Reduced Interaction of Aggregated α-Synuclein and VAMP2 by Environmental Enrichment Alleviates Hyperactivity and Anxiety in a Model of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Transgenic Mouse Model of Parkinson’s Disease
2.2. Genotyping
2.3. Housing Conditions
2.4. Behavioral Assessment
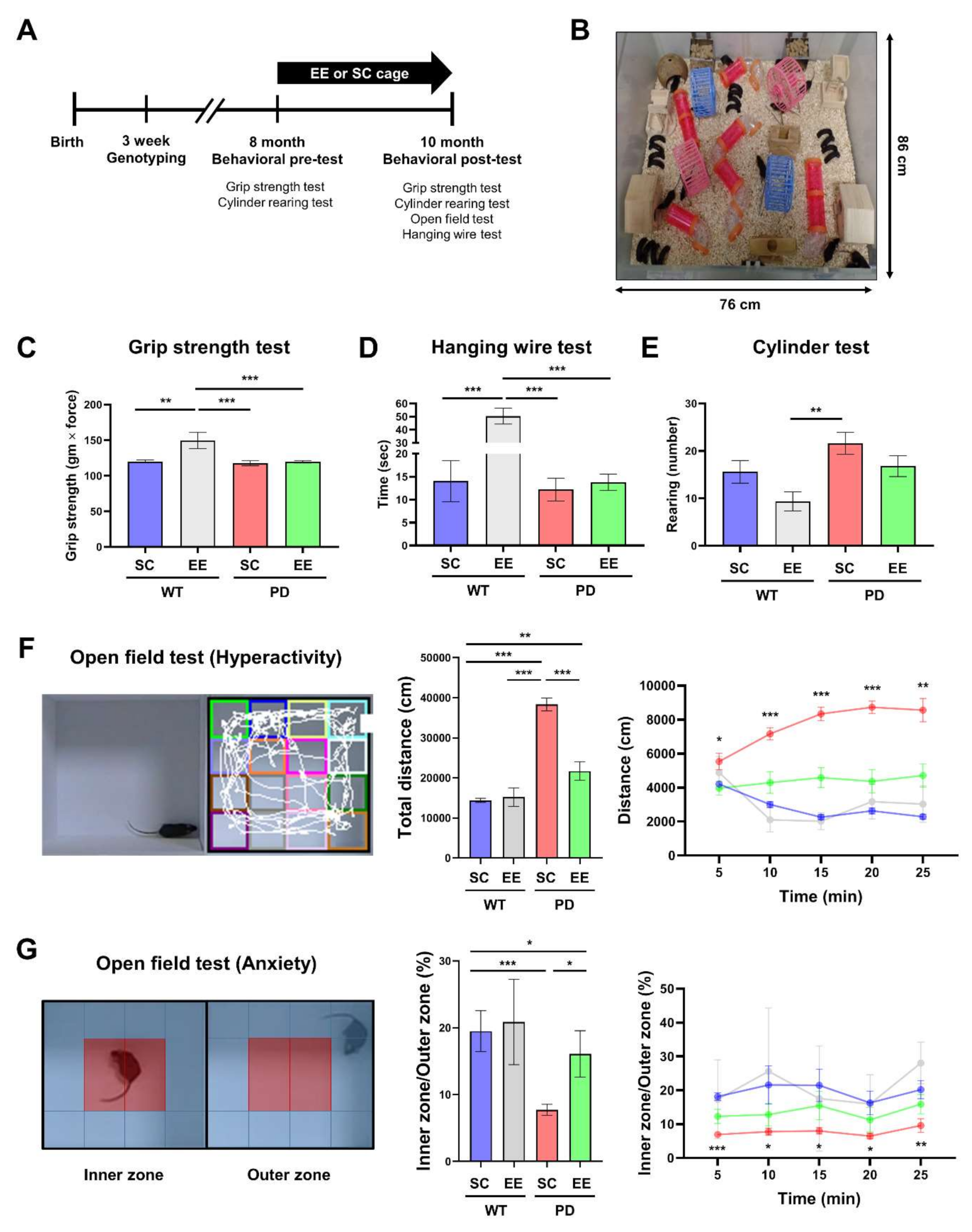
2.4.1. Grip Strength Test
2.4.2. Hanging Wire Test
2.4.3. Cylinder Test
2.4.4. Open Field Test
2.5. RNA Extraction
2.6. Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
2.7. Western Blot Analysis
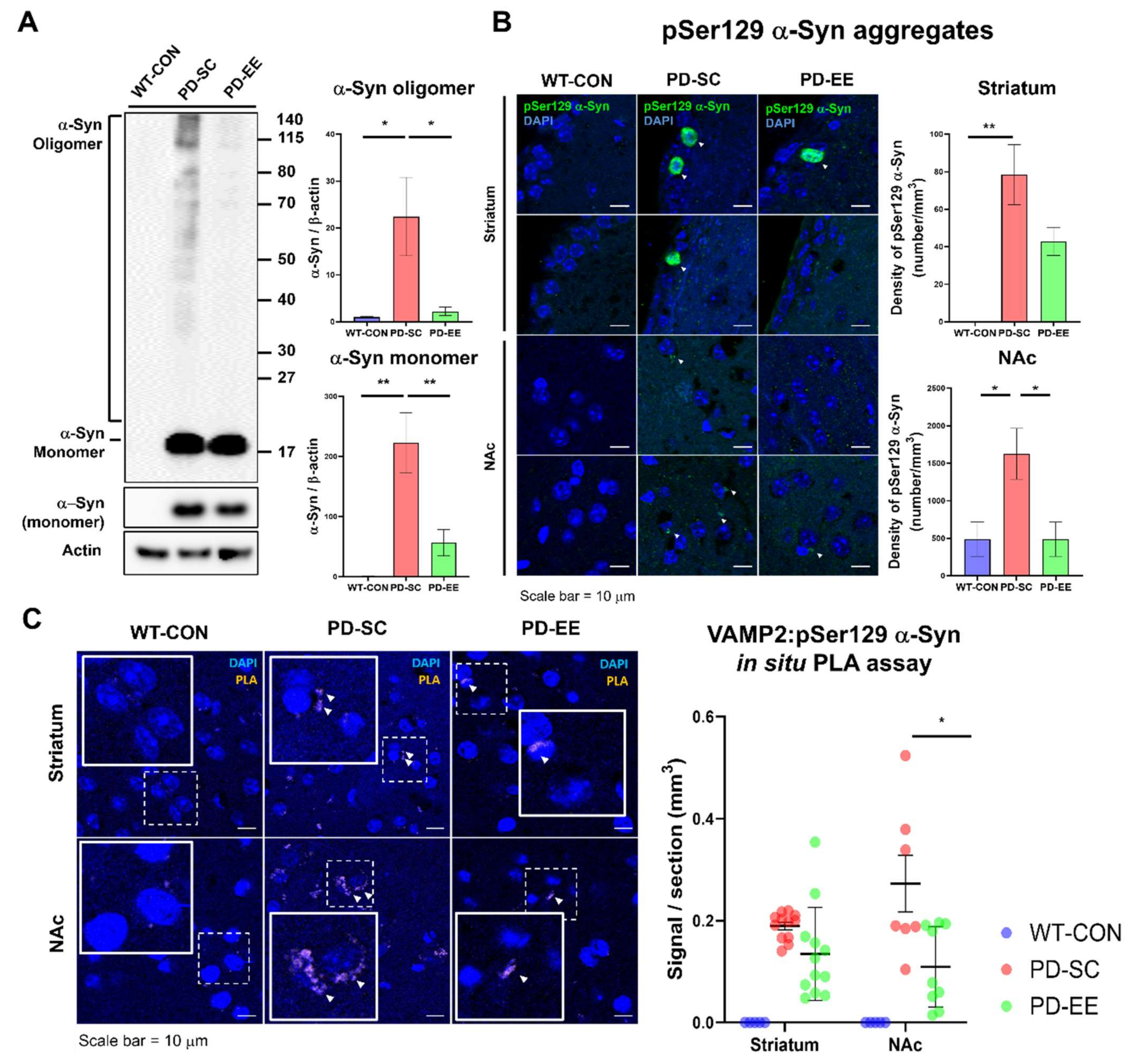
2.8. Immunohistochemistry (IHC)
2.9. In Situ Proximity Ligation Assay (PLA)
2.10. Statistical Analysis
3. Results
3.1. EE Ameliorates Hyperactivity and Anxiety but Not Motor Function in hA53T α-Syn-Overexpressing Transgenic Mice
3.2. EE Reduces the Degeneration of Dopaminergic Nerve Terminals in the NAc of hA53T α-Syn-Overexpressing Transgenic Mice
3.3. EE Increases Soluble N-Ethylmaleimide-Sensitive Factor Attachment Protein Receptor (SNARE) Expression and Alters Dopamine Transporters and Receptors in the NAc of hA53T α-Syn-Overexpressing Transgenic Mice
3.4. EE Reduces Aggregated α-Syn Levels and the Interaction Between α-Syn and VAMP-2 in the NAc of hA53T α-Syn-Overexpressing Transgenic Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Cairns, N.J.; Lantos, P.L.; Goedert, M. Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci. Lett. 1998, 251, 205–208. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.-Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.D.; Rueter, S.M.; Trojanowski, J.Q.; Lee, V.M.-Y. Synucleins are developmentally expressed, and α-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 2000, 20, 3214–3220. [Google Scholar] [CrossRef] [PubMed]
- Yavich, L.; Tanila, H.; Vepsäläinen, S.; Jäkälä, P. Role of α-synuclein in presynaptic dopamine recruitment. J. Neurosci. 2004, 24, 11165–11170. [Google Scholar] [CrossRef]
- Cabin, D.E.; Shimazu, K.; Murphy, D.; Cole, N.B.; Gottschalk, W.; McIlwain, K.L.; Orrison, B.; Chen, A.; Ellis, C.E.; Paylor, R. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J. Neurosci. 2002, 22, 8797–8807. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-K.; Choi, M.-G.; Kim, J.-Y.; Yang, Y.; Lai, Y.; Kweon, D.-H.; Lee, N.K.; Shin, Y.-K. Large α-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc. Natl. Acad. Sci. USA 2013, 110, 4087–4092. [Google Scholar] [CrossRef] [PubMed]
- Nemani, V.M.; Lu, W.; Berge, V.; Nakamura, K.; Onoa, B.; Lee, M.K.; Chaudhry, F.A.; Nicoll, R.A.; Edwards, R.H. Increased expression of α-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 2010, 65, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Roy, S. α-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J. Neurosci. 2012, 32, 10129–10135. [Google Scholar] [CrossRef] [PubMed]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. α-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Kim, J.; Hawk, B.J.; Shin, Y.-K. α-Synuclein may cross-bridge v-SNARE and acidic phospholipids to facilitate SNARE-dependent vesicle docking. Biochem. J. 2017, 474, 2039–2049. [Google Scholar] [CrossRef]
- Giasson, B.I.; Duda, J.E.; Quinn, S.M.; Zhang, B.; Trojanowski, J.Q.; Lee, V.M.-Y. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron 2002, 34, 521–533. [Google Scholar] [CrossRef]
- Lee, M.K.; Stirling, W.; Xu, Y.; Xu, X.; Qui, D.; Mandir, A.S.; Dawson, T.M.; Copeland, N.G.; Jenkins, N.A.; Price, D.L. Human α-synuclein-harboring familial Parkinson’s disease-linked Ala-53→ Thr mutation causes neurodegenerative disease with α-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 8968–8973. [Google Scholar] [CrossRef] [PubMed]
- Farrell, K.F.; Krishnamachari, S.; Villanueva, E.; Lou, H.; Alerte, T.N.; Peet, E.; Drolet, R.E.; Perez, R.G. Non-motor parkinsonian pathology in aging A53T α-Synuclein mice is associated with progressive synucleinopathy and altered enzymatic function. J. Neurochem. 2014, 128, 536–546. [Google Scholar] [CrossRef]
- Unger, E.L.; Eve, D.J.; Perez, X.A.; Reichenbach, D.K.; Xu, Y.; Lee, M.K.; Andrews, A.M. Locomotor hyperactivity and alterations in dopamine neurotransmission are associated with overexpression of A53T mutant human α-synuclein in mice. Neurobiol. Dis. 2006, 21, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Song, N.; Jia, F.; Tang, T.; Bao, W.; Zuo, C.; Xie, J.; Jiang, H. Genomic DNA levels of mutant alpha-synuclein correlate with non-motor symptoms in an A53T Parkinson’s disease mouse model. Neurochem. Int. 2018, 114, 71–79. [Google Scholar] [CrossRef]
- Koch, J.; Bitow, F.; Haack, J.; d’Hedouville, Z.; Zhang, J.; Tönges, L.; Michel, U.; Oliveira, L.; Jovin, T.; Liman, J. Alpha-Synuclein affects neurite morphology, autophagy, vesicle transport and axonal degeneration in CNS neurons. Cell Death Dis. 2015, 6, e1811. [Google Scholar] [CrossRef] [PubMed]
- Riley, B.E.; Gardai, S.J.; Emig-Agius, D.; Bessarabova, M.; Ivliev, A.E.; Schüle, B.; Alexander, J.; Wallace, W.; Halliday, G.M.; Langston, J.W. Systems-based analyses of brain regions functionally impacted in Parkinson’s disease reveals underlying causal mechanisms. PLoS ONE 2014, 9, e102909. [Google Scholar] [CrossRef] [PubMed]
- Carriere, N.; Besson, P.; Dujardin, K.; Duhamel, A.; Defebvre, L.; Delmaire, C.; Devos, D. Apathy in Parkinson’s disease is associated with nucleus accumbens atrophy: A magnetic resonance imaging shape analysis. Mov. Disord. 2014, 29, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Juárez Olguín, H.; Calderon Guzman, D.; Hernandez Garcia, E.; Barragán Mejía, G. The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxid. Med. Cell. Longevity 2016, 2016, 9730467. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.J.; Nestler, E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013, 14, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Kano, O.; Ikeda, K.; Cridebring, D.; Takazawa, T.; Yoshii, Y.; Iwasaki, Y. Neurobiology of depression and anxiety in Parkinson’s disease. Parkinson’s Dis. 2011, 2011, 143547. [Google Scholar] [CrossRef] [PubMed]
- Iarkov, A.; Barreto, G.E.; Grizzell, J.A.; Echeverria, V. Strategies for the treatment of Parkinson’s disease: Beyond dopamine. Front. Aging Neurosci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Salgado, S.; Kaplitt, M.G. The nucleus accumbens: A comprehensive review. Stereotact. Funct. Neurosurg. 2015, 93, 75–93. [Google Scholar] [CrossRef]
- Williams, N.; Short, B.; Williams, E.; Jeffery, A.; Kerns, S.; Sahlem, G.; Hanlon, C.; Revuelta, G.; Takacs, I.; George, M. Deep Brain Stimulation of the Nucleus Accumbens Has Positive Effects on Parkinson’s Disease-Related Apathy (P7.050). Neurology 2014, 82, 7.050. [Google Scholar]
- Rosenzweig, M.R.; Bennett, E.L.; Hebert, M.; Morimoto, H. Social grouping cannot account for cerebral effects of enriched environments. Brain Res. 1978, 153, 563–576. [Google Scholar] [CrossRef]
- Janssen, H.; Ada, L.; Bernhardt, J.; McElduff, P.; Pollack, M.; Nilsson, M.; Spratt, N.J. An enriched environment increases activity in stroke patients undergoing rehabilitation in a mixed rehabilitation unit: A pilot non-randomized controlled trial. Disabil. Rehabil. 2014, 36, 255–262. [Google Scholar] [CrossRef]
- White, J.H.; Alborough, K.; Janssen, H.; Spratt, N.; Jordan, L.; Pollack, M. Exploring staff experience of an “enriched environment” within stroke rehabilitation: A qualitative sub-study. Disabil. Rehabil. 2014, 36, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, S.; Schwarzschild, M.; Hernan, M.; Ascherio, A. Physical activity and the risk of Parkinson disease. Neurology 2005, 64, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Park, Y.; Huang, X.; Hollenbeck, A.; Blair, A.; Schatzkin, A.; Chen, H. Physical activities and future risk of Parkinson disease. Neurology 2010, 75, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Castello, N.; Cotman, C.W. Exercise and time-dependent benefits to learning and memory. Neuroscience 2010, 167, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C. Physical activity and the maintenance of cognition: Learning from animal models. Alzheimers Dement 2007, 3, S30–S37. [Google Scholar] [CrossRef] [PubMed]
- Petzinger, G.M.; Fisher, B.E.; Van Leeuwen, J.E.; Vukovic, M.; Akopian, G.; Meshul, C.K.; Holschneider, D.P.; Nacca, A.; Walsh, J.P.; Jakowec, M.W. Enhancing neuroplasticity in the basal ganglia: The role of exercise in Parkinson’s disease. Mov. Disord. 2010, 25, S141–S145. [Google Scholar] [CrossRef]
- Faherty, C.J.; Shepherd, K.R.; Herasimtschuk, A.; Smeyne, R.J. Environmental enrichment in adulthood eliminates neuronal death in experimental Parkinsonism. Mol. Brain. Res. 2005, 134, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, N.; Haack, A.; Meshul, C. Enriched environment promotes similar neuronal and behavioral recovery in a young and aged mouse model of Parkinson’s disease. Neuroscience 2011, 172, 443–452. [Google Scholar] [CrossRef]
- Fan, L.-W.; Lin, S.; Pang, Y.; Lei, M.; Zhang, F.; Rhodes, P.G.; Cai, Z. Hypoxia-ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behav. Brain Res. 2005, 165, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Im, S.; Yu, J.; Park, E.; Lee, J.; Kim, H.; Park, K.I.; Kim, G.; Park, C.; Cho, S.-R. Induction of striatal neurogenesis enhances functional recovery in an adult animal model of neonatal hypoxic-ischemic brain injury. Neuroscience 2010, 169, 259–268. [Google Scholar] [CrossRef]
- Carola, V.; D’Olimpio, F.; Brunamonti, E.; Mangia, F.; Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 2002, 134, 49–57. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Park, H.; Chang, K.-A. Therapeutic Potential of Repeated Intravenous Transplantation of Human Adipose-Derived Stem Cells in Subchronic MPTP-Induced Parkinson’s Disease Mouse Model. Int. J. Mol. Sci. 2020, 21, 8129. [Google Scholar] [CrossRef]
- Che, Y.; Hou, L.; Sun, F.; Zhang, C.; Liu, X.; Piao, F.; Zhang, D.; Li, H.; Wang, Q. Taurine protects dopaminergic neurons in a mouse Parkinson’s disease model through inhibition of microglial M1 polarization. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Denenberg, V.H. Open-field behavior in the rat: What does it mean? Ann. N. Y. Acad. Sci. 1969, 159, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.N.; Cummins, R.A. The open-field test: A critical review. Psychol. Bull. 1976, 83, 482. [Google Scholar] [CrossRef] [PubMed]
- Valvassori, S.S.; Varela, R.B.; Quevedo, J. Animal models of mood disorders: Focus on bipolar disorder and depression. In Animal Models for the Study of Human Disease; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2017; pp. 991–1001. [Google Scholar]
- Ostrerova-Golts, N.; Petrucelli, L.; Hardy, J.; Lee, J.M.; Farer, M.; Wolozin, B. The A53T α-synuclein mutation increases iron-dependent aggregation and toxicity. J. Neurosci. 2000, 20, 6048–6054. [Google Scholar] [CrossRef]
- Zhou, W.; Barkow, J.C.; Freed, C.R. Running wheel exercise reduces α-synuclein aggregation and improves motor and cognitive function in a transgenic mouse model of Parkinson’s disease. PLoS ONE 2017, 12, e0190160. [Google Scholar] [CrossRef]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Daher, J.P.L.; Pletnikova, O.; Biskup, S.; Musso, A.; Gellhaar, S.; Galter, D.; Troncoso, J.C.; Lee, M.K.; Dawson, T.M.; Dawson, V.L. Neurodegenerative phenotypes in an A53T α-synuclein transgenic mouse model are independent of LRRK2. Hum. Mol. Genet. 2012, 21, 2420–2431. [Google Scholar] [CrossRef] [PubMed]
- Pellicano, C.; Benincasa, D.; Pisani, V.; Buttarelli, F.R.; Giovannelli, M.; Pontieri, F.E. Prodromal non-motor symptoms of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2007, 3, 145. [Google Scholar] [CrossRef] [PubMed]
- Shiba, M.; Bower, J.H.; Maraganore, D.M.; McDonnell, S.K.; Peterson, B.J.; Ahlskog, J.E.; Schaid, D.J.; Rocca, W.A. Anxiety disorders and depressive disorders preceding Parkinson’s disease: A case-control study. Mov. Disord. 2000, 15, 669–677. [Google Scholar] [CrossRef]
- Dagher, A.; Robbins, T.W. Personality, addiction, dopamine: Insights from Parkinson’s disease. Neuron 2009, 61, 502–510. [Google Scholar] [CrossRef]
- Barbosa, P.; Hapuarachchi, B.; Djamshidian, A.; Strand, K.; Lees, A.J.; de Silva, R.; Holton, J.L.; Warner, T.T. Lower nucleus accumbens α-synuclein load and D3 receptor levels in Parkinson’s disease with impulsive compulsive behaviours. Brain 2019, 142, 3580–3591. [Google Scholar] [CrossRef]
- Guerreiro, P.S.; Coelho, J.E.; Sousa-Lima, I.; Macedo, P.; Lopes, L.V.; Outeiro, T.F.; Pais, T.F. Mutant A53T α-Synuclein Improves Rotarod Performance Before Motor Deficits and Affects Metabolic Pathways. Neuromol. Med. 2017, 19, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.T.; Fahrenkopf, A.; Choquette, J.M.; Vermilyea, S.C.; Lee, M.K.; Vossel, K. Ablating tau reduces hyperexcitability and moderates electroencephalographic slowing in transgenic mice expressing A53T human α-synuclein. Front. Neurol. 2020, 11, 563. [Google Scholar] [CrossRef] [PubMed]
- Darios, F.; Ruiperez, V.; Lopez, I.; Villanueva, J.; Gutierrez, L.M.; Davletov, B. α-Synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis. EMBO Rep. 2010, 11, 528–533. [Google Scholar] [CrossRef]
- Chandra, S.; Gallardo, G.; Fernández-Chacón, R.; Schlüter, O.M.; Südhof, T.C. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 2005, 123, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Reitböck, P.; Anichtchik, O.; Bellucci, A.; Iovino, M.; Ballini, C.; Fineberg, E.; Ghetti, B.; Della Corte, L.; Spano, P.; Tofaris, G.K. SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain 2010, 133, 2032–2044. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.; Double, K.L.; Lastres-Becker, I.; Tozzi, A.; Tantucci, M.; Bockhart, V.; Bonin, M.; García-Arencibia, M.; Nuber, S.; Schlaudraff, F. A53T-alpha-synuclein overexpression impairs dopamine signaling and striatal synaptic plasticity in old mice. PLoS ONE 2010, 5, e11464. [Google Scholar] [CrossRef]
- Seeman, P.; Niznik, H.B. Dopamine receptors and transporters in Parkinson’s disease and schizophrenia. FASEB J. 1990, 4, 2737–2744. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Yu, J.H.; Kim, J.Y.; Seo, J.H.; Park, E.S.; Kim, C.H.; Kim, H.; Cho, S.-R. Alteration of synaptic activity–regulating genes underlying functional improvement by long-term exposure to an enriched environment in the adult brain. Neurorehabilit. Neural Repair 2013, 27, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Yu, J.H.; Kim, C.H.; Choi, J.Y.; Seo, J.H.; Lee, M.-Y.; Yi, C.H.; Choi, T.H.; Ryu, Y.H.; Lee, J.E. Environmental enrichment enhances synaptic plasticity by internalization of striatal dopamine transporters. J. Cereb. Blood Flow Metab. 2016, 36, 2122–2133. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-Y.; Chae, M.; Yu, J.H.; Lee, M.Y.; Pyo, S.; Shin, Y.-K.; Baek, A.; Park, J.-W.; Park, E.S.; Choi, J.Y. Environmental enrichment upregulates striatal synaptic vesicle-associated proteins and improves motor function. Front. Neurol. 2018, 9, 465. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Wi, S.; Seo, J.H.; Pyo, S.; Cho, S.-R. Reduced Interaction of Aggregated α-Synuclein and VAMP2 by Environmental Enrichment Alleviates Hyperactivity and Anxiety in a Model of Parkinson’s Disease. Genes 2021, 12, 392. https://doi.org/10.3390/genes12030392
Kim K, Wi S, Seo JH, Pyo S, Cho S-R. Reduced Interaction of Aggregated α-Synuclein and VAMP2 by Environmental Enrichment Alleviates Hyperactivity and Anxiety in a Model of Parkinson’s Disease. Genes. 2021; 12(3):392. https://doi.org/10.3390/genes12030392
Chicago/Turabian StyleKim, Kyungri, Soohyun Wi, Jung Hwa Seo, Soonil Pyo, and Sung-Rae Cho. 2021. "Reduced Interaction of Aggregated α-Synuclein and VAMP2 by Environmental Enrichment Alleviates Hyperactivity and Anxiety in a Model of Parkinson’s Disease" Genes 12, no. 3: 392. https://doi.org/10.3390/genes12030392
APA StyleKim, K., Wi, S., Seo, J. H., Pyo, S., & Cho, S.-R. (2021). Reduced Interaction of Aggregated α-Synuclein and VAMP2 by Environmental Enrichment Alleviates Hyperactivity and Anxiety in a Model of Parkinson’s Disease. Genes, 12(3), 392. https://doi.org/10.3390/genes12030392






