High PD-L1/IDO-2 and PD-L2/IDO-1 Co-Expression Levels Are Associated with Worse Overall Survival in Resected Non-Small Cell Lung Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. RNA Extraction and Array Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
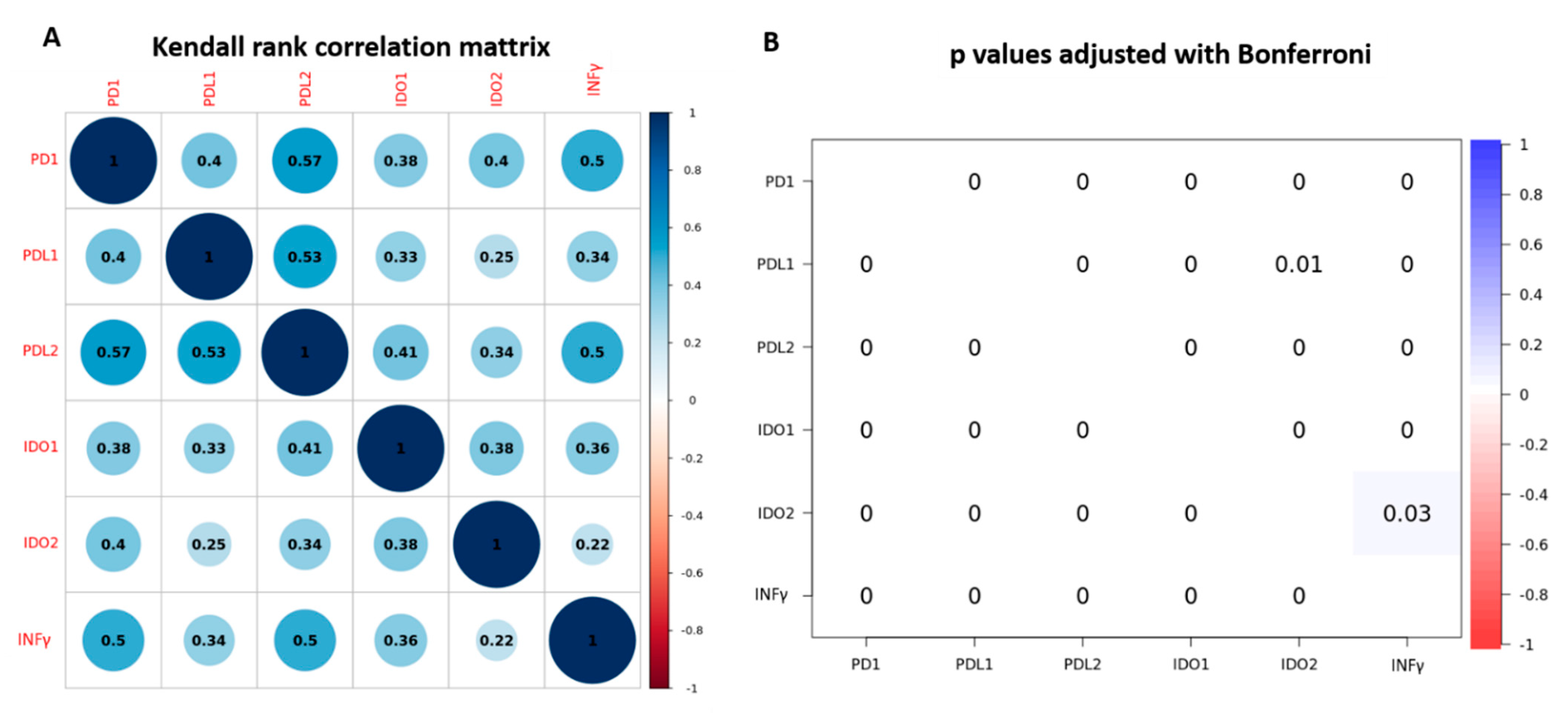
3.2. PD-1, PD-L1, PD-L2, IDO-1, IDO-2 and INFγ Gene Expression and Association with Clinic-Pathological Characteristics
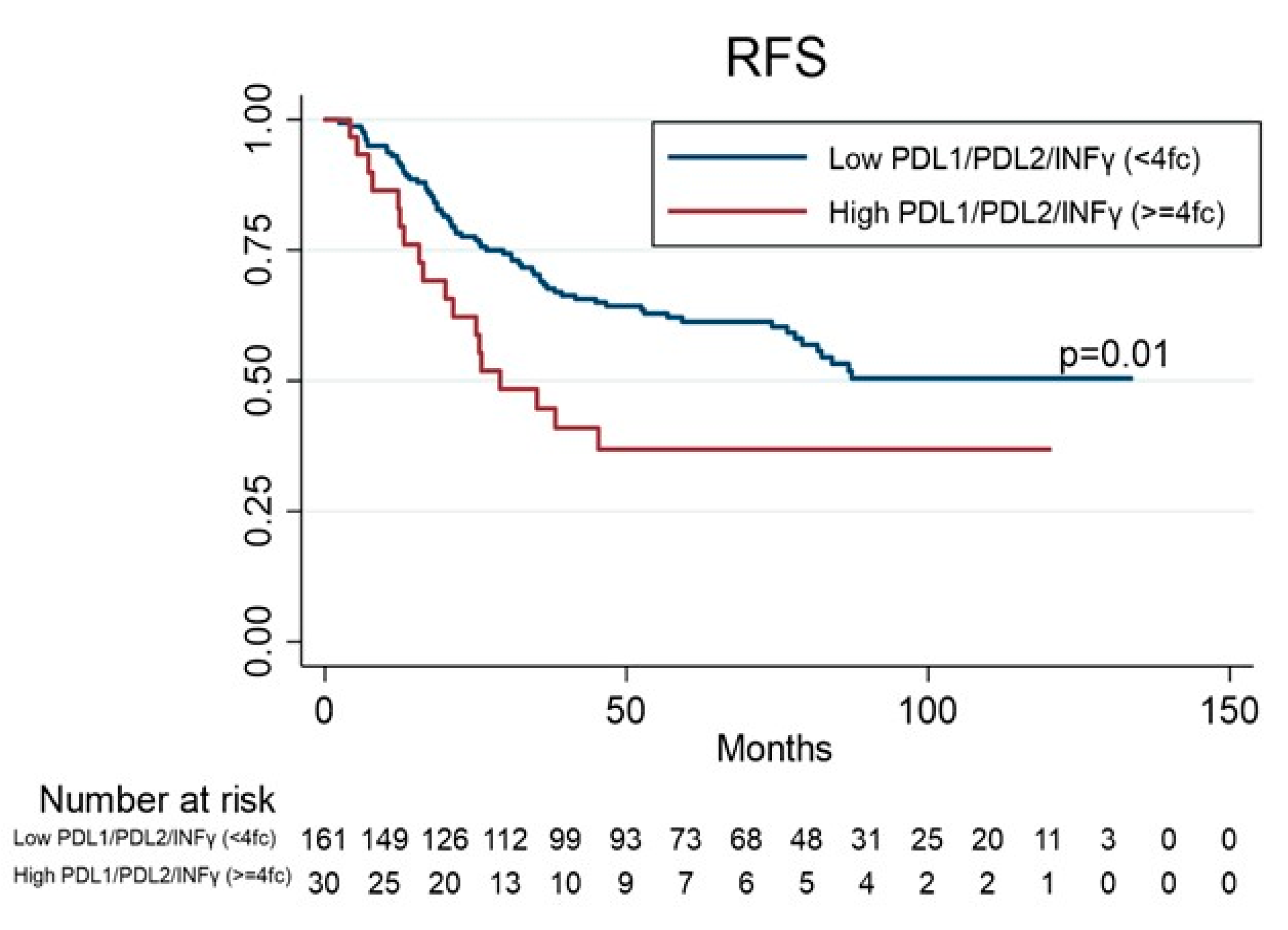
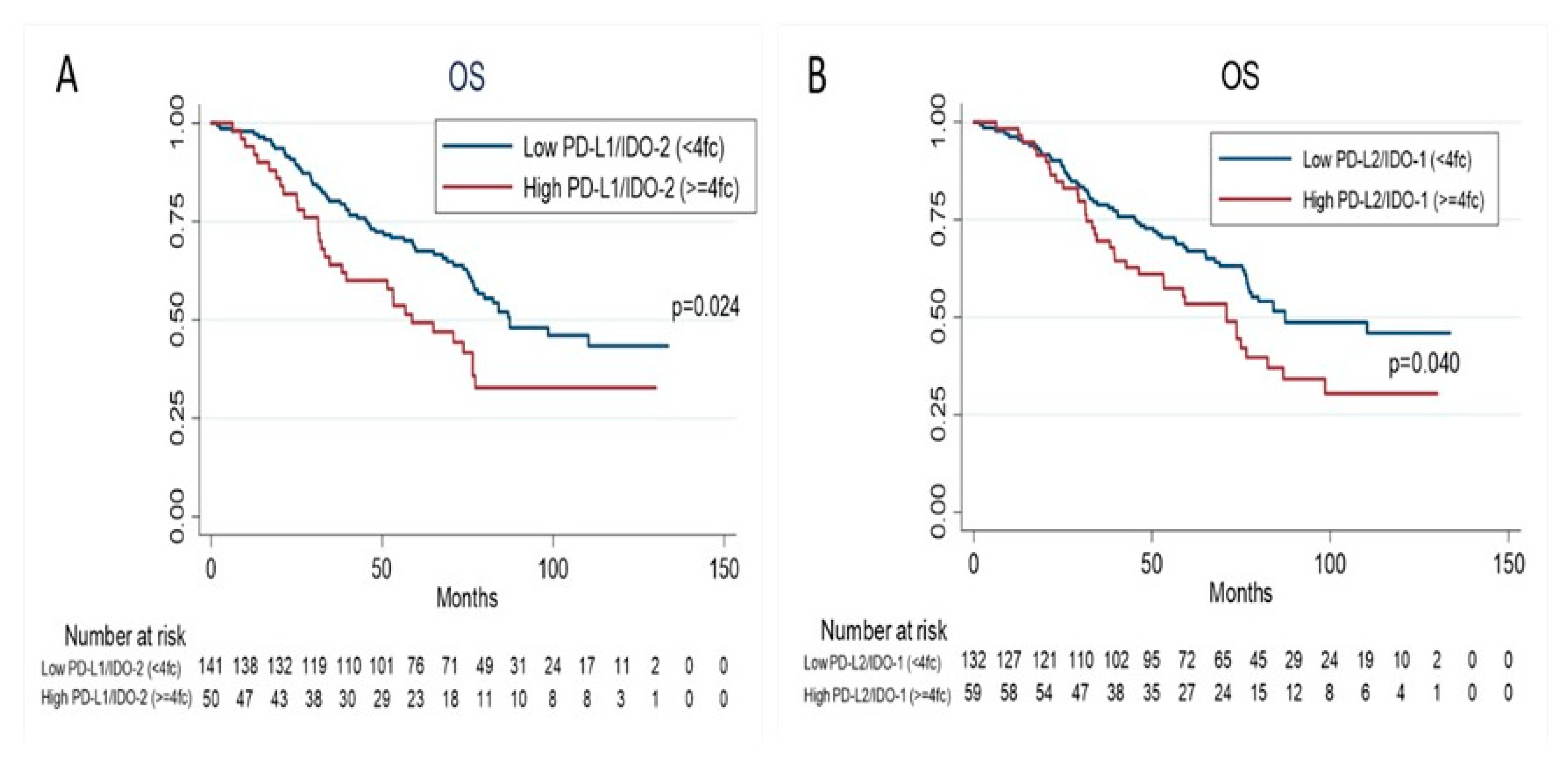
3.3. Prognostic Value of PD-1, PD-L1, PD-L2, IDO-1, IDO-2 and INFγ Gene Expression
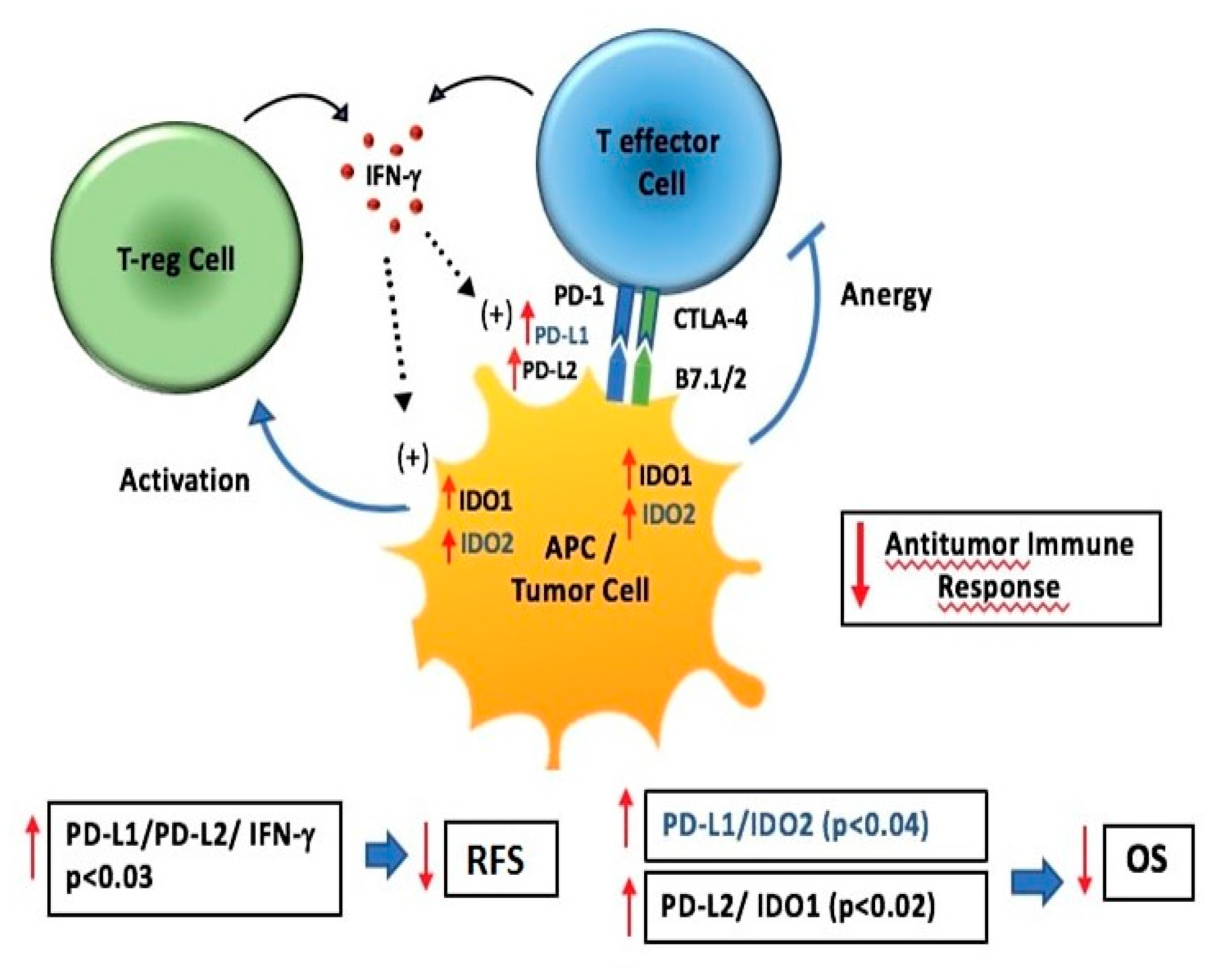
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung Cancer Statistics. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2016; Volume 893, pp. 1–19. [Google Scholar]
- Pao, W.; Hutchinson, K.E. Chipping away at the lung cancer genome. Nat. Med. 2012, 18, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef]
- Zavala, V.A.; Kalergis, A.M. New clinical advances in immunotherapy for the treatment of solid tumours. Immunology 2015, 145, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Gettinger, S.N.; Horn, L.; Gandhi, L.; Spigel, D.R.; Antonia, S.J.; Rizvi, N.A.; Powderly, J.D.; Heist, R.S.; Carvajal, R.D.; Jackman, D.M.; et al. Overall Survival and Long-Term Safety of Nivolumab (Anti–Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2015, 33, 2004–2012. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Schietinger, A.; Greenberg, P.D. Tolerance and exhaustion: Defining mechanisms of T cell dysfunction. Trends Immunol. 2014, 35, 51–60. [Google Scholar] [CrossRef]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Spranger, S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int. Immunol. 2016, 28, 383–391. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Mu, C.Y.; Huang, J.A.; Chen, Y.; Chen, C.; Zhang, X.G. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med. Oncol. 2011, 28, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Konishi, J.; Yamazaki, K.; Azuma, M.; Kinoshita, I.; Dosaka-Akita, H.; Nishimura, M. B7-H1 Expression on Non-Small Cell Lung Cancer Cells and Its Relationship with Tumor-Infiltrating Lymphocytes and Their PD-1 Expression. Clin. Cancer Res. 2004, 10, 5094–5100. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, C.-Y.; Huang, J.-A. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: A 5-year-follow-up study. Tumori 2012, 98, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Boland, J.M.; Kwon, E.D.; Harrington, S.M.; Wampfler, J.A.; Tang, H.; Yang, P.; Aubry, M.C. Tumor B7-H1 and B7-H3 Expression in Squamous Cell Carcinoma of the Lung. Clin. Lung Cancer 2013, 14, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Mellor, A.L.; Regents, G.; Place, F. Regulation and Tolerance. Trends Immunol. 2017, 37, 193–207. [Google Scholar] [CrossRef]
- Isla Larrain, M.T.; Rabassa, M.E.; Lacunza, E.; Barbera, A.; Cretón, A.; Segal-Eiras, A.; Croce, M.V. IDO is highly expressed in breast cancer and breast cancer-derived circulating microvesicles and associated to aggressive types of tumors by in silico analysis. Tumor Biol. 2014, 35, 6511–6519. [Google Scholar] [CrossRef]
- Schalper, K.A.; Carvajal-Hausdorf, D.; McLaughlin, J.; Altan, M.; Velcheti, V.; Gaule, P.; Sanmamed, M.F.; Chen, L.; Herbst, R.S.; Rimm, D.L. Differential Expression and Significance of PD-L1, IDO-1, and B7-H4 in Human Lung Cancer. Clin. Cancer Res. 2017, 23, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Litzenburger, U.M.; Opitz, U.; Sahm, F.; Ochs, K.; Lutz, C.; Wick, W.; Platten, M. The Indoleamine-2,3-Dioxygenase (IDO) Inhibitor 1-Methyl-D-tryptophan Upregulates IDO1 in Human Cancer Cells. PLoS ONE 2011, 6, e19823. [Google Scholar] [CrossRef]
- Dunn, G.P.; Koebel, C.M.; Schreiber, R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006, 6, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.S.; Kudelka, A.P.; Kavanagh, J.J.; Verschraegen, C.; Edwards, C.L.; Nash, M.; Levy, L.; Atkinson, E.N.; Zhang, H.Z.; Melichar, B.; et al. Clinical and biological effects of intraperitoneal injections of recombinant interferon-gamma and recombinant interleukin 2 with or without tumor-infiltrating lymphocytes in patients with ovarian or peritoneal carcinoma. Clin. Cancer Res. 2000, 6, 2268–2278. [Google Scholar] [PubMed]
- Blank, C.; Brown, I.; Peterson, A.C.; Spiotto, M.; Iwai, Y.; Honjo, T.; Gajewski, T.F. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004, 64, 1140–1145. [Google Scholar] [CrossRef]
- Spranger, S.; Spaapen, R.M.; Zha, Y.; Williams, J.; Meng, Y.; Ha, T.T.; Gajewski, T.F. Up-Regulation of PD-L1, IDO, and Tregs in the Melanoma Tumor Microenvironment Is Driven by CD8+ T Cells. Sci. Transl. Med. 2013, 5, 200ra116. [Google Scholar] [CrossRef]
- Zheng, S.; Luo, X.; Dong, C.; Zheng, D.; Xie, J.; Zhuge, L.; Sun, Y.; Chen, H. A B7-CD28 family based signature demonstrates significantly different prognoses and tumor immune landscapes in lung adenocarcinoma. Int. J. Cancer 2018, 143, 2592–2601. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Chansky, K.; Groome, P.; Bolejack, V.; Crowley, J.; Shemanski, L.; Kennedy, C.; Krasnik, M.; Peake, M.; Rami-Porta, R. The IASLC lung cancer staging project: Methodology and validation used in the development of proposals for revision of the stage classification of NSCLC in the Forthcoming (Eighth) Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2016, 11, 1433–1446. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Ludovini, V.; Bianconi, F.; Siggillino, A.; Piobbico, D.; Vannucci, J.; Metro, G.; Chiari, R.; Bellezza, G.; Puma, F.; Della Fazia, M.A.; et al. Gene identification for risk of relapse in stage I lung adenocarcinoma patients: A combined methodology of gene expression profiling and computational gene network analysis. Oncotarget 2016, 7, 30561–30574. [Google Scholar] [CrossRef]
- Thiele, C.; Hirschfeld, G. Cutpointr: Improved estimation and validation of optimal cutpoints in R. arXiv 2020, VV, 2002.09209. [Google Scholar] [CrossRef]
- Mandarano, M.; Bellezza, G.; Belladonna, M.L.; Vannucci, J.; Mondanelli, G.; Ludovini, V.; Ferri, I.; Bianconi, F.; Del Sordo, R.; Cagini, L.; et al. Assessment of TILs, IDO-1, and PD-L1 in resected non-small cell lung cancer: An immunohistochemical study with clinicopathological and prognostic implications. Virchows Arch. 2019, 474, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cui, Y.; Diehn, M.; Li, R. Development and validation of an individualized immune prognostic signature in early-stage nonsquamous non–small cell lung cancer. JAMA Oncol. 2017, 3, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Velcheti, V.; Schalper, K.A.; Carvajal, D.E.; Anagnostou, V.K.; Syrigos, K.N.; Sznol, M.; Herbst, R.S.; Gettinger, S.N.; Chen, L.; Rimm, D.L. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Investig. 2014, 94, 107–116. [Google Scholar] [CrossRef]
- Zhu, M.M.T.; Dancsok, A.R.; Nielsen, T.O. Indoleamine Dioxygenase Inhibitors: Clinical Rationale and Current Development. Curr. Oncol. Rep. 2019, 21. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Hamid, O.; Smith, D.C.; Bauer, T.M.; Wasser, J.S.; Olszanski, A.J.; Luke, J.J.; Balmanoukian, A.S.; Schmidt, E.V.; Zhao, Y.; et al. Epacadostat plus pembrolizumab in patients with advanced solid tumors: Phase I results from a multicenter, open-label phase I/II trial (ECHO-202/KEYNOTE-037). J. Clin. Oncol. 2018, 36, 3223–3230. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef]
- Ramos, R.N.; De Moraes, C.J.; Zelante, B.; Barbuto, J.A.M. What are the molecules involved in regulatory T-cells induction by dendritic cells in cancer? Clin. Dev. Immunol. 2013, 2013. [Google Scholar] [CrossRef]
- Ma, G.; Deng, Y.; Jiang, H.; Li, W.; Wu, Q.; Zhou, Q. The prognostic role of programmed cell death-ligand 1 expression in non-small cell lung cancer patients: An updated meta-analysis. Clin. Chim. Acta 2018, 482, 101–107. [Google Scholar] [CrossRef]
- Zhou, C.; Tang, J.; Sun, H.; Zheng, X.; Li, Z.; Sun, T.; Li, J.; Wang, S.; Zhou, X.; Sun, H.; et al. PD-L1 expression as poor prognostic factor in patients with nonsquamous non-small cell lung cancer. Oncotarget 2017, 8, 58457–58468. [Google Scholar] [CrossRef]
- Wu, S.; Shi, X.; Sun, J.; Liu, Y.; Luo, Y.; Liang, Z.; Wang, J.; Zeng, X. The significance of programmed cell death ligand 1 expression in resected lung adenocarcinoma. Oncotarget 2017, 8, 16421–16429. [Google Scholar] [CrossRef] [PubMed]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.; Zhang, N.; Zhuang, M.; Zong, Z.; Zou, J.; Li, G.; Wang, X.; Zhou, H.; Zhang, L.; et al. Cross-talk between TNF-α and IFN-γ signaling in induction of B7-H1 expression in hepatocellular carcinoma cells. Cancer Immunol. Immunother. 2018, 67, 271–283. [Google Scholar] [CrossRef]
- Lai, Q.; Wang, H.; Li, A.; Xu, Y.; Tang, L.; Chen, Q.; Zhang, C.; Gao, Y.; Song, J.; Du, Z. Decitibine improve the efficiency of anti-PD-1 therapy via activating the response to IFN/PD-L1 signal of lung cancer cells. Oncogene 2018, 37, 2302–2312. [Google Scholar] [CrossRef]
- Katz, J.B.; Muller, A.J.; Prendergast, G.C. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol. Rev. 2008, 222, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Mandarano, M.; Bellezza, G.; Belladonna, M.L.; Vannucci, J.; Gili, A.; Ferri, I.; Lupi, C.; Ludovini, V.; Falabella, G.; Metro, G.; et al. Indoleamine 2,3-Dioxygenase 2 Immunohistochemical Expression in Resected Human Non-small Cell Lung Cancer: A Potential New Prognostic Tool. Front. Immunol. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Lindström, V.; Aittoniemi, J.; Jylhävä, J.; Eklund, C.; Hurme, M.; Paavonen, T.; Oja, S.S.; Itälä-Remes, M.; Sinisalo, M. Indoleamine 2,3-dioxygenase activity and expression in patients with chronic lymphocytic leukemia. Clin. Lymphoma Myeloma Leuk. 2012, 12, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zheng, X.; Zhang, X.; Wang, H.; Li, Q.; Yuan, K.; Zhou, N.; Yu, Y.; Song, N.; et al. Gene silencing of indoleamine 2,3-dioxygenase 2 in melanoma cells induces apoptosis through the suppression of NAD+ and inhibits in vivo tumor growth. Oncotarget 2016, 7, 32329–32340. [Google Scholar] [CrossRef]
- Endesfelder, D.; Math, D.; Gronroos, E.; Ph, D.; Martinez, P.; Ph, D.; Matthews, N.; Sc, B.; Stewart, A.; Sc, M.; et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar]
- Zhang, J.; Fujimoto, J.; Zhang, J.; Wedge, D.C.; Song, X.; Zhang, J.; Seth, S.; Chow, C.W.; Cao, Y.; Gumbs, C.; et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science 2014, 346, 256–259. [Google Scholar] [CrossRef]
| Characteristics | Patients | |
|---|---|---|
| N = 191 | % | |
| Median Age, years (range) | 67.6 (38.7–84.3) | |
| Gender | ||
| Female | 54 | 28.3 |
| Male | 137 | 71.7 |
| Smoking History | ||
| Never smokers | 16 | 8.4 |
| Former smokers | 76 | 39.8 |
| Current smokers | 99 | 51.8 |
| Histology | ||
| Invasive Adenocarcinomas | 120 | 62.8 |
| Squamous cell carcinomas | 69 | 36.1 |
| Adenosquamous carcinomas | 2 | 1.1 |
| Grading | ||
| 1 | 11 | 5.8 |
| 2 | 99 | 51.8 |
| 3 | 81 | 42.4 |
| pStage | ||
| I | 101 | 52.9 |
| II | 56 | 29.3 |
| III | 34 | 17.8 |
| Type of Resection | ||
| Lobectomy | 183 | 95.8 |
| Pneumonectomy | 4 | 2.1 |
| Other | 4 | 2.1 |
| Relapse | ||
| No | 105 | 55.0 |
| Yes | 86 | 45.0 |
| Exitus | ||
| Live | 95 | 49.7 |
| Dead | 96 | 50.3 |
| Variables | PD-1, N. (%) | PD-L1, N. (%) | PD-L2, N. (%) | IDO-1, N. (%) | IDO-2, N. (%) | INFγ, N. (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | Low | High | Low | High | Low | High | |
| N | 151 (79.1) | 40 (20.9) | 77 (40.3) | 114 (59.7) | 103 (53.4) | 88 (46.1) | 110 (57.6) | 81 (42.4) | 130 (68.1) | 61 (31.9) | 157 (82.2) | 34 (17.8) |
| Age (years) | ||||||||||||
| <60 | 24 (12.6) | 10 (5.2) | 11 (5.8) | 23 (12.0) | 12 (6.3) | 22 (11.5) | 16 (8.4) | 18 (9.4) | 22 (11.5) | 12 (6.3) | 25 (13.1) | 9 (4.7) |
| ≥60 | 127 (66.5) | 30 (15.7) | 66 (34.6) | 91 (47.6) | 91 (47.6) | 66 (34.6) | 94 (49.2) | 63 (33.0) | 108 (56.5) | 49 (25.7) | 132 (69.1) | 25 (13.1) |
| p = 0.181 | p = 0.297 | p = 0.016 | p = 0.170 | p = 0.643 | p = 0.145 | |||||||
| Sex | ||||||||||||
| Female | 45 (23.6) | 9 (4.7) | 18 (9.4) | 36 (18.9) | 32 (16.8) | 22 (11.5) | 31 (16.2) | 23 (12.0) | 42 (22.0) | 12 (6.3) | 46 (24.1) | 8 (4.2) |
| Male | 106 (55.5) | 31 (16.2) | 59 (30.9) | 78 (40.8) | 71 (37.2) | 66 (34.5) | 79 (41.4) | 58 (30.4) | 88 (46.1) | 49 (25.6) | 111 (58.1) | 26 (13.6) |
| p = 0.362 | p = 0.217 | p = 0.353 | p = 0.974 | p = 0.071 | p = 0.675 | |||||||
| Smoking | ||||||||||||
| Never | 15 (7.9) | 1 (0.5) | 6 (3.1) | 10 (5.2) | 8 (4.2) | 8 (4.2) | 7 (3.7) | 9 (4.7) | 14 (7.3) | 2 (1.1) | 14 (7.3) | 2 (1.1) |
| Current | 59 (30.9) | 17 (8.9) | 30 (15.7) | 46 (24.1) | 40 (20.9) | 36 (18.9) | 44 (23.0) | 31 (16.8) | 48 (25.1) | 28 (14.7) | 62 (32.5) | 14 (7.3) |
| Former | 77 (40.3) | 22 (11.5) | 41 (21.5) | 58 (30.4) | 55 (28.8) | 44 (23.0) | 59 (30.9) | 40 (20.9) | 68 (35.6) | 31 (16.2) | 81 (42.4) | 18 (9.4) |
| p = 0.388 | p = 0.975 | p = 0.882 | p = 0.502 | p = 0.162 | p = 0.920 | |||||||
| Histology | ||||||||||||
| Invasive Adenocarcinomas | 104 (54.4) | 16 (8.4) | 54 (28.3) | 66 (34.6) | 73 (38.2) | 47 (24.6) | 73 (38.2) | 47 (24.6) | 95 (49.7) | 25 (13.1) | 100 (52.4) | 20 (10.5) |
| Squamous | 45 (23.6) | 24 (12.6) | 23 (12.0) | 46 (24.1) | 29 (15.2) | 40 (21.0) | 36 (18.9) | 33 (17.3) | 33 (17.3) | 36 (18.9) | 55 (28.8) | 14 (7.3) |
| Adenosquamous | 2 (1.0) | 0 (0.0) | 0 (0.0) | 2 (1.0) | 1 (0.5) | 1 (0.5) | 1 (0.5) | 1 (0.5) | 2 (1.0) | 0 (0.0) | 2 (1.0) | 0 (0.0) |
| p = 0.001 | p = 0.146 | p = 0.021 | p = 0.498 | p < 0.001 | p = 0.702 | |||||||
| Grading | ||||||||||||
| 1 | 8 (4.2) | 3 (1.6) | 2 (1.0) | 9 (4.7) | 5 (2.6) | 6 (3.2) | 3 (1.6) | 8 (4.2) | 7 (3.7) | 4 (2.1) | 7 (3.7) | 4 (2.1) |
| 2 | 81 (42.4) | 18 (9.4) | 43 (22.5) | 56 (29.3) | 55 (28.8) | 44 (23.0) | 61 (31.9) | 38 (19.9) | 74 (38.7) | 25 (13.1) | 82 (43) | 17 (8.9) |
| 3 | 62 (32.5) | 19 (9.9) | 32 (16.8) | 49 (25.7) | 43 (22.5) | 38 (19.9) | 46 (24.1) | 35 (18.3) | 49 (25.7) | 32 (16.7) | 68 (35.6) | 13 (6.8) |
| p = 0.540 | p = 0.299 | p = 0.818 | p = 0.094 | p = 0.107 | p = 0.261 | |||||||
| p-Stage | ||||||||||||
| I | 89 (46.6) | 12 (6.3) | 49 (25.7) | 52 (27.2) | 59 (30.9) | 42 (22.0) | 65 (34.0) | 36 (18.9) | 80 (41.9) | 21 (11.0) | 87 (45,5) | 14 (7.3) |
| II | 39 (20.4) | 17 (8.9) | 18 (9.4) | 38 (19.9) | 28 (14.7) | 28 (14.7) | 31 (16.2) | 25 (13.1) | 31 (16.2) | 25 (13.1) | 45 (23.6) | 11 (5.8) |
| III | 23 (12.0) | 11 (5.8) | 10 (5.2) | 24 (12.6) | 16 (8.3) | 18 (9.4) | 14 (7.3) | 20 (10.5) | 19 (9.9) | 15 (7.9) | 25 (13.1) | 9 (4.7) |
| p = 0.005 | p = 0.048 | p = 0.404 | p = 0.059 | p = 0.002 | p = 0.208 | |||||||
| RFS | ||||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||||
| Variables | N. Pts at risck | HR | 95% CI | * p | HR | 95% CI | * p | |
| Age | <60 (Ref) | 34 | ||||||
| ≥60 | 157 | 0.84 | 0.49–1.44 | 0.54 | ||||
| Sex | ||||||||
| F (Ref) | 54 | |||||||
| M | 137 | 1.03 | 0.65–1.65 | 0.88 | ||||
| Smoking | ||||||||
| Never (Ref) | 16 | |||||||
| Current | 76 | 2.09 | 0.82–5.30 | 0.12 | ||||
| Former | 99 | 1.58 | 0.62–4.00 | 0.33 | ||||
| Histology | ||||||||
| Squamous (Ref) | 69 | |||||||
| Adenocarcinoma | 120 | 1.62 | 1.02–2.58 | 0.03 | 2.16 | 1.30–3.60 | 0.003 | |
| Adenosquamous | 2 | 1.39 | 0.18–10.25 | 0.32 | 1.36 | 0.17–10.46 | 0.76 | |
| p-Stage | ||||||||
| I (Ref) | 101 | |||||||
| II | 56 | 1.71 | 1.05–2.79 | 0.03 | 2.13 | 1.26–3.62 | 0.005 | |
| III | 34 | 2.01 | 1.17–3.41 | 0.01 | 2.13 | 1.20–3.80 | 0.01 | |
| PD-1 | ||||||||
| Low < 4fc (Ref) | 151 | |||||||
| High ≥ 4fc | 40 | 1.17 | 0.70–21.96 | 0.52 | ||||
| PD-L1 | ||||||||
| Low < 4fc (Ref) | 77 | |||||||
| High ≥ 4fc | 114 | 1.22 | 0.79–1.90 | 0.35 | ||||
| PD-L2 | ||||||||
| Low < 4fc (Ref) | 103 | |||||||
| High ≥ 4fc | 88 | 1.41 | 0.92–2.15 | 0.11 | ||||
| IDO-1 | ||||||||
| Low < 4fc (Ref) | 110 | |||||||
| High ≥ 4fc | 81 | 1.26 | 0.832–1.93 | 0.27 | ||||
| IDO-2 | ||||||||
| Low < 4fc (Ref) | 130 | |||||||
| High ≥ 4fc | 61 | 1.33 | 0.85–2.08 | 0.20 | ||||
| INFγ | ||||||||
| Low < 4fc (Ref) | 157 | |||||||
| High ≥ 4fc | 34 | 1.55 | 0.93–2.59 | 0.08 | ||||
| PDL1/PDL2/INFγ | ||||||||
| Low < 4fc (Ref) | 161 | |||||||
| High ≥ 4fc | 30 | 1.90 | 1.13–3.21 | 0.01 | 1.98 | 1.06–3.71 | 0.03 | |
| OS | ||||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||||
| Variables | N. Pts at risk | HR | 95% CI | * p | HR | 95% CI | * p | |
| Age | ||||||||
| <60 | 34 | |||||||
| ≥60 | 157 | 1.04 | 0.60–178 | 0.88 | ||||
| Sex | ||||||||
| F (Ref) | 54 | |||||||
| M | 137 | 1.62 | 1.00–1.64 | 0.04 | 1.53 | 0.94 | 0.08 | |
| Smoking | ||||||||
| Never (Ref) | 16 | 1 | ||||||
| Current | 76 | 1.81 | 0.77–4.28 | 0.17 | ||||
| Former | 99 | 1.62 | 0.69–3.78 | 0.26 | ||||
| Histology | ||||||||
| Squamous (Ref) | 69 | |||||||
| Adenocarcinoma | 120 | 1.14 | 0.75–173 | 0.53 | ||||
| Adenosquamous | 2 | 0.93 | 0.12–6.85 | 0.94 | ||||
| p-Stage | ||||||||
| I (Ref) | 101 | 1 | ||||||
| II | 56 | 1.68 | 1.07–2.64 | 0.02 | 1.65 | 1.03–2.63 | 0.03 | |
| III | 34 | 1.55 | 0.90–2.66 | 0.10 | 1.46 | 0.83–2.57 | 0.18 | |
| PD-1 | ||||||||
| Low < 4fc (Ref) | 151 | |||||||
| High ≥ 4fc | 40 | 1.26 | 0.78–2.04 | 0.29 | ||||
| PD-L1 | ||||||||
| Low < 4fc (Ref) | 77 | |||||||
| High ≥ 4fc | 114 | 1.45 | 0.95–2.21 | 0.08 | ||||
| PD-L2 | ||||||||
| Low < 4fc (Ref) | 103 | |||||||
| High ≥ 4fc | 88 | 1.37 | 0.91–2.04 | 0.12 | ||||
| IDO-1 | ||||||||
| Low < 4fc (Ref) | 110 | |||||||
| High ≥ 4fc | 81 | 1.31 | 0.88–1.96 | 0.17 | ||||
| IDO-2 | ||||||||
| Low < 4fc (Ref) | 130 | |||||||
| High ≥ 4fc | 61 | 1.47 | 0.97–2.22 | 0.06 | ||||
| INFγ | ||||||||
| Low < 4fc (Ref) | 157 | |||||||
| High ≥ 4fc | 34 | 1.33 | 0.80–2.20 | 0.26 | ||||
| PD-L1/IDO-2 | ||||||||
| Low < 4fc (Ref) | 141 | |||||||
| High ≥ 4fc | 50 | 1.63 | 1.06–2.51 | 0.02 | 1.98 | 1.02–3.83 | 0.04 | |
| PD-L2/IDO-1 | ||||||||
| Low < 4fc (Ref) | 132 | |||||||
| High ≥ 4fc | 59 | 1.54 | 1.02–2.33 | 0.04 | 1.92 | 1.10–3.35 | 0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ludovini, V.; Bianconi, F.; Siggillino, A.; Vannucci, J.; Baglivo, S.; Berti, V.; Tofanetti, F.R.; Reda, M.S.; Bellezza, G.; Mandarano, M.; et al. High PD-L1/IDO-2 and PD-L2/IDO-1 Co-Expression Levels Are Associated with Worse Overall Survival in Resected Non-Small Cell Lung Cancer Patients. Genes 2021, 12, 273. https://doi.org/10.3390/genes12020273
Ludovini V, Bianconi F, Siggillino A, Vannucci J, Baglivo S, Berti V, Tofanetti FR, Reda MS, Bellezza G, Mandarano M, et al. High PD-L1/IDO-2 and PD-L2/IDO-1 Co-Expression Levels Are Associated with Worse Overall Survival in Resected Non-Small Cell Lung Cancer Patients. Genes. 2021; 12(2):273. https://doi.org/10.3390/genes12020273
Chicago/Turabian StyleLudovini, Vienna, Fortunato Bianconi, Annamaria Siggillino, Jacopo Vannucci, Sara Baglivo, Valeria Berti, Francesca Romana Tofanetti, Maria Sole Reda, Guido Bellezza, Martina Mandarano, and et al. 2021. "High PD-L1/IDO-2 and PD-L2/IDO-1 Co-Expression Levels Are Associated with Worse Overall Survival in Resected Non-Small Cell Lung Cancer Patients" Genes 12, no. 2: 273. https://doi.org/10.3390/genes12020273
APA StyleLudovini, V., Bianconi, F., Siggillino, A., Vannucci, J., Baglivo, S., Berti, V., Tofanetti, F. R., Reda, M. S., Bellezza, G., Mandarano, M., Belladonna, M. L., Metro, G., Chiari, R., Sidoni, A., Puma, F., Minotti, V., & Roila, F. (2021). High PD-L1/IDO-2 and PD-L2/IDO-1 Co-Expression Levels Are Associated with Worse Overall Survival in Resected Non-Small Cell Lung Cancer Patients. Genes, 12(2), 273. https://doi.org/10.3390/genes12020273








