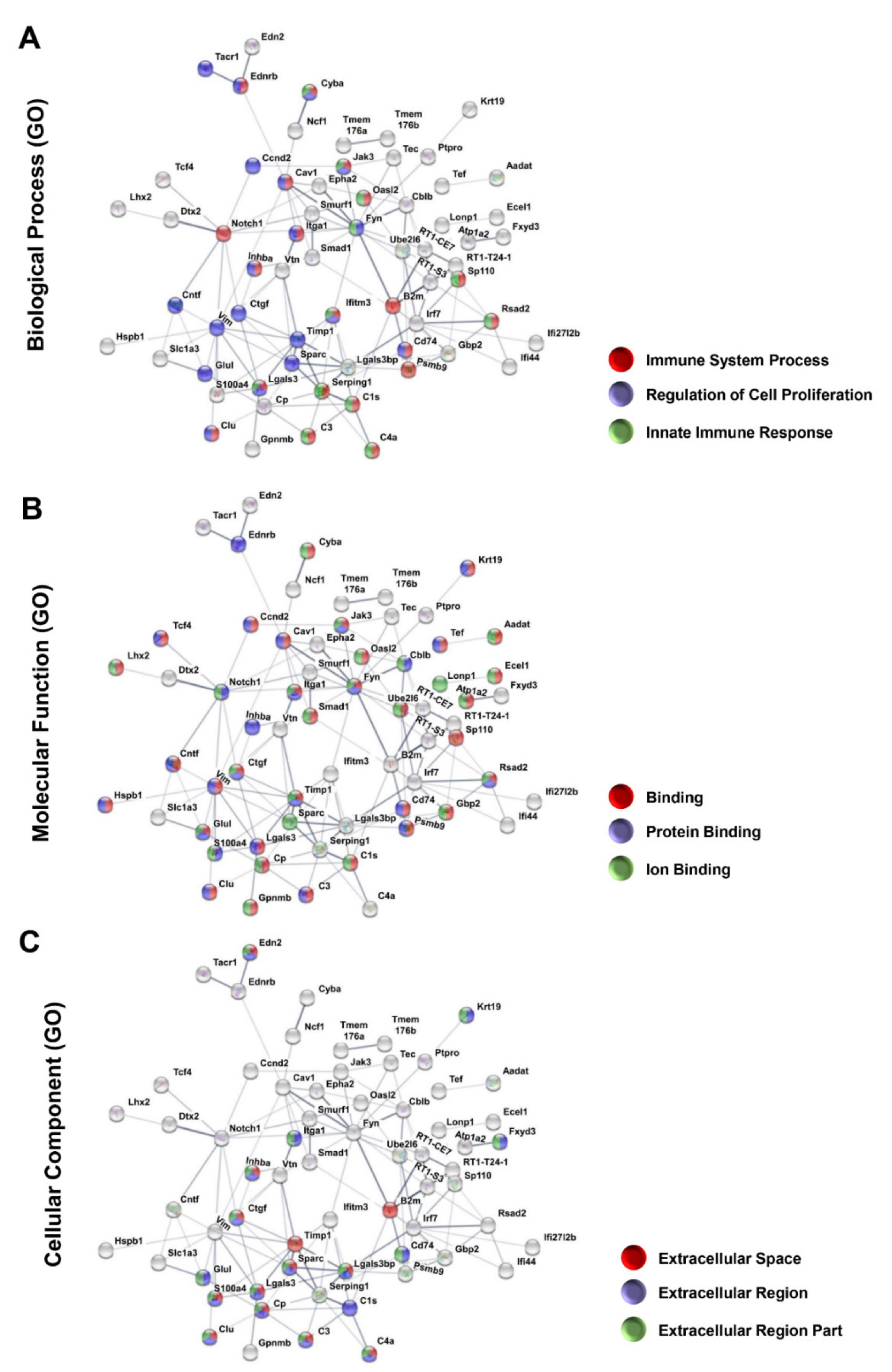
3.4. Protein–Protein Interaction Encoded by The Most Upregulated Genes
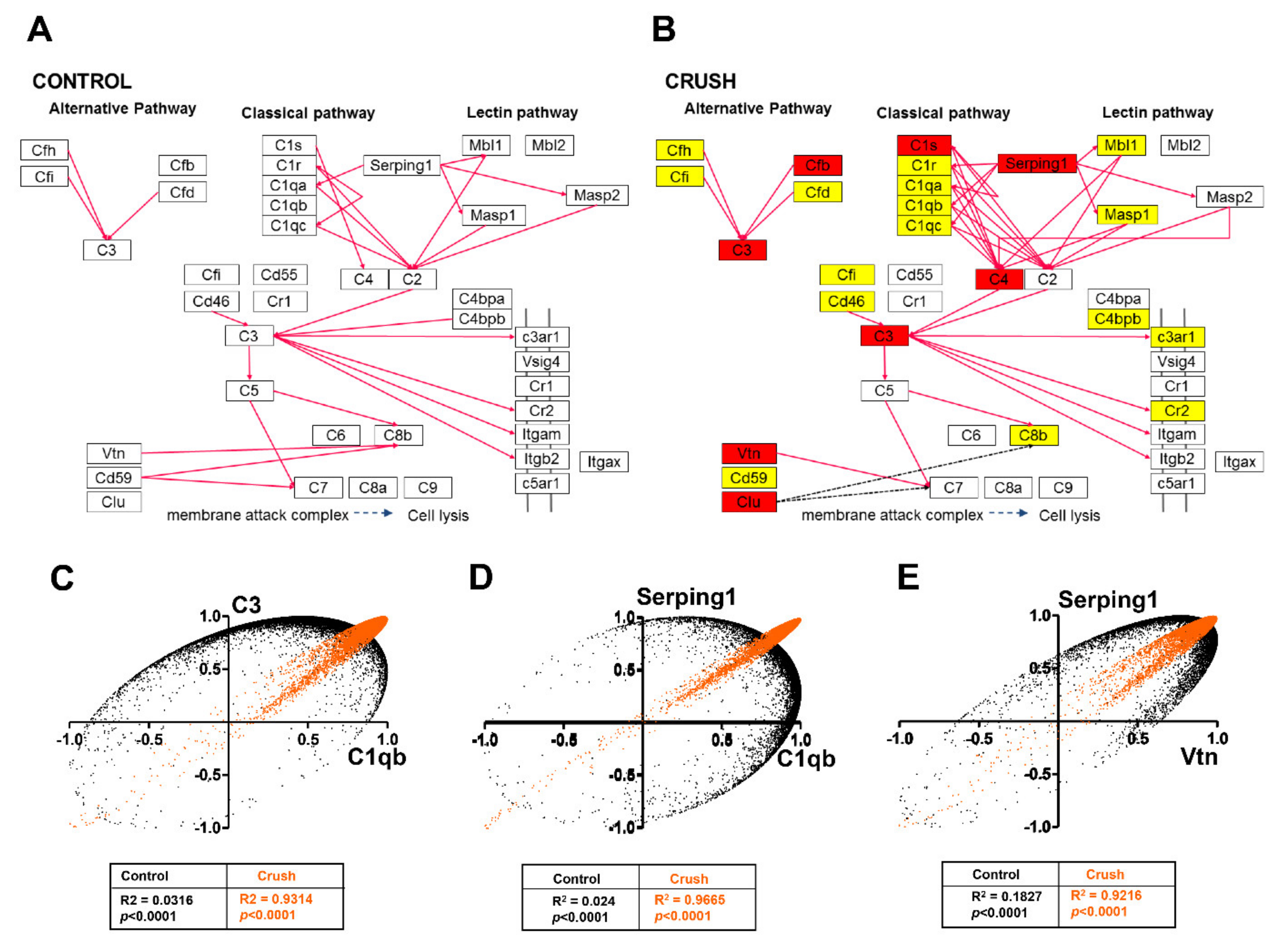
3.5. ONC Altered The Stability of Gene Expression
3.6. Pathway Enrichment Analysis in ONC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Foster, P.J.; Buhrmann, R.; Quigley, H.A.; Johnson, G.J. The definition and classification of glaucoma in prevalence surveys. Br. J. Ophthalmol. 2002, 86, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.V.; Tomarev, S.I. Rodent models of glaucoma. Brain Res. Bull. 2010, 81, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Yoshitomi, T.; Zorumski, C.F.; Izumi, Y. Experimentally Induced Mammalian Models of Glaucoma. Biomed. Res. Int. 2015, 281214. [Google Scholar] [CrossRef]
- Harada, C.; Kimura, A.; Guo, X.; Namekata, K.; Harada, T. Recent advances in genetically modified animal models of glaucoma and their roles in drug repositioning. Br. J. Ophthalmol. 2019, 103, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Rheaume, B.A.; Jereen, A.; Bolisetty, M.; Sajid, M.S.; Yang, Y.; Renna, K.; Sun, L.; Robson, P.; Trakhtenberg, E.F. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat. Commun. 2018, 9, 2759. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Ray, A.; Rodgers, K.; Ergorul, C.; Hyman, B.T.; Huang, W.; Grosskreutz, C.L. Global gene expression changes in rat retinal ganglion cells in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2010, 51, 4084–4095. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Monti, C.; Alberio, T. A systems biology-led insight into the role of the proteome in neurodegenerative diseases. Expert Rev. Proteomics 2016, 13, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Karpe, A.; Zinkernagel, M.S.; Munk, M.R. Inner retinal layer change in glaucoma patients receiving anti-VEGF for neovascular age related macular degeneration. Arch. Clin. Exp. Ophthalmol. 2017, 255, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Vien, L.; DalPorto, C.; Yang, D. Retrograde Degeneration of Retinal Ganglion Cells Secondary to Head Trauma. Optom. Vis. Sci. 2017, 94, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Chiha, W.; LeVaillant, C.J.; Bartlett, C.A.; Hewitt, A.W.; Melton, P.E.; Fitzgerald, M.; Harvey, A.R. Retinal genes are differentially expressed in areas of primary versus secondary degeneration following partial optic nerve injury. PLoS ONE 2018, 13, e0192348. [Google Scholar] [CrossRef]
- McCurley, A.T.; Callard, G.V. Time course analysis of gene expression patterns in zebrafish eye during optic nerve regeneration. J. Exp. Neurosci. 2010, 4, 17–33. [Google Scholar] [CrossRef]
- Whitworth, G.B.; Misaghi, B.C.; Rosenthal, D.M.; Mills, E.A.; Heinen, D.J.; Watson, A.H.; Ives, C.W.; Ali, S.H.; Bezold, K.; Marsh-Armstrong, N.; et al. Translational profiling of retinal ganglion cell optic nerve regeneration in Xenopus laevis. Dev. Biol. 2017, 426, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Kompass, K.S.; Agapova, O.A.; Li, W.; Kaufman, P.L.; Rasmussen, C.A.; Hernandez, M.R. Bioinformatic and statistical analysis of the optic nerve head in a primate model of ocular hypertension. BMC Neurosci. 2008, 9, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.R.; Inman, D.M.; Calkins, D.J.; Horner, P.J.; Vetter, M.L. Microarray Analysis of Retinal Gene Expression in the DBA/2J Model of Glaucoma. Invest. Ophthalmol. Vis. Sci. 2006, 47, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Brown, K.M.; Stephan, D.A.; Morrison, J.C.; Johnson, E.C.; Tomarev, S.I. Microarray Analysis of Changes in mRNA Levels in the Rat Retina after Experimental Elevation of Intraocular Pressure. Invest. Ophthalmol. Vis. Sci. 2004, 45, 1247–1258. [Google Scholar] [CrossRef][Green Version]
- Vázquez-Chona, F.; Song, B.K.; Geisert, E.E., Jr. Temporal Changes in Gene Expression after Injury in the Rat Retina. Invest. Ophthalmol. Vis. Sci. 2004, 45, 2737–2746. [Google Scholar] [CrossRef]
- Piri, N.; Kwong, J.M.K.; Song, M.; Elashoff, D.; Caprioli, J. Gene expression changes in the retina following optic nerve transection. Mol. Vis. 2006, 12, 1660–1673. [Google Scholar] [PubMed]
- Kamphuis, W.; Dijk, F.; van Soest, S.; Bergen, A.A. Global gene expression profiling of ischemic preconditioning in the rat retina. Mol. Vis. 2007, 13, 1020–1030. [Google Scholar] [PubMed]
- Howell, G.R.; Macalinao, D.G.; Sousa, G.L.; Walden, M.; Soto, I.; Kneeland, S.C.; Barbay, J.M.; King, B.L.; Marchant, J.K.; Hibbs, M.; et al. Molecular clustering identifies complement and Endothelin induction as early events in a mouse model of glaucoma. J. Clin. Invest. 2011, 121, 1429–1444. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Johnson, E.C.; Cepurna, W.O.; Dyck, J.A.; Doser, T.; Morrison, J.C. Early Gene Expression Changes in the Retinal Ganglion Cell Layer of a Rat Glaucoma Model. Invest. Ophthalmol. Vis. Sci. 2011, 52, 1460–1473. [Google Scholar] [CrossRef][Green Version]
- Yang, Z.; Quigley, H.A.; Pease, M.E.; Yang, Y.; Qian, J.; Valenta, D.; Zack, D.J. Changes in Gene Expression in Experimental Glaucoma and Optic Nerve Transection: The Equilibrium between Protective and Detrimental Mechanisms. Invest. Ophthalmol. Vis. Sci. 2007, 48, 5539–5548. [Google Scholar] [CrossRef]
- Yoshimura, N.; Kikuchi, T.; Kuroiwa, S.; Gaun, S. Differential temporal and spatial expression of immediate early genes in retinal neurons after ischemia-reperfusion injury. Invest. Ophthalmol. Vis. Sci. 2003, 44, 2211–2220. [Google Scholar] [CrossRef][Green Version]
- Wilson, A.S.; Hobbs, B.G.; Shen, W.Y.; Speed, T.P.; Schmidt, U.; Begley, C.G.; Rakoczy, P.E. Argon laser photocoagulation-induced modification of gene expression in the retina. Invest. Ophthalmol. Vis. Sci. 2003, 44, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, W.; Dentchev, T.; Zeng, Y.; Wang, J.; Tsui, I.; Tobias, J.W.; Bennett, J.; Baldwin, D.; Dunaief, J.L. Light damage induced changes in mouse retinal gene expression. Exp. Eye Res. 2004, 79, 239–247. [Google Scholar] [CrossRef]
- Agudo, M.; Pérez-Marín, M.C.; Sobrado-Calvo, P.; Lonngren, U.; Salinas-Navarro, M.; Cánovas, I.; Nadal-Nicolás, F.M.; Miralles-Imperial, J.; Hallbook, F.; Vidal-Sanz, M. Immediate up-regulation of proteins belonging to different branches of the apoptotic cascade in the retina after optic nerve transection and optic nerve crush. Invest. Ophthalmol. Vis. Sci. 2009, 50, 424–431. [Google Scholar] [CrossRef]
- Grimm, C.; Wenzel, A.; Hafezi, F.; Reme, C.E. Gene expression in the mouse retina: The effect of damaging light. Mol. Vis. 2000, 6, 252–260. [Google Scholar] [PubMed]
- Yasuda, M.; Tanaka, Y.; Ryu, M.; Tsuda, S.; Nakazawa, T. RNA sequence reveals mouse retinal transcriptome changes early after axonal injury. PLoS ONE 2014, 9, e93258. [Google Scholar] [CrossRef] [PubMed]
- Frebel, K.; Wiese, S. Signalling molecules essential for neuronal survival and differentiation. Biochem. Soc. Trans. 2006, 34, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Agudo, M.; Pérez-Marín, M.C.; Lönngren, U.; Sobrado, P.; Conesa, A.; Cánovas, I.; Salinas-Navarro, M.; Miralles-Imperial, J.; Hallböök, F.; Vidal-Sanz, M. Time course profiling of the retinal transcriptome after optic nerve transection and optic nerve crush. Mol. Vis. 2008, 14, 1050–1063. [Google Scholar] [PubMed]
- Sharma, T.P.; McDowell, C.M.; Liu, Y.; Wagner, A.H.; Thole, D.; Faga, B.P.; Wordinger, R.J.; Braun, T.A.; Clark, A.F. Optic nerve crush induces spatial and temporal gene expression patterns in retina and optic nerve of BALB/cJ mice. Mol. Neurodegeneration 2014, 9, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cepurna, W.O.; Dyck, J.A.; Doser, T.A.; Johnson, E.C.; Morrison, J.C. Retinal cell responses to elevated intraocular pressure: A gene array comparison between the whole retina and retinal ganglion cell layer. Invest. Ophthalmol. Vis. Sci. 2010, 51, 3003–3018. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grozdanic, S.D.; Lazic, T.; Kuehn, M.H.; Harper, M.M.; Kardon, R.H.; Kwon, Y.H.; Lavik, E.B.; Sakaguchi, D.S. Exogenous modulation of intrinsic optic nerve neuroprotective activity. Graefes. Arch. Clin. Exp. Ophthalmol. 2010, 248, 1105–1116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goldblum, D.; Mittag, T. Prospects for relevant glaucoma models with retinal ganglion cell damage in the rodent eye. Vis. Res. 2002, 42, 471–478. [Google Scholar] [CrossRef][Green Version]
- Calkins, D.J. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog. Retin. Eye Res. 2012, 31, 702–719. [Google Scholar] [CrossRef]
- Mykkänen, O.T.; Kalesnykas, G.; Adriaens, M.; Evelo, C.T.; Törrönen, R.; Kaarniranta, K. Bilberries potentially alleviate stress-related retinal gene expression induced by a high-fat diet in mice. Mol. Vis. 2012, 18, 2338–2351. [Google Scholar]
- van Iersel, M.P.; Kelder, T.; Pico, A.R.; Hanspers, K.; Coort, S.; Conklin, B.R.; Evelo, C. Presenting and exploring biological pathways with PathVisio. BMC Bioinform. 2008, 9, 399. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Iacobas, S.; Tanowitz, H.B.; de Carvalho, A.C.; Spray, D.C. Functional genomic fabrics are remodeled in a mouse model of Chagasic cardiomyopathy and restored following cell therapy. Microbes. Infect. 2018, 20, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Linden, R.; Perry, V.H. Massive retinotectal projection in rats. Brain Res. 1983, 272, 145–149. [Google Scholar] [CrossRef]
- Kravchick, D.O.; Hrdinka, M.; Iacobas, S.; Iacobas, D.A.; Kreutz, M.R.; Jordan, B.A. Synaptonuclear messenger PRR7 inhibits c-Jun ubiquitination and regulates NMDA mediated excitotoxicity. EMBOJ 2016, 35, 1923–1934. [Google Scholar] [CrossRef]
- Lee, P.R.; Cohen, J.E.; Iacobas, D.A.; Iacobas, S.; Fields, R.D. Gene networks activated by pattern-specific generation of action potentials in dorsal root ganglia neurons. Sci. Rep. 2017, 7, 43765. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Iacobas, S.; Nebieridze, N.; Velisek, L.; Veliskova, J. Estrogen protects neurotransmission transcriptome during status epilepticus. Front. Neurosci. 2018, 12, 332. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Urban, M.; Iacobas, S.; Scemes, E.; Spray, D.C. Array analysis of gene expression in connexin43 null astrocytes. Physiol. Genomics 2003, 15, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Iacobas, D.A.; Iacobas, S.; Urban-Maldonado, M.; Spray, D. Sensitivity of the brain transcriptome to connexin ablation. Biochim. Biofis. Acta 2005, 1711, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Iacobas, D.A.; Chachua, T.; Iacobas, S.; Benson, M.; Borges, K.; Veliskova, J.; Velisek, L. ACTH and PMX53 recover synaptic transcriptome alterations in a rat model of infantile spasms. Sci. Rep. 2018, 8, 5722. [Google Scholar] [CrossRef] [PubMed]
- Iacobas, D.A.; Iacobas, S.; Spray, D.C. Connexin43 and the brain transcriptome of the newborn mice. Genomics 2007, 89, 113–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iacobas, S.; Ede, N.; Iacobas, D.A. The Gene Master Regulators (GMR) Approach Provides Legitimate Targets for Personalized, Time-Sensitive Cancer Gene Therapy. Genes 2019, 10, E560. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Kutmon, M.; van Iersel, M.P.; Bohler, A.; Kelder, T.; Nunes, N.; Pico, A.R.; Evelo, C.T. PathVisio 3: An extendable pathway analysis toolbox. PLoS Comput. Biol. 2015, 11, e1004085. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Sharma, T.P.; Liu, Y.; Wordinger, R.J.; Pang, I.H.; Clark, A.F. Neuritin 1 promotes retinal ganglion cell survival and axonal regeneration following optic nerve crush. Cell Death Dis. 2015, 6, e1661. [Google Scholar] [CrossRef] [PubMed]
- Spray, D.C.; Iacobas, D.A. Organizational principles of the connexin-related brain transcriptome. J. Membr. Biol. 2007, 218, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Iacobas, D.A.; Iacobas, S.; Spray, D.C. Connexin-dependent transcellular transcriptomic networks in mouse brain. Prog. Biophys. Mol. Biol. 2007, 94, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Adesse, D.; Goldenberg, R.C.; Fortes, F.S.; Iacobas, D.A.; Iacobas, S.; Campos de Carvalho, A.C.; de Narareth, M.; Huang, H.; Tanowitz, H.B.; Garzoni, L.R.; et al. Gap junctions and Chagas’ disease. Adv. Parasitol. 2011, 76, 63–81. [Google Scholar] [CrossRef]
- Piri, N.; Kwong, J.M.; Caprioli, J. Crystallins in retinal ganglion cell survival and regeneration. Mol. Neurobiol. 2013, 48, 819–828. [Google Scholar] [CrossRef]
- Morozov, V.; Wawrousek, E.F. Caspase-dependent secondary lens fiber cell disintegration in αA-/αB-crystallin double-knockout mice. Development 2006, 133, 813–821. [Google Scholar] [CrossRef]
- Kamradt, M.C.; Lu, M.; Werner, M.E. The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J. Biol. Chem. 2005, 280, 11059–11066. [Google Scholar] [CrossRef]
- Mao, Y.W.; Liu, J.P.; Xiang, H.; Li, D.W. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004, 11, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Munemasa, Y.; Kwong, J.M.; Caprioli, J.; Piri, N. The Role of αA- and αB-Crystallins in the Survival of Retinal Ganglion Cells after Optic Nerve Axotomy. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3869–3875. [Google Scholar] [CrossRef]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef]
- Nishikura, K. A-to-I editing of coding and non-coding RNAs by ADARs. Nat. Rev. Mol. Cell. Biol. 2016, 17, 83–96. [Google Scholar] [CrossRef]
- Dávalos, V.; Súarez-López, L.; Castaño, J.; Messent, A.; Abasolo, I.; Fernandez, Y.; Guerra-Moreno, A.; Espín, E.; Armengol, M.; Musulen, E.; et al. Human SMC2 Protein, a Core Subunit of Human Condensin Complex, Is a Novel Transcriptional Target of the WNT Signaling Pathway and a New Therapeutic Target. J. Biol. Chem. 2012, 287, 43472–43481. [Google Scholar] [CrossRef] [PubMed]
- Eyer, J.; Peterson, A. Neurofilament-deficient axons and perikaryal aggregates in viable transgenic mice expressing a neurofilament-B-galactosidase fusion protein. Neuron 1994, 12, 398–405. [Google Scholar] [CrossRef]
- Ohara, O.; Gahara, Y.; Miyake, T.; Teraoka, H.; Kitamura, T. Neurofilament deficiency in quail caused by nonsense mutation in neurofilament-L gene. J. Cell Biol. 1993, 121, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Yum, S.W.; Zhang, J.; Mo, K.; Li, J.; Scherer, S.S. A novel recessive Nefl mutation causes a severe, early-onset axonal neuropathy. Ann. Neurol. 2009, 66, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Kittur, S.; Hoh, J.; Endo, H.; Tourtellotte, W.; Weeks, B.S.; Markesbery, W.; Adler, W. Cytoskeletal neurofilament gene expression in brain tissue from Alzheimer’s disease patients. I. Decrease in NF-L and NF-M message. J. Geriatr. Psychiatry Neurol. 1994, 7, 153–158. [Google Scholar] [CrossRef]
- Torres, V.I.; Vallejo, D.; Inestrosa, N.C. Emerging Synaptic Molecules as Candidates in the Etiology of Neurological Disorders. Neural. Plast. 2017, 2017, 8081758. [Google Scholar] [CrossRef] [PubMed]
- Nudo, R.J. Recovery after brain injury: Mechanisms and principles. Front. Hum. Neurosci. 2013, 7, 887. [Google Scholar] [CrossRef]
- Risner, M.L.; Pasini, S.; Cooper, M.L.; Lambert, W.S.; Calkins, D.J. Axogenic mechanism enhances retinal ganglion cell excitability during earlyprogression in glaucoma. Proc. Natl. Acad. Sci. USA 2018, 115, E2393–E2402. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.J.; Harman, C.D. Structure-function relations of parasol cells in the normal and glaucomatous primate retina. Invest. Ophthalmol. Vis. Sci. 2005, 46, 3197–3200. [Google Scholar] [CrossRef]
- Yuan, A.; Nixon, R.A. Specialized roles of neurofilament proteins in synapses: Relevance to neuropsychiatric disorders. Brain Res. Bull. 2016, 126, 334–346. [Google Scholar] [CrossRef]
- Yuan, A.; Sershen, H.; Veeranna Basavarajappa, B.S.; Kumar, A.; Hashim, A.; Berg, M.; Lee, J.H.; Sato, Y.; Rao, M.V.; Mohan, P.S.; et al. Neurofilament subunits are integral components of synapses and modulate neurotransmission and behavior in vivo. Mol. Psychiatry 2015, 20, 986–994. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Iacobas, S.; Werner, P.; Scemes, E.; Spray, D.C. Alteration of transcriptomic networks in adoptive-transfer experimental autoimmune encephalomyelitis. Front. Integr. Neurosci. 2007, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Templeton, J.P.; Freeman, N.E.; Nickerson, J.M.; Jablonski, M.M.; Rex, T.S.; Williams, R.W.; Geisert, E.E. Innate immune network in the retina activated by optic nerve crush. Invest. Ophthalmol. Vis. Sci. 2013, 54, 2599–2606. [Google Scholar] [CrossRef] [PubMed]
- Panagis, L.; Zhao, X.; Ge, Y.; Ren, L.; Mittag, T.W.; Danias, J. Retinal gene expression changes related to IOP exposure and axonal loss in DBA/2J mice. Invest. Ophthalmol. Vis. Sci. 2011, 52, 7807–7816. [Google Scholar] [CrossRef]
- Berry, R.H.; Qu, J.; John, S.W.M.; Howell, G.R.; Jakobs, T.C. Synapse Loss and Dendrite Remodeling in a Mouse Model of Glaucoma. PLoS ONE 2015, 10, e0144341. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.H.; Barres, B.A.; Stevens, B. The complement system: An unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 2012, 35, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Pisalyaput, K.; Tenner, A.J. Complement component C1q inhibits beta-amyloid- and serum amyloid P-induced neurotoxicity via caspase- and calpain-independent mechanisms. J. Neurochem. 2008, 104, 696–707. [Google Scholar] [CrossRef]
- Anderson, S.R.; Zhang, J.; Steele, M.R.; Romero, C.O.; Kautzman, A.G.; Schafer, D.P.; Vetter, M.L. Complement Targets Newborn Retinal Ganglion Cells for Phagocytic Elimination by Microglia. J. Neurosci. 2019, 39, 2025–2040. [Google Scholar] [CrossRef] [PubMed]
- Shagdarsuren, E.; Bidzhekov, K.; Djalali-Talab, Y.; Liehn, E.A.; Hristov, M.; Matthijsen, R.A.; Buurman, W.A.; Zernecke, A.; Weber, C. C1-esterase inhibitor protects against neointima formation after arterial injury in atherosclerosis-prone mice. Circulation 2008, 117, 70–78. [Google Scholar] [CrossRef]
- Nelson, B.R.; Gumuscu, B.; Hartman, B.H.; Reh, T.A. Notch activity is downregulated just prior to retinal ganglion cell differentiation. Dev. Neurosci. 2006, 28, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.; Nelson, B.R.; Buckingham, B.; Reh, T.A. Notch signaling regulates regeneration in the avian retina. Dev. Biol. 2007, 312, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Ghai, K.; Zelinka, C.; Fischer, A.J. Notch Signaling Influences Neuroprotective and Proliferative Properties of Mature Müller Glia. J. Neurosci. 2010, 30, 3101–3112. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, M.H.; Viehman, A.; Lippa, S.M.; Lippa, C.F. Notch-1 immunoexpression is increased in Alzheimer’s and Pick’s disease. J. Neurol. Sci. 2006, 244, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Mattson, M.P.; Cheng, A. Notch: From neural development to neurological disorders. J. Neurochem. 2008, 107, 1471–1481. [Google Scholar] [CrossRef]
- Dutta, D.; Mutsuddi, M.; Mukherjee, A. Synergistic interaction of Deltex and Hrp48 leads to JNK activation. Cell Biol. Int. 2019, 43, 350–357. [Google Scholar] [CrossRef]
- Eagar, T.N.; Tang, Q.; Wolfe, M.; He, Y.; Pear, W.S.; Bluestone, J.A. Notch 1 signaling regulates peripheral T cell activation. Immunity 2004, 20, 407–415. [Google Scholar] [CrossRef]
- Sestan, N.; Artavanis-Tsakonas, S.; Rakic, P. Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science 1999, 286, 741–746. [Google Scholar] [CrossRef]
- Berezovska, O.; McLean, P.; Knowles, R.; Frosh, M.; Lu, F.M.; Lux, S.E.; Hyman, B.T. Notch1 inhibits neurite outgrowth in postmitotic primary neurons. Neuroscience 1999, 93, 433–439. [Google Scholar] [CrossRef]
- Dvoriantchikova, G.; Grant, J.; Santos, A.R.; Hernandez, E.; Ivanov, D. Neuronal NAD(P)H oxidases contribute to ROS production and mediate RGC death after ischemia. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2823–2830. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yamagishi, S.; Matsui, T.; Jinnouchi, Y.; Fukami, K.; Imaizumi, T.; Yamakawa, R. Protective role of pigment epithelium-derived factor (PEDF) in early phase of experimental diabetic retinopathy. Diabetes/Metab. Res. Rev. 2009, 25, 678–686. [Google Scholar] [CrossRef]
- Qiu, Y.; Tao, L.; Lei, C.; Wang, J.; Yang, P.; Li, Q.; Lei, B. Downregulating p22phox ameliorates inflammatory response in Angiotensin II-induced oxidative stress by regulating MAPK and NF-κB pathways in ARPE-19 cells. Sci. Rep. 2015, 5, 14362. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.S.; Stein-O’Brien, G.L.; Shiau, F.; Cannon, G.H.; Davis-Marcisak, E.; Sherman, T.; Santiago, C.P.; Hoang, T.V.; Rajaii, F.; James-Esposito, R.E.; et al. Single-Cell RNA-Seq Analysis of Retinal Development Identifies NFI Factors as Regulating Mitotic Exit and Late-Born Cell Specification. Neuron 2019, 102, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.; Gronostajski, R.; Messina, G. Nuclear Factor One X in Development and Disease. Trends Cell Biol. 2019, 29, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Messina, G.; Biressi, S.; Monteverde, S.; Magli, A.; Cassano, M.; Perani, L.; Roncaglia, E.; Tagliafico, E.; Starnes, L.; Campbell, C.E.; et al. Nfix regulates fetal-specific transcription in developing skeletal muscle. Cell 2010, 140, 554–566. [Google Scholar] [CrossRef]
- Palacios, D.; Puri, P.L. Switch NFix developmental myogenesis. Dev. Cell 2010, 18, 340–341. [Google Scholar] [CrossRef][Green Version]
- Rossi, G.; Bonfanti, C.; Antonini, S.; Bastoni, M.; Monteverde, S.; Innocenzi, A.; Saclier, M.; Taglietti, V.; Messina, G. Silencing Nfix rescues muscular dystrophy by delaying muscle regeneration. Nat. Commun. 2017, 8, 1055. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Dong, Y.; Li, M.; Wang, X.; Jiang, M.; Yang, W.; Liu, G.; Sun, S.; Xu, W. A circular RNA from NFIX facilitates oxidative stress-induced H9c2 cells apoptosis. In vitro cellular & developmental biology. Animal 2020, 56, 715–722. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H.; Harris-Cerruti, C.; Groner, Y.; Wheeler, L.A.; Schwartz, M.; Yoles, E. RGC death in mice after optic nerve crush injury: Oxidative stress and neuroprotection. Invest. Ophthalmol. Vis. Sci. 2020, 41, 4169–4174. [Google Scholar]
- Vernazza, S.; Tirendi, S.; Bassi, A.M.; Traverso, C.E.; Saccà, S.C. Neuroinflammation in Primary Open-Angle Glaucoma. J. Clin. Med. 2020, 9, 3172. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.Y.F.; Rawji, K.S.; Ghorbani, S.; Xue, M.; Yong, V.W. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell. Mol. Immunol. 2019, 16, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Bishop, P.N. The eye as a complement dysregulation hotspot. Semin. Immunopathol. 2018, 40, 65–74. [Google Scholar] [CrossRef]
- Tezel, G.; Yang, X.; Luo, C.; Kain, A.D.; Powell, D.W.; Kuehn, M.H.; Kaplan, H.J. Oxidative stress and the regulation of complement activation in human glaucoma. Invest. Ophthalmol. Vis. Sci. 2010, 51, 5071–5082. [Google Scholar] [CrossRef]
- Tezel, G. Fourth ARVO/Pfizer Ophthalmics Research Institute Conference Working Group. The role of glia, mitochondria, and the immune system in glaucoma. IOVS 2009, 50, 1001–1012. [Google Scholar] [CrossRef]
- Hess, C.; Kemper, C. Complement-Mediated Regulation of Metabolism and Basic Cellular Processes. Immunity 2016, 45, 240–254. [Google Scholar] [CrossRef]
- Ries, A.; Goldberg, J.L.; Grimpe, B. A novel biological function for CD44 in axon growth of retinal ganglion cells identified by a bioinformatics approach. J. Neurochem. 2007, 103, 1491–1505. [Google Scholar] [CrossRef]
| Gene Name | Gene Symbol | Fold Change | p-Value |
|---|---|---|---|
| Neuritin 1 | Nrn1 | −18.82 | 0.0080 |
| Solute carrier family 17 (sodium-dependent inorganic phosphate cotransporter) member 6 | Slc17a6 | −14.95 | 0.0037 |
| Serine (or cysteine) peptidase inhibitor, clade B, member 1b | Serpinb1b | −12.79 | 0.0040 |
| Peripherin | Prph | −12.69 | 0.00009 |
| Neurofilament, medium polypeptide | Nefm | −11.77 | 0.0010 |
| Neurofilament, heavy polypeptide | Nefh | -11.42 | 0.0079 |
| Synuclein, gamma (breast cancer-specific protein 1) | Sncg | −10.76 | 0.0023 |
| POU class 4 homeobox 2 | Pou4f2 | −7.30 | 0.0088 |
| Neurofilament, light polypeptide | Nefl | −6.79 | 0.0027 |
| Serine (or cysteine) proteinase inhibitor, clade B, member 1a | Serpinb1a | −4.94 | 0.0028 |
| Tubulin polymerization-promoting protein family member 3 | Tppp3 | −4.56 | 0.0013 |
| Transmembrane protein 163 | Tmem163 | −4.32 | 0.0010 |
| Thy-1 cell surface antigen | Thy1 | −3.88 | 0.0272 |
| Major facilitator superfamily domain containing 6 | Mfsd6 | −3.73 | 0.0008 |
| Chemokine (C-X-C motif) ligand 13 | Cxcl13 | −3.05 | 0.0142 |
| Sodium channel, voltage-gated, type IV, beta | Scn4b | −2.91 | 0.0050 |
| ISL LIM homeobox 2 | Isl2 | −2.90 | 0.0067 |
| Sodium channel, voltage-gated, type I, beta | Scn1b | −2.83 | 0.033 |
| Calpain 1, (mu/I) large subunit | Capn1 | −2.79 | 0.0149 |
| RAS guanyl releasing protein 2 (calcium and DAG-regulated) | Rasgrp2 | −2.69 | 0.0153 |
| Sodium channel, voltage-gated, type II, alpha 1 | Scn2a1 | −2.59 | 0.0301 |
| Regulator of G-protein signaling 4 | Rgs4 | −2.48 | 0.0052 |
| Visinin-like 1 | Vsnl1 | −2.45 | 0.0212 |
| Synaptotagmin II | Syt2 | −2.43 | 0.0250 |
| NEL-like 2 (chicken) | Nell2 | −2.40 | 0.0006 |
| L1 cell adhesion molecule | L1cam | −2.35 | 0.0466 |
| Plastin 3 | Pls3 | −2.29 | 0.0141 |
| F-box protein 2 | Fbxo2 | −2.26 | 0.0041 |
| Complexin 1 | Cplx1 | −2.25 | 0.0393 |
| ELAV (embryonic lethal, abnormal vision, Drosophila)-like 2 (Hu antigen B) | Elavl2 | −2.21 | 0.0221 |
| Microsomal glutathione S-transferase 3 | Mgst3 | −2.19 | 0.0044 |
| N-acetyltransferase 8-like | Nat8l | −2.17 | 0.0155 |
| Annexin A6 | Anxa6 | −2.10 | 0.0047 |
| Rho GTPase activating protein 32 | Arhgap32 | −2.08 | 0.0288 |
| Leucine-rich repeat LGI family, member 3 | Lgi3 | −2.08 | 0.0132 |
| Tyrosine 3-monooxygenase/tryptophan 5- monooxygenase activation protein, eta polypeptide | Ywhah | −2.05 | 0.0008 |
| Ly6/neurotoxin 1 | Lynx1 | −2.03 | 0.0070 |
| gb|Rattus norvegicus similar to glyceraldehyde-3-phosphate dehydrogenase (LOC304769), mRNA [XM_222600] | XM_222600 | −2.00 | 0.0275 |
| Receptor (G protein-coupled) activity modifying protein 3 | Ramp3 | −1.98 | 0.0331 |
| FXYD domain-containing ion transport regulator 7 | Fxyd7 | −1.95 | 0.0023 |
| Sulfotransferase family 4A, member 1 | Sult4a1 | −1.93 | 0.0115 |
| Tubulin, beta 3 class III | Tubb3 | −1.93 | 0.0163 |
| Internexin neuronal intermediate filament protein, alpha | Ina | −1.93 | 0.0163 |
| Parvalbumin | Pvalb | −1.91 | 0.0048 |
| Potassium voltage-gated channel, Shal-related subfamily, member 2 | Kcnd2 | −1.89 | 0.0406 |
| Heme binding protein 2 | Hebp2 | −1.89 | 0.0306 |
| Pannexin 2 | Panx2 | −1.89 | 0.0050 |
| Gremlin 2 | Grem2 | −1.86 | 0.0147 |
| Prostaglandin F2 receptor negative regulator | Ptgfrn | −1.82 | 0.0021 |
| Tropomodulin 4 | Tmod4 | −1.81 | 0.0412 |
| Gene Name | Gene Symbol | Fold Change | p-Value |
|---|---|---|---|
| Cd74 molecule, major histocompatibility complex, class II invariant chain | Cd74 | 25.60 | 0.0030 |
| tc|HG2A_RAT (P10247) H-2 class II histocompatibility antigen gamma chain (MHC class II-associated invariant chain) (Ia antigen-associated invariant chain) (Ii) (CD74 antigen), partial (31%) [TC588776] | TC588776 | 11.55 | 0.0100 |
| Follistatin-like 3 (secreted glycoprotein) | Fstl3 | 5.31 | 0.0176 |
| Serpin peptidase inhibitor, clade G, member 1 | Serping1 | 3.49 | 0.0031 |
| Eph receptor A2 | Epha2 | 3.31 | 0.0061 |
| Ceruloplasmin (ferroxidase) | Cp | 3.29 | 0.0086 |
| ADAM metallopeptidase with thrombospondin type 1 motif, 1 | Adamts1 | 3.26 | 0.0097 |
| Chitinase 3-like 1 (cartilage glycoprotein-39) | Chi3l1 | 2.89 | 0.0051 |
| Cysteine and glycine-rich protein 3 (cardiac LIM protein) | Csrp3 | 2.69 | 0.0271 |
| Leucine rich repeat containing 15 | Lrrc15 | 2.61 | 0.0036 |
| Protein tyrosine phosphatase, receptor type, O | Ptpro | 2.58 | 0.0031 |
| Keratin 19 | Krt19 | 2.57 | 0.0013 |
| Solute carrier family 17 (anion/sugar transporter), member 5 | Slc17a5 | 2.54 | 0.0175 |
| Scavenger receptor class A, member 3 | Scara3 | 2.47 | 0.0086 |
| Ellis van Creveld syndrome 2 homolog (human) | Evc2 | 2.47 | 0.0449 |
| Cysteine and glycine-rich protein 2 | Csrp2 | 2.44 | 0.0121 |
| Complement component 1, s subcomponent | C1s | 2.43 | 0.0157 |
| S100 calcium binding protein A3 | S100a3 | 2.42 | 0.0366 |
| Complement component 4A (Rodgers blood group) | C4a | 2.41 | 0.0118 |
| PR domain containing 9 | Prdm9 | 2.36 | 0.0225 |
| Unknown | A_64_P105338 | 2.35 | 0.0068 |
| Endothelin converting enzyme-like 1 | Ecel1 | 2.34 | 0.0199 |
| TIMP metallopeptidase inhibitor 1 | Timp1 | 2.33 | 0.0115 |
| Complement factor B | Cfb | 2.26 | 0.0046 |
| Transmembrane protein 176A | Tmem176a | 2.19 | 0.0108 |
| Solute carrier family 6 (neurotransmitter transporter, creatine), member 8 | Slc6a8 | 2.18 | 0.0179 |
| Janus kinase 3 | Jak3 | 2.17 | 0.0144 |
| Complement component 3 | C3 | 2.17 | 0.0104 |
| Interferon regulatory factor 2 binding protein-like | Irf2bpl | 2.16 | 0.0412 |
| SMAD family member 1 | Smad1 | 2.12 | 0.0045 |
| Aminoadipate aminotransferase | Aadat | 2.09 | 0.0316 |
| Plexin D1 | Plxnd1 | 2.09 | 0.0250 |
| Transmembrane BAX inhibitor motif containing 1 | Tmbim1 | 2.08 | 0.0143 |
| S100 calcium-binding protein A4 | S100a4 | 2.03 | 0.0038 |
| EGF-containing fibulin-like extracellular matrix protein 1 | Efemp1 | 2.02 | 0.0229 |
| Interferon induced transmembrane protein 3 | Ifitm3 | 2.00 | 0.0251 |
| Interferon, alpha-inducible protein 27-like 2B | Ifi27l2b | 1.99 | 0.0214 |
| Guanylate binding protein 2, interferon-inducible | Gbp2 | 1.96 | 0.0295 |
| Ectonucleoside triphosphate diphosphohydrolase 2 | Entpd2 | 1.96 | 0.0153 |
| Interferon-induced protein 44-like | Ifi44l | 1.95 | 0.0349 |
| Lipopolysaccharide-induced TNF factor | Litaf | 1.94 | 0.0245 |
| Connective tissue growth factor | Ctgf | 1.94 | 0.0261 |
| Notch1 | Notch1 | 1.94 | 0.0087 |
| Cytochrome b-245, alpha polypeptide | Cyba | 1.93 | 0.0306 |
| SP110 nuclear body protein | Sp110 | 1.93 | 0.0346 |
| biogenesis of lysosomal organelles complex-1, subunit 3 | Bloc1s3 | 1.91 | 0.0152 |
| Tachykinin receptor 1 | Tacr1 | 1.91 | 0.0335 |
| Coiled-coil domain containing 87 | Ccdc87 | 1.90 | 0.0331 |
| Ataxin 7-like 2 | Atxn7l2 | 1.90 | 0.0320 |
| G protein-coupled receptor 37 | Gpr37 | 1.87 | 0.0286 |
| Microarray | qPCR | ||||
|---|---|---|---|---|---|
| Gene Name | Pathway | ONC vs. CTR | p-Value | ONC vs. CTR | p-Value |
| Cd74 | N/A | 25.60 | 0.003 | 28.56 | 0.010 |
| C3 | Complement Cascade | 2.17 | 0.010 | 3.242 | 0.046 |
| Cyba | Oxidative stress | 1.94 | 0.03 | 1.41 | 0.01 |
| Fyn | Kit receptor Signaling | 1.63 | 0.045 | 1.47 | 0.04 |
| Tubb3 | RGC marker | −1.94 | 0.016 | −3.93 | 0.004 |
| Nefm | RGC marker | −11.78 | 0.001 | −19,27 | <0.0001 |
| Nell2 | RGC marker | −2.41 | 0.0006 | −4.15 | 0.0012 |
| Gene Name | Gene Symbol | Fold Change | p-Value |
|---|---|---|---|
| Neuritin 1 | Nrn1 | −18.82 | 0.0080 |
| Solute carrier family 17 (sodium-dependent inorganic phosphate cotransporter) member 6 | Slc17a6 | −14.95 | 0.0037 |
| Peripherin | Prph | −12.07 | 0.00009 |
| Neurofilament, medium polypeptide | Nefm | −11.77 | 0.001 |
| Neurofilament, heavy polypeptide | Nefh | −11.42 | 0.0079 |
| Synuclein, gamma (breast cancer-specific protein 1) | Sncg | −10.76 | 0.0023 |
| POU class 4 homeobox 2 | Pou4f2 | −7.30 | 0.0088 |
| Neurofilament, light polypeptide | Nefl | −6.79 | 0.0027 |
| Tubulin polymerization-promoting protein family member 3 | Tppp3 | −4.56 | 0.0013 |
| Thy-1 cell surface antigen | Thy1 | −3.88 | 0.0272 |
| Regulator of G-protein signaling 4 | Rgs4 | −2.48 | 0.005 |
| Visinin-like 1 | Vsnl1 | −2.46 | 0.021 |
| Synaptogmin 2 | Syt2 | −2.43 | 0.025 |
| F-box protein 2 | Fbxo2 | −2.27 | 0.004 |
| Tyrosine-3-monooxygenase/tryptophan 5-monooxygenase activation protein, eta polypeptide | Ywhah | −2.05 | 0.0008 |
| Tubulin, beta 3 class III | Tubb3 | −1.93 | 0.016 |
| Pannexin 2 | Panx2 | −1.89 | 0.005 |
| Gene Ontology | Number of Genes | Genes |
|---|---|---|
| Biological Process | ||
| Lens development in camera-type eye | 4 | Crygb, Crygc, Crygd, and Crygs |
| Sensory organ development | 7 | Cryba4, Crybb2, Crygb, Crygc, Crygd, Crygs, and Krt13 |
| Camera-type eye development | 6 | Cryba4, Crybb2, Crygb, Crygc, Crygd, and Crygs |
| Molecular Function | ||
| Structural constituent of eye lens | 9 | Cryaa, Cryba4, Crybb2, Crybb3, Crygb, Crygc, Crygd, Crygs, and Lim2 |
| Structural molecule activity | 13 | Anxa1, Cryaa, Cryba4, Crybb2, Crybb3, Crygb, Crygc, Crygd, Crygs, Krt12, Krt13, Krt80, and Lim2 |
| Pathway | Positive (r) | Measured (n) | Total | % | Z Score | p-Value (Permuted) |
|---|---|---|---|---|---|---|
| Complement activation, classical pathway | 3 | 15 | 18 | 20.00% | 6.27 | 0 |
| Complement and coagulation cascades | 5 | 56 | 63 | 8.93% | 4.96 | 0 |
| Delta-Notch signaling pathway | 4 | 72 | 82 | 5.56% | 3.13 | 0.009 |
| Oxidative stress | 2 | 26 | 28 | 7.69% | 2.81 | 0.031 |
| Notch signaling pathway | 2 | 32 | 45 | 6.25% | 2.41 | 0.017 |
| Kit receptor signaling pathway | 3 | 64 | 68 | 4.69% | 2.34 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Victorino, P.H.; Marra, C.; Iacobas, D.A.; Iacobas, S.; Spray, D.C.; Linden, R.; Adesse, D.; Petrs-Silva, H. Retinal Genomic Fabric Remodeling after Optic Nerve Injury. Genes 2021, 12, 403. https://doi.org/10.3390/genes12030403
Victorino PH, Marra C, Iacobas DA, Iacobas S, Spray DC, Linden R, Adesse D, Petrs-Silva H. Retinal Genomic Fabric Remodeling after Optic Nerve Injury. Genes. 2021; 12(3):403. https://doi.org/10.3390/genes12030403
Chicago/Turabian StyleVictorino, Pedro Henrique, Camila Marra, Dumitru Andrei Iacobas, Sanda Iacobas, David C. Spray, Rafael Linden, Daniel Adesse, and Hilda Petrs-Silva. 2021. "Retinal Genomic Fabric Remodeling after Optic Nerve Injury" Genes 12, no. 3: 403. https://doi.org/10.3390/genes12030403
APA StyleVictorino, P. H., Marra, C., Iacobas, D. A., Iacobas, S., Spray, D. C., Linden, R., Adesse, D., & Petrs-Silva, H. (2021). Retinal Genomic Fabric Remodeling after Optic Nerve Injury. Genes, 12(3), 403. https://doi.org/10.3390/genes12030403








