Optic Atrophy and Inner Retinal Thinning in CACNA1F-Related Congenital Stationary Night Blindness
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Demographics and Clinical Features
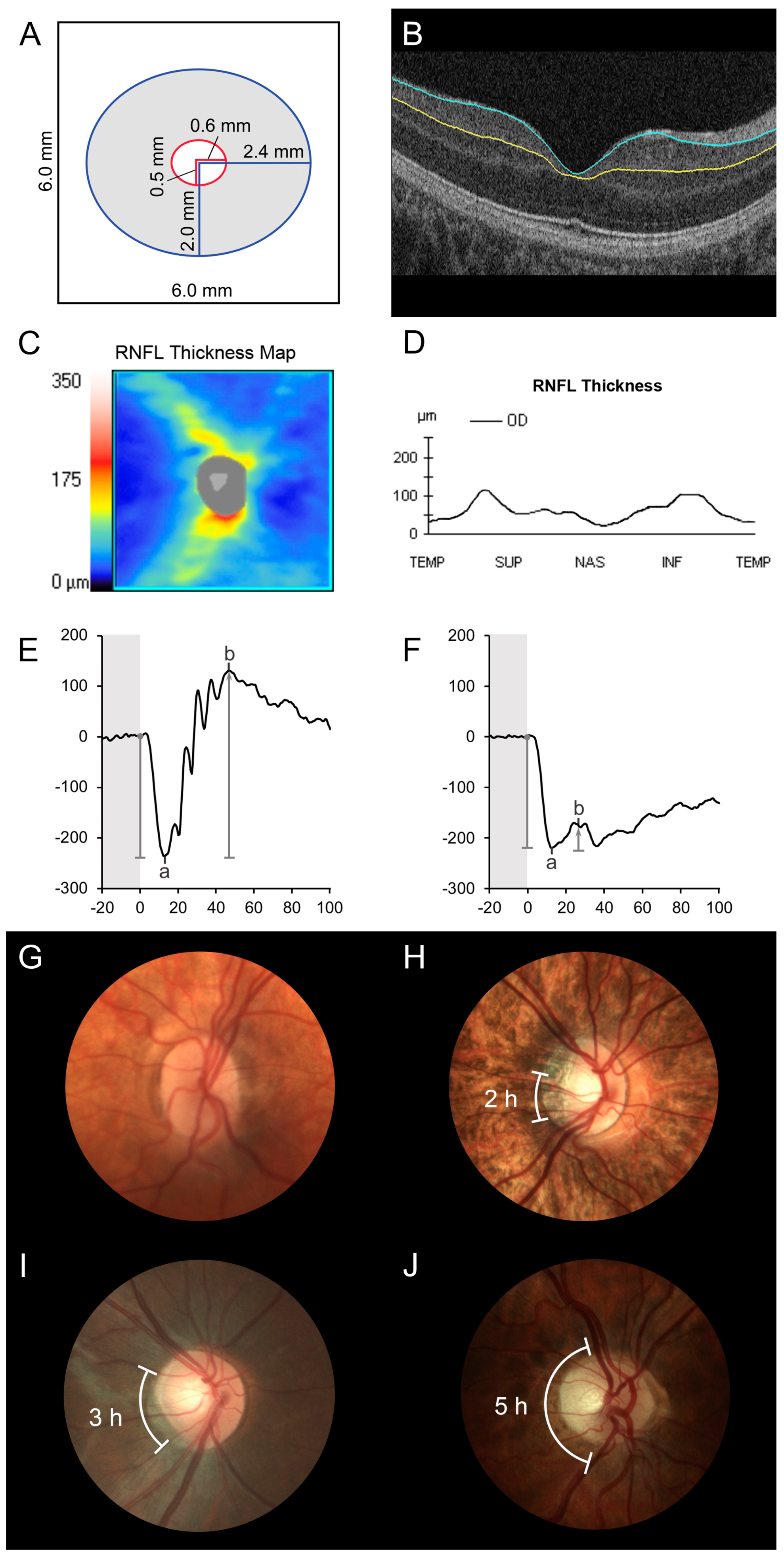
3.2. Optical Coherence Tomography
3.3. Full-Field Electroretinogram Results
3.4. Optic Disc Evaluation and Fundus Findings
3.5. Genetic Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zeitz, C.; Robson, A.G.; Audo, I. Congenital stationary night blindness: An analysis and update of genotype–phenotype correlations and pathogenic mechanisms. Prog. Retin. Eye Res. 2015, 45, 58–110. [Google Scholar] [CrossRef]
- Riggs, L.A. Electroretinography in Cases of Night Blindness. Am. J. Ophthalmol. 1954, 38, 70–78. [Google Scholar] [CrossRef]
- Schubert, G.; Bornschein, H. Beitrag zur Analyse des menschlichen Elektroretinogramms. Ophthalmologica 1952, 123, 396–413. [Google Scholar] [CrossRef]
- Marmor, M.F. Fundus albipunctatus: A clinical study of the fundus lesions, the physiologic deficit, and the vitamin a metabolism. Doc. Ophthalmol. 1977, 43, 277–302. [Google Scholar] [CrossRef]
- Fuchs, S.; Nakazawa, M.; Maw, M.; Tamai, M.; Oguchi, Y.; Gal, A. A homozygous 1–base pair deletion in the arrestin gene is a frequent cause of Oguchi disease in Japanese. Nat. Genet. 1995, 10, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Sippel, K.C.; Berson, E.L.; Dryja, T.P. Defects in the rhodopsin kinase gene in the Oguchi form of stationary night blindness. Nat. Genet. 1997, 15, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Yagasaki, K.; Horiguchi, M.; Kawase, Y.; Kanda, T. Congenital Stationary Night Blindness with Negative Electroretinogram. Arch. Ophthalmol. 1986, 104, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Krill, A.E.; Martin, D. Photopic abnormalities in congenital stationary nightblindness. Investig. Ophthalmol. 1971, 10, 625–636. [Google Scholar]
- Lachapelle, P.; Little, J.M.; Polomeno, R.C. The photopic electroretinogram in congenital stationary night blindness with myopia. Investig. Ophthalmol. Vis. Sci. 1983, 24, 442–450. [Google Scholar]
- Bijveld, M.M.; Florijn, R.J.; Bergen, A.A.; Born, L.I.V.D.; Kamermans, M.; Prick, L.; Riemslag, F.C.; Van Schooneveld, M.J.; Kappers, A.M.; Van Genderen, M.M. Genotype and Phenotype of 101 Dutch Patients with Congenital Stationary Night Blindness. Ophthalmologica 2013, 120, 2072–2081. [Google Scholar] [CrossRef]
- Khan, A.O.; Alrashed, M.; Alkuraya, F.S. Clinical characterisation of the CABP4-related retinal phenotype. Br. J. Ophthalmol. 2012, 97, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Zeitz, C.; Kloeckener-Gruissem, B.; Forster, U.; Kohl, S.; Magyar, I.; Wissinger, B.; Mátyás, G.; Borruat, F.-X.; Schorderet, D.F.; Zrenner, E.; et al. Mutations in CABP4, the Gene Encoding the Ca2+-Binding Protein 4, Cause Autosomal Recessive Night Blindness. Am. J. Hum. Genet. 2006, 79, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Wycisk, K.A.; Zeitz, C.; Feil, S.; Wittmer, M.; Forster, U.; Neidhardt, J.; Wissinger, B.; Zrenner, E.; Wilke, R.; Kohl, S.; et al. Mutation in the Auxiliary Calcium-Channel Subunit CACNA2D4 Causes Autosomal Recessive Cone Dystrophy. Am. J. Hum. Genet. 2006, 79, 973–977. [Google Scholar] [CrossRef]
- Ba-Abbad, R.; Arno, G.; Carss, K.; Stirrups, K.; Penkett, C.J.; Moore, A.T.; Michaelides, M.; Raymond, F.L.; Webster, A.R.; Holder, G.E. Mutations in CACNA2D4 Cause Distinctive Retinal Dysfunction in Humans. Ophthalmology 2016, 123, 668–671.e2. [Google Scholar] [CrossRef]
- Mechaussier, S.; Almoallem, B.; Zeitz, C.; Van Schil, K.; Jeddawi, L.; Van Dorpe, J.; Rey, A.D.; Condroyer, C.; Pelle, O.; Polak, M.; et al. Loss of Function of RIMS2 Causes a Syndromic Congenital Cone-Rod Synaptic Disease with Neurodevelopmental and Pancreatic Involvement. Am. J. Hum. Genet. 2020, 106, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y. Establishment of the concept of new clinical entities—Complete and incomplete form of congenital stationary night blindness. Nippon. Ganka Gakkai Zasshi 2002, 106, 737–755. [Google Scholar]
- Riemslag, F.C.C. Visually impaired children: “coming to better terms”. Doc. Ophthalmol. 2009, 119, 1–7. [Google Scholar] [CrossRef]
- Pasutto, F.; Ekici, A.; Reis, A.; Kremers, J.; Huchzermeyer, C. Novel truncating mutation in CACNA1F in a young male patient diagnosed with optic atrophy. Ophthalmic Genet. 2018, 39, 741–748. [Google Scholar] [CrossRef]
- Nakamura, M.; Ito, S.; Piao, C.-H.; Terasaki, H.; Miyake, Y. Retinal and Optic Disc Atrophy Associated With a CACNA1F Mutation in a Japanese Family. Arch. Ophthalmol. 2003, 121, 1028–1033. [Google Scholar] [CrossRef]
- Wutz, K.; Sauer, C.; Zrenner, E.; Lorenz, B.; Alitalo, T.; Broghammer, M.; Hergersberg, M.; De La Chapelle, A.; Weber, B.H.F.; Wissinger, B.; et al. Thirty distinct CACNA1F mutations in 33 families with incomplete type of XLCSNB and Cacna1f expression profiling in mouse retina. Eur. J. Hum. Genet. 2002, 10, 449–456. [Google Scholar] [CrossRef]
- Chen, R.W.; Greenberg, J.P.; Lazow, M.A.; Ramachandran, R.; Lima, L.H.; Hwang, J.C.; Schubert, C.; Braunstein, A.; Allikmets, R.; Tsang, S.H. Autofluorescence Imaging and Spectral-Domain Optical Coherence Tomography in Incomplete Congenital Stationary Night Blindness and Comparison with Retinitis Pigmentosa. Am. J. Ophthalmol. 2012, 153, 143–154.e2. [Google Scholar] [CrossRef]
- Mwanza, J.-C.; Durbin, M.K.; Budenz, D.L.; Girkin, C.A.; Leung, C.K.; Liebmann, J.M.; Peace, J.H.; Werner, J.S.; Wollstein, G. Profile and Predictors of Normal Ganglion Cell–Inner Plexiform Layer Thickness Measured with Frequency-Domain Optical Coherence Tomography. Investig. Opthalmol. Vis. Sci. 2011, 52, 7872–7879. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.-C.; Cheung, C.Y.; Koh, V.T.; Cheng, C.-Y.; Sidhartha, E.; Strouthidis, N.G.; Wong, T.Y.; Aung, T. Relationship between ganglion cell-inner plexiform layer and optic disc/retinal nerve fibre layer parameters in non-glaucomatous eyes. Br. J. Ophthalmol. 2013, 97, 1592–1597. [Google Scholar] [CrossRef]
- González-López, J.J.; Rebolleda, G.; Leal, M.; Oblanca, N.; Muñoz-Negrete, F.J.; Costa-Frossard, L.; Álvarez-Cermeño, J.C. Comparative Diagnostic Accuracy of Ganglion Cell-Inner Plexiform and Retinal Nerve Fiber Layer Thickness Measures by Cirrus and Spectralis Optical Coherence Tomography in Relapsing-Remitting Multiple Sclerosis. BioMed. Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef][Green Version]
- Zmyslowska, A.; Waszczykowska, A.; Baranska, D.; Stawiski, K.; Borowiec, M.; Jurowski, P.; Fendler, W.; Mlynarski, W. Optical coherence tomography and magnetic resonance imaging visual pathway evaluation in Wolfram syndrome. Dev. Med. Child Neurol. 2019, 61, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Rönnbäck, C.; Milea, D.; Larsen, M. Imaging of the Macula Indicates Early Completion of Structural Deficit in Autosomal-Dominant Optic Atrophy. Ophthalmology 2013, 120, 2672–2677. [Google Scholar] [CrossRef]
- Kupersmith, M.J.; Garvin, M.K.; Wang, J.-K.; Durbin, M.; Kardon, R. Retinal Ganglion Cell Layer Thinning Within One Month of Presentation for Non-Arteritic Anterior Ischemic Optic Neuropathy. Investig. Opthalmol. Vis. Sci. 2016, 57, 3588–3593. [Google Scholar] [CrossRef] [PubMed]
- Mwanza, J.-C.; Durbin, M.K.; Budenz, D.L.; Sayyad, F.E.; Chang, R.T.; Neelakantan, A.; Godfrey, D.G.; Carter, R.; Crandall, A.S. Glaucoma Diagnostic Accuracy of Ganglion Cell–Inner Plexiform Layer Thickness: Comparison with Nerve Fiber Layer and Optic Nerve Head. Ophthalmology 2012, 119, 1151–1158. [Google Scholar] [CrossRef]
- Vincent, A.; Wright, T.; Day, M.A.; Westall, C.A.; Héon, E. A novel p. Gly603Arg mutation in CACNA1F causes Åland island eye disease and incomplete congenital stationary night blindness phenotypes in a family. Mol. Vis. 2011, 17, 3262–3270. [Google Scholar]
- Vincent, A.; Héon, E. Outer retinal structural anomaly due to frameshift mutation in CACNA1F gene. Eye 2012, 26, 1278–1280. [Google Scholar] [CrossRef][Green Version]
- Chandrakumar, M.; Colpa, L.; Reginald, Y.A.; Goltz, H.C.; Wong, A.M. Measuring Contrast Sensitivity Using the M&S Smart System II versus the Pelli-Robson Chart. Ophthalmology 2013, 120, 2160–21610. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Garvin, M.K.; Sonka, M. Retinal Imaging and Image Analysis. IEEE Rev. Biomed. Eng. 2010, 3, 169–208. [Google Scholar] [CrossRef]
- Garvin, M.K.; Abramoff, M.D.; Wu, X.; Russell, S.R.; Burns, T.L.; Sonka, M. Automated 3-D Intraretinal Layer Segmentation of Macular Spectral-Domain Optical Coherence Tomography Images. IEEE Trans. Med. Imaging 2009, 28, 1436–1447. [Google Scholar] [CrossRef]
- Antony, B.; Abràmoff, M.D.; Tang, L.; Ramdas, W.D.; Vingerling, J.R.; Jansonius, N.M.; Lee, K.; Kwon, Y.H.; Sonka, M.; Garvin, M.K. Automated 3-D method for the correction of axial artifacts in spectral-domain optical coherence tomography images. Biomed. Opt. Express 2011, 2, 2403–2416. [Google Scholar] [CrossRef]
- Clark, A.; Wright, T.; Isaac, M.; Westall, C.; Mireskandari, K.; Tehrani, N.N. Macular morphology following unilateral bevacizumab injection for retinopathy of prematurity: An OCT study. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2017, 21, 499–501.e1. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.; Kumarappah, A.; Stavropoulos, A.; Reginald, A.; Buncic, J.R.; Westall, C.A. Vigabatrin Toxicity in Infancy Is Associated with Retinal Defect in Adolescence. Retina 2017, 37, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Flitcroft, D.I.; He, M.; Jonas, J.B.; Jong, M.; Naidoo, K.; Ohno-Matsui, K.; Rahi, J.; Resnikoff, S.; Vitale, S.; Yannuzzi, L. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investig. Opthalmol. Vis. Sci. 2019, 60, M20–M30. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, D.L.; Marmor, M.F.; Brigell, M.G.; Hamilton, R.; Holder, G.E.; Tzekov, R.; Bach, M. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc. Ophthalmol. 2015, 130, 1–12. [Google Scholar] [CrossRef]
- Marmor, M.F.; Holder, G.E.; Seeliger, M.W.; Yamamoto, S. Standard for clinical electroretinography (2004 update). Doc. Ophthalmol. 2004, 108, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Ohno-Matsui, K.; Yannuzzi, L.A. Pathologic Myopia; Springer: New York, NY, USA, 2013. [Google Scholar]
- Simonsz, H.; Florijn, R.; Van Minderhout, H.; Bergen, A.; Kamermans, M. Nightblindness-Associated Transient Tonic Downgaze (NATTD) in Infant Boys with Chin-Up Head Posture. Strabismus 2009, 17, 158–164. [Google Scholar] [CrossRef]
- Zeitz, C.; Michiels, C.; Neuillé, M.; Friedburg, C.; Condroyer, C.; Boyard, F.; Antonio, A.; Bouzidi, N.; Milicevic, D.; Veaux, R.; et al. Where are the missing gene defects in inherited retinal disorders? Intronic and synonymous variants contribute at least to 4% of CACNA1F -mediated inherited retinal disorders. Hum. Mutat. 2019, 40, 765–787. [Google Scholar] [CrossRef] [PubMed]
- Lodha, N.; Loucks, C.M.; Beaulieu, C.; Parboosingh, J.S.; Bech-Hansen, N.T. Congenital Stationary Night Blindness: Mutation Update and Clinical Variability. Adv. Exp. Med. Biol. 2011, 723, 371–379. [Google Scholar] [CrossRef]
- Bech-Hansen, N.T.; Naylor, M.J.; Maybaum, T.A.; Pearce, W.G.; Koop, B.; Fishman, G.A.; Mets, M.; Musarella, M.A.; Boycott, K.M. Loss-of-function mutations in a calcium-channel α1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat. Genet. 1998, 19, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.M.; Nyakatura, G.; Apfelstedt-Sylla, E.; Hellebrand, H.; Lorenz, B.; Weber, B.H.F.; Wutz, K.; Gutwillinger, N.; Rüther, K.; Drescher, B.; et al. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat. Genet. 1998, 19, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Zeitz, C.; Labs, S.; Lorenz, B.; Forster, U.; Üksti, J.; Kroes, H.Y.; De Baere, E.; Leroy, B.P.; Cremers, F.P.M.; Wittmer, M.; et al. Genotyping Microarray for CSNB-Associated Genes. Investig. Opthalmol. Vis. Sci. 2009, 50, 5919–5926. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.J.; Guo, Y.; Li, W.N.; Wang, H.Z.; Wang, Y.X.; Thomas, R.; Wang, N.L. Age-related changes in and determinants of macular ganglion cell-inner plexiform layer thickness in normal Chinese adults. Clin. Exp. Ophthalmol. 2018, 46, 400–406. [Google Scholar] [CrossRef]
- Ueda, K.; Kanamori, A.; Akashi, A.; Tomioka, M.; Kawaka, Y.; Nakamura, M. Effects of Axial Length and Age on Circumpapillary Retinal Nerve Fiber Layer and Inner Macular Parameters Measured by 3 Types of SD-OCT Instruments. J. Glaucoma 2016, 25, 383–389. [Google Scholar] [CrossRef]
- Leung, C.K.; Ye, C.; Weinreb, R.N.; Yu, M.; Lai, G.; Lam, D.S. Impact of Age-related Change of Retinal Nerve Fiber Layer and Macular Thicknesses on Evaluation of Glaucoma Progression. Ophthalmology 2013, 120, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Koh, V.T.; Tham, Y.-C.; Cheung, C.Y.; Wong, W.-L.; Baskaran, M.; Saw, S.-M.; Wong, T.Y.; Aung, T. Determinants of Ganglion Cell–Inner Plexiform Layer Thickness Measured by High-Definition Optical Coherence Tomography. Investig. Opthalmol. Vis. Sci. 2012, 53, 5853–5859. [Google Scholar] [CrossRef]
- Gürağaç, F.B.; Totan, Y.; Güler, E.; Tenlik, A.; Ertuğrul, I.G. Normative Spectral Domain Optical Coherence Tomography Data in Healthy Turkish Children. Semin. Ophthalmol. 2016, 32, 216–222. [Google Scholar] [CrossRef]
- Al-Haddad, C.; Barikian, A.; Jaroudi, M.; Massoud, V.; Tamim, H.; Noureddin, B. Spectral domain optical coherence tomography in children: Normative data and biometric correlations. BMC Ophthalmol. 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Barrio-Barrio, J.; Noval, S.; Galdós, M.; Ruiz-Canela, M.; Bonet, E.; Capote, M.; Lopez, M. Multicenter Spanish study of spectral-domain optical coherence tomography in normal children. Acta Ophthalmol. 2013, 91, e56–e63. [Google Scholar] [CrossRef]
- Elía, N.; Pueyo, V.; Altemir, I.; Oros, D.; Pablo, L.E. Normal reference ranges of optical coherence tomography parameters in childhood. Br. J. Ophthalmol. 2012, 96, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Nam, K.Y.; Park, H.J.; Lim, H.-B.; Kim, J.-Y. Longitudinal changes in the ganglion cell-inner plexiform layer thickness in high myopia: A prospective observational study. Br. J. Ophthalmol. 2019, 104, 604–609. [Google Scholar] [CrossRef]
- Biswas, S.; Lin, C.; Leung, C.K.S. Evaluation of a Myopic Normative Database for Analysis of Retinal Nerve Fiber Layer Thickness. JAMA Ophthalmol. 2016, 134, 1032–1039. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Yoo, C.; Kim, Y.Y. Myopic Optic Disc Tilt and the Characteristics of Peripapillary Retinal Nerve Fiber Layer Thickness Measured by Spectral-domain Optical Coherence Tomography. J. Glaucoma 2012, 21, 260–265. [Google Scholar] [CrossRef]
- Thomas, M.G.; Kumar, A.; Mohammad, S.; Proudlock, F.A.; Engle, E.C.; Andrews, C.; Chan, W.-M.; Thomas, S.; Gottlob, I. Structural Grading of Foveal Hypoplasia Using Spectral-Domain Optical Coherence Tomography. Ophthalmology 2011, 118, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Jalkanen, R.; Tobias, R.; Mäntyjärvi, M.; De La Chapelle, A.; Bech-Hansen, N.T.; Sankila, E.-M.; Forsius, H.; Alitalo, T. A Novel CACNA1F Gene Mutation Causes Aland Island Eye Disease. Investig. Opthalmol. Vis. Sci. 2007, 48, 2498–2502. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Pollard, K.S.; Hubisz, M.J.; Rosenbloom, K.R.; Siepel, A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res. 2009, 20, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Eng, L.; Coutinho, G.; Nahas, S.; Yeo, G.; Tanouye, R.; Babaei, M.; Dörk, T.; Burge, C.; Gatti, R.A. Nonclassical splicing mutations in the coding and noncoding regions of the ATM Gene: Maximum entropy estimates of splice junction strengths. Hum. Mutat. 2003, 23, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Hebsgaard, S.M. Splice site prediction in Arabidopsis thaliana pre-mRNA by combining local and global sequence information. Nucleic Acids Res. 1996, 24, 3439–3452. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.B.; Senapathy, P. RNA splice junctions of different classes of eukaryotes: Sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987, 15, 7155–7174. [Google Scholar] [CrossRef]
- Jaganathan, K.; Panagiotopoulou, S.K.; McRae, J.F.; Darbandi, S.F.; Knowles, D.; Li, Y.I.; Kosmicki, J.A.; Arbelaez, J.; Cui, W.; Schwartz, G.B.; et al. Predicting Splicing from Primary Sequence with Deep Learning. Cell 2019, 176, 535–548.e24. [Google Scholar] [CrossRef]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009, 4, 1073–1081. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A meth-od and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Mathe, E.; Olivier, M.; Kato, S.; Ishioka, C.; Hainaut, P.; Tavtigian, S.V. Computational approaches for predicting the biological effect of p53 missense mutations: A comparison of three sequence analysis based methods. Nucleic Acids Res. 2006, 34, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; Rödelsperger, C.; Schuelke, M.; Seelow, D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods 2010, 7, 575–576. [Google Scholar] [CrossRef]
- Ioannidis, N.M.; Rothstein, J.H.; Pejaver, V.; Middha, S.; McDonnell, S.K.; Baheti, S.; Musolf, A.; Li, Q.; Holzinger, E.; Karyadi, D.; et al. REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am. J. Hum. Genet. 2016, 99, 877–885. [Google Scholar] [CrossRef]
- Nakamura, M.; Ito, S.; Terasaki, H.; Miyake, Y. Novel CACNA1F mutations in Japanese patients with incomplete congenital stationary night blindness. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1610–1616. [Google Scholar]
- Boycott, K.M.; Pearce, W.G.; Bech-Hansen, N.T. Clinical variability among patients with incomplete X-linked congenital stationary night blindness and a founder mutation in CACNA1F. Can. J. Ophthalmol. 2000, 35, 204–213. [Google Scholar] [CrossRef]
- Allen, L.E.; Zito, I.; Bradshaw, K.; Patel, R.J.; Bird, A.C.; Fitzke, F.; Yates, J.R.; Trump, D.; Hardcastle, A.J.; Moore, A.T. Genotype-phenotype correlation in British families with X linked congenital stationary night blindness. Br. J. Ophthalmol. 2003, 87, 1413–1420. [Google Scholar] [CrossRef]
- Hope, C.I.; Sharp, D.M.; Hemara-Wahanui, A.; Sissingh, J.I.; Lundon, P.; Mitchell, E.A.; Maw, M.A.; Clover, G.M. Clinical manifestations of a unique X-linked retinal disorder in a large New Zealand family with a novel mutation in CACNA1F, the gene responsible for CSNB2. Clin. Exp. Ophthalmol. 2005, 33, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Godara, P.; Cooper, R.F.; Sergouniotis, P.I.; Diederichs, M.A.; Streb, M.R.; Genead, M.A.; McAnany, J.J.; Webster, A.R.; Moore, A.T.; Dubis, A.M.; et al. Assessing Retinal Structure in Complete Congenital Stationary Night Blindness and Oguchi Disease. Am. J. Ophthalmol. 2012, 154, 987–1001.e1. [Google Scholar] [CrossRef]
- Chang, B.; Heckenlively, J.R.; Bayley, P.R.; Brecha, N.C.; Davisson, M.T.; Hawes, N.L.; Hirano, A.A.; Hurd, R.E.; Ikeda, A.; Johnson, B.A.; et al. Thenob2mouse, a null mutation in Cacna1f: Anatomical and functional abnormalities in the outer retina and their consequences on ganglion cell visual responses. Vis. Neurosci. 2006, 23, 11–24. [Google Scholar] [CrossRef]
- Haeseleer, F.; Imanishi, Y.; Maeda, T.; Possin, D.E.; Maeda, A.; Lee, A.; Rieke, F.; Palczewski, K. Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nat. Neurosci. 2004, 7, 1079–1087. [Google Scholar] [CrossRef]
- Kerov, V.; Laird, J.G.; Joiner, M.-L.; Knecht, S.; Soh, D.; Hagen, J.; Gardner, S.H.; Gutierrez, W.; Yoshimatsu, T.; Bhattarai, S.; et al. α2δ-4 Is Required for the Molecular and Structural Organization of Rod and Cone Photoreceptor Synapses. J. Neurosci. 2018, 38, 6145–6160. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, K.; Allen, L.; Trump, R.; Hardcastle, A.; George, N.; Moore, A. A comparison of ERG abnormalities in XLRS and XLCSNB. Doc. Ophthalmol. 2004, 108, 135–145. [Google Scholar] [CrossRef]
- Heckenlively, J.R.; Martin, D.A.; Rosenbaum, A.L. Loss of Electroretinographic Oscillatory Potentials, Optic Atrophy, and Dysplasia in Congenital Stationary Night Blindness. Am. J. Ophthalmol. 1983, 96, 526–534. [Google Scholar] [CrossRef]
- Wang, Y.X.; Panda-Jonas, S.; Jonas, J.B. Optic nerve head anatomy in myopia and glaucoma, including parapapillary zones α, β, γ and delta: Histology and clinical features. Prog. Retin. Eye Res. 2020, 100933. [Google Scholar] [CrossRef]
- Chang, L.; Pan, C.-W.; Ohno-Matsui, K.; Lin, X.; Cheung, G.C.; Gazzard, G.; Koh, V.; Hamzah, H.; Tai, E.S.; Lim, S.C.; et al. Myopia-Related Fundus Changes in Singapore Adults with High Myopia. Am. J. Ophthalmol. 2013, 155, 991–999.e1. [Google Scholar] [CrossRef]
- Cheng, S.C.; Lam, C.S.; Yap, M.K. Prevalence of myopia-related retinal changes among 12–18 year old Hong Kong Chinese high myopes. Ophthalmic Physiol. Opt. 2013, 33, 652–660. [Google Scholar] [CrossRef]
- Waldner, D.M.; Bech-Hansen, N.T.; Stell, W.K. Channeling Vision: CaV1.4—A Critical Link in Retinal Signal Transmission. BioMed. Res. Int. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Baert, A.; Machackova, E.; Coene, I.; Cremin, C.; Turner, K.; Portigal-Todd, C.; Asrat, M.J.; Nuk, J.; Mindlin, A.; Young, S.; et al. Thorough in silico and in vitro cDNA analysis of 21 putativeBRCA1andBRCA2splice variants and a complex tandem duplication inBRCA2allowing the identification of activated cryptic splice donor sites inBRCA2exon 11. Hum. Mutat. 2017, 39, 515–526. [Google Scholar] [CrossRef]
- Mucaki, E.J.; Ainsworth, P.; Rogan, P.K. Comprehensive prediction of mRNA splicing effects of BRCA1 and BRCA2 variants. Hum. Mutat. 2011, 32, 735–742. [Google Scholar] [CrossRef]
- Morgans, C.W. Localization of the α(1F) calcium channel subunit in the rat retina. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2414–2418. [Google Scholar]
- Liu, X.; Kerov, V.; Haeseleer, F.; Majumder, A.; Artemyev, N.; Baker, S.A.; Lee, A. Dysregulation of Cav1.4 channels disrupts the maturation of photoreceptor synaptic ribbons in congenital stationary night blindness type 2. Channels 2013, 7, 514–523. [Google Scholar] [CrossRef]
- Naylor, M.J.; Rancourt, D.E.; Bech-Hansen, N. Isolation and Characterization of a Calcium Channel Gene, Cacna1f, the Murine Orthologue of the Gene for Incomplete X-Linked Congenital Stationary Night Blindness. Genomics 2000, 66, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Morgans, C.W.; Gaughwin, P.; Maleszka, R. Expression of the alpha1F calcium channel subunit by photoreceptors in the rat retina. Mol. Vis. 2001, 7, 202–209. [Google Scholar] [PubMed]
- Knoflach, D.; Kerov, V.; Sartori, S.B.; Obermair, G.J.; Schmuckermair, C.; Liu, X.; Sothilingam, V.; Garrido, M.G.; Baker, S.A.; Glösmann, M.; et al. Cav1.4 IT mouse as model for vision impairment in human congenital stationary night blindness type 2. Channels 2013, 7, 503–513. [Google Scholar] [CrossRef]
- Shi, L.; Chang, J.Y.-A.; Yu, F.; Ko, M.L.; Ko, G.Y.-P. The Contribution of L-Type Cav1.3 Channels to Retinal Light Responses. Front. Mol. Neurosci. 2017, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Waldner, D.M.; Ito, K.; Chen, L.-L.; Nguyen, L.; Chow, R.L.; Lee, A.; Rancourt, D.E.; Tremblay, F.; Stell, W.K.; Bech-Hansen, N.T. Transgenic Expression of Cacna1f Rescues Vision and Retinal Morphology in a Mouse Model of Congenital Stationary Night Blindness 2A (CSNB2A). Transl. Vis. Sci. Technol. 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]

| Case | Age, y | NM_005183.3 cDNA and AA Variations | VA, OD; OS | Refraction, OD; OS | Color, HRR, OU | CS, OD; OS | Symptoms | ||
|---|---|---|---|---|---|---|---|---|---|
| Nystagmus | Nyctalopia | Photophobia | |||||||
| 1 | 10 | c.245G>A; p.(Arg82Gln) [1] | 0.40; 0.40 | +1.00/+1.25; +2.00/+0.75 | mild RG | 1.30; 1.30 | - | - | - |
| 2 | 15 | c.1047G>A; p.(Trp349*) [1,41] | 0.50; 0.50 | −9.25/+4.25; −9.50/+4.00 | normal | 1.40; 1.50 | - | - | + |
| 3 a | 14 | c.1681C>T; p.(Gln561*) N | 0.20; 0.20 | −9.75/+2.50; −8.75/+1.50 | normal | 1.50; 1.50 | - | - | |
| 4 a | 16 | c.1681C>T; p.(Gln561*) N | 0.50; 0.60 | −15.75/+1.50; −14.75/+1.25 | normal | 1.30; 1.20 | - | + | - |
| 5 b | 11 | c.1807G>C; p.(Gly603Arg) [29] | 0.40; 0.48 | −12.25/+3.00; −12.25/+3.00 | strong RG | 1.50; 1.50 | + | + | |
| 6 b | 58 | c.1807G>C; p.(Gly603Arg) | 0.40; 0.40 | plano; plano | mild RG | 1.65; 1.65 | + | ||
| 7 | 9 | c.1832del; p.(Val611Glyfs*32) N | 0.48; 0.60 | −12.50/+4.00; −11.50/+4.00 | normal | 1.40; 1.50 | + | + | + |
| 8 | 14 | c.1849C>T; p.(Gln617*) [1] | 0.30; 0.40 | +1.00/+3.00; +1.00/+3.00 | normal | 1.30; 1.30 | + | + | + |
| 9 | 6 | c.2094_2095del; p.(Gln699Glyfs*12) [42] | 0.80; 0.80 | −7.50/+2.50; −5.50/+3.50 | mild RG | 1.20; 1.20 | - | - | |
| 10 | 8 | c.2576+1G>A; p.? [42,43] | 0.30; 0.48 | −0.25/+1.00; −1.00/+0.75 | normal | 1.20; 1.20 | - | + | |
| 11 | 9 | c.2576+1G>A; p.? | 0.80; 0.90 | −9.00; −9.75 | 0.90; 0.90 | - | + | - | |
| 12 | 8 | c.3166dup; p.(Leu1056Profs*11) [1,44,45] | 0.40; 0.40 | −14.00/+2.00; −14.00/+2.75 | normal | 1.50; 1.50 | + | - | |
| 13 c | 13 | c.3166dup; p.(Leu1056Profs*11) | 0.40; 0.30 | −9.25/+3.25; −9.50/+2.75 | normal | 1.40; 1.40 | - | ||
| 14 c | 13 | c.3166dup; p.(Leu1056Profs*11) | 0.48; 0.60 | −11.00/+3.25; −10.00/+3.25 | mild RG | 1.30; 1.20 | + | ||
| 15 c | 16 | c.3166dup; p.(Leu1056Profs*11) | 0.30; 0.30 | −7.25/+5.00; −6.75/+4.50 | normal | 1.40; 1.40 | |||
| 16 | 17 | c.3166dup; p.(Leu1056Profs*11) | 0.18; 0.18 | +0.25; +0.25 | medium RG | 1.40; 1.30 | + | + | + |
| 17 | 9 | c.3471G>A; p.(Gln1157Gln) N, S | 0.40; 0.40 | −14.25/+4.75; −14.25/+4.75 | normal | 1.30; 1.30 | - | ||
| 18 | 17 | c.3741+2T>C; p.? N | 0.40; 0.40 | −10.25/+2.75; −9.75/+2.75 | normal | 1.30; 1.30 | + | - | |
| 19 | 20 | c.4474C>G; p.(Pro1492Ala) [1,46] | 0.48; 0.30 | −21.75/+4.00; −22.75/+4.50 | normal | 1.30; 1.50 | - | - | |
| 20 | 16 | c.4474C>T; p.(Pro1492Ser) N | 0.48; 0.30 | −6.75/+2.00; −6.50/+2.00 | normal | 1.30; 1.30 | - | - | + |
| 21 | 7 | c.4504C>T; p.(Arg1502*) [42] | 0.54; 0.54 | −1.50/+3.50; −3.00/+4.00 | normal | 1.10; 1.10 | + | ||
| 22 | 10 | c.5156G>T; p.(Arg1719Met) [42] | 0.10; 0.10 | −1.00/+3.00; −2.00/+3.00 | normal | 1.80; 1.80 | + | - | - |
| Case | Study Eye | Average Macular GCL-IPL Thickness, µm | Average (Temporal) RNFL Thickness, µm | ERG b/a Ratio DA Standard Flash | ERG b/a Ratio DA Bright Flash | ERG a-Wave; b-Wave amplitude (µV) LA Standard Flash | ERG b/a Ratio LA Standard Flash | Disc Pallor, OD; OS, h (Description) | Fundus Findings, OU |
|---|---|---|---|---|---|---|---|---|---|
| 1 | OD | 64 | 0.56 | 0.41 | –20; 27 | 1.35 | 5; 4 | Normal | |
| 2 | OD | 52 | 0.51 | 0.44 | –48; 36 | 0.75 | 3 (PC); 4 (PC) | Tessellated, FH (1) | |
| 3 | OS | 54 | 0.52 | 0.54 | –26; 32 | 1.23 | 5 (PC); 4 (PC) | Tessellated, WWP | |
| 4 | OD | 47 | 0.66 | 0.56 | –29; 34 | 1.17 | 3 (PC); 3 (PC) | Tessellated, WWP, staphyloma, FH (1) | |
| 5 | OD | 50 | 0.48 | 0.54 | –24; 28 | 1.17 | 5 (PC); 5 (PC) | Blonde, FH (2) | |
| 6 | OD | 53 | 0.75 | –35; 35 | 1.00 | 2; 2 | Normal | ||
| 7 | OS | 55 | 0.48 | –35; 25 | 0.71 | 5; 5 | Tessellated | ||
| 8 | OD | 58 | 68 (40) | 0.60 | 0.99 | –27; 39 | 1.44 | 4; 4 | Peripheral atrophy |
| 9 | OS | 51 | 66 (51) | 0.25 | 0.22 | –14; 9 | 0.64 | 3; 0 | Appearance of sheathing around superotemporal vascular arcades |
| 10 | OD | 56 | 0.37 | 0.54 | 3; 0 | Tessellated, blonde, FH (1) | |||
| 11 | OD | 56 | 0.84 | 0.63 | –24; 32 | 1.33 | 3 (PC); 3 (PC) | Blonde | |
| 12 | OD | 49 | 0.47 | 0.40 | –12; 14 | 1.17 | 2 (PC); 4 (PC) | Tessellated, WWP | |
| 13 | OD | 55 | 0.53 | 0.55 | –22; 30 | 1.07 | 5 (PC); 3 (PC) | Tessellated | |
| 14 | OD | 52 | 61 (44) | 0.70 | 0.59 | –32; 41 | 1.28 | Tessellated, lattice degeneration | |
| 15 | OD | 51 | 1.16 | 0.94 | –28; 39 | 1.39 | 5 (PC); 5 (PC) | Tessellated, FH (1) | |
| 16 | OD | 52 | 72 (39) | 0.41 | 0.40 | –12; 12 | 1.00 | 5 (PC); 5 (PC) | Tessellated |
| 17 | OD | 60 | 71 (75) | 0.67 | 0.68 | –31; 37 | 1.19 | 4 (PC); 5 (PC) | Tessellated |
| 18 | OS | 55 | 0.37 | 0.38 | –23; 25 | 1.09 | 3 (PC); 2 | Tessellated, WWP | |
| 19 | OS | 46 | 0.51 | 0.39 | –14; 21 | 1.50 | 2 (PC); 3 (PC) | Tessellated, blonde, staphyloma. Fuchs spot (OD) | |
| 20 | OD | 54 | 74 (59) | 0.29 | 0.27 | –10; 15 | 1.50 | 3 (PC); 4 | Tessellated, blonde, FH (2) |
| 21 | OD | 70 | 1.14 | 1.06 | –27; 35 | 1.30 | 5; 4 | Normal | |
| 22 | OD | 70 | 0.71 | 0.67 | –24; 38 | 1.58 | 3; 3 | Appearance of perivascular sheathing along some vessels |
| Source | Age, y | n | Refraction, SE (D) | Average RNFL (mm) | Temporal RNFL |
|---|---|---|---|---|---|
| Guragac et al., 2017 [51] | 3–17 a | 318 | –4.00 to +3.00 a | 96.49 (10.10) b; 80.00–114.00 d | 67.60 (9.93) b; 53.95–87.00 d |
| Al-Haddad et al., 2014 [52] | 6–17 a | 108 | –4.25 to +5.00 a | 95.6 (8.7) b; 80–111 d | 66.4 (8.9) b; 54–84 d |
| Barrio-Barrio et al., 2013 [53] | 4–17 a | 283 | –4.88 to +5.25 a | 97.40 (9) b; 77.0–121.7 a; 82.4–113.3 d | 67.4 b; 51.8–83.3 d |
| Elia et al., 2012 [54] | 6–14 a | 344 | –2.50 to +6.25 a | 98.46 (10.79) b | 69.35 (11.28) b |
| Lee et al., 2020 [55] | 39.3 (12.2) b | 80 | –6.50 (–8.50 to –4.25) c | 89.88 (8.87) b | |
| Biswas et al., 2016 [56] | 36.0 (12.3) b | 180 | –17.1 to –6.0D a | 89.6 (7.2) b; 70–110 a | |
| Hwang et al., 2012 [57] | 19–25 a | 255 | –11.00 to 0.00 a | 97.14 (6.63) b; 72–118 a | 74.03 (11.13) b; 53–105 a |
| Present study | 6–17 a | 6 | –11.88 to +2.50a | 68.67 (4.72) b; 61–74 a | 51.33 (13.81) b; 39–75 a; 39.25–71.00 d |
| Genomic Position (hg19, chr X) | NM_005183.3—cDNA; Protein Position | Predicted Effect | Splicing or Pathogenicity Scores | GnomAD [60] (v2.1.1) | PhyloP [61] | ACMG Criteria; Classification [62] |
|---|---|---|---|---|---|---|
| g.49082374G>A | c.1681C>T; p.(Gln561*) | nonsense | N/A | N/A | 4.73 | PVS1, PM2, PP1; pathogenic |
| g.49081301del | c.1832del; p.(Val611Glyfs*32) | frameshift stop | N/A | N/A | 3.76 | PVS1, PM2; likely pathogenic |
| g.49071802C>T | c.3471G>A; p.(Gln1157Gln) | splicing, predicted loss of donor site 1 bp downstream | MaxEntScan [63]: −100.0%, NNSPLICE [64]: −95.8%, SSF [65]: −15.7%, spliceAI [66]: 0.96 | N/A | 2.47 | PM2, PP3; uncertain significance |
| g.49070617A>G | c.3741+2T>C; p.? | splicing, predicted loss of donor site 2 bp upstream | MaxEntScan [63]: −100.0%, NNSPLICE [64]: 0.0%, SSF [65]: −13.8%, spliceAI [66]: 0.97 | N/A | 4.08 | PVS1, PM2, PP1, PP3; pathogenic |
| g.49067094G>A | c.4474C>T; p.(Pro1492Ser) | missense | SIFT [67]: 0 (deleterious), Poly Phen-2 [68]: 1 (PD), Align GVGD [69]: C65, MutationTaster [70]: 1(Del), Revel [71]: 0.83(Del) | N/A | 5.45 | PM1, PM2, PM5, PP3; likely pathogenic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leahy, K.E.; Wright, T.; Grudzinska Pechhacker, M.K.; Audo, I.; Tumber, A.; Tavares, E.; MacDonald, H.; Locke, J.; VandenHoven, C.; Zeitz, C.; et al. Optic Atrophy and Inner Retinal Thinning in CACNA1F-Related Congenital Stationary Night Blindness. Genes 2021, 12, 330. https://doi.org/10.3390/genes12030330
Leahy KE, Wright T, Grudzinska Pechhacker MK, Audo I, Tumber A, Tavares E, MacDonald H, Locke J, VandenHoven C, Zeitz C, et al. Optic Atrophy and Inner Retinal Thinning in CACNA1F-Related Congenital Stationary Night Blindness. Genes. 2021; 12(3):330. https://doi.org/10.3390/genes12030330
Chicago/Turabian StyleLeahy, Kate E, Tom Wright, Monika K Grudzinska Pechhacker, Isabelle Audo, Anupreet Tumber, Erika Tavares, Heather MacDonald, Jeff Locke, Cynthia VandenHoven, Christina Zeitz, and et al. 2021. "Optic Atrophy and Inner Retinal Thinning in CACNA1F-Related Congenital Stationary Night Blindness" Genes 12, no. 3: 330. https://doi.org/10.3390/genes12030330
APA StyleLeahy, K. E., Wright, T., Grudzinska Pechhacker, M. K., Audo, I., Tumber, A., Tavares, E., MacDonald, H., Locke, J., VandenHoven, C., Zeitz, C., Heon, E., Buncic, J. R., & Vincent, A. (2021). Optic Atrophy and Inner Retinal Thinning in CACNA1F-Related Congenital Stationary Night Blindness. Genes, 12(3), 330. https://doi.org/10.3390/genes12030330







