Eicosanoid Signaling in Insect Immunology: New Genes and Unresolved Issues
Abstract
1. Introduction
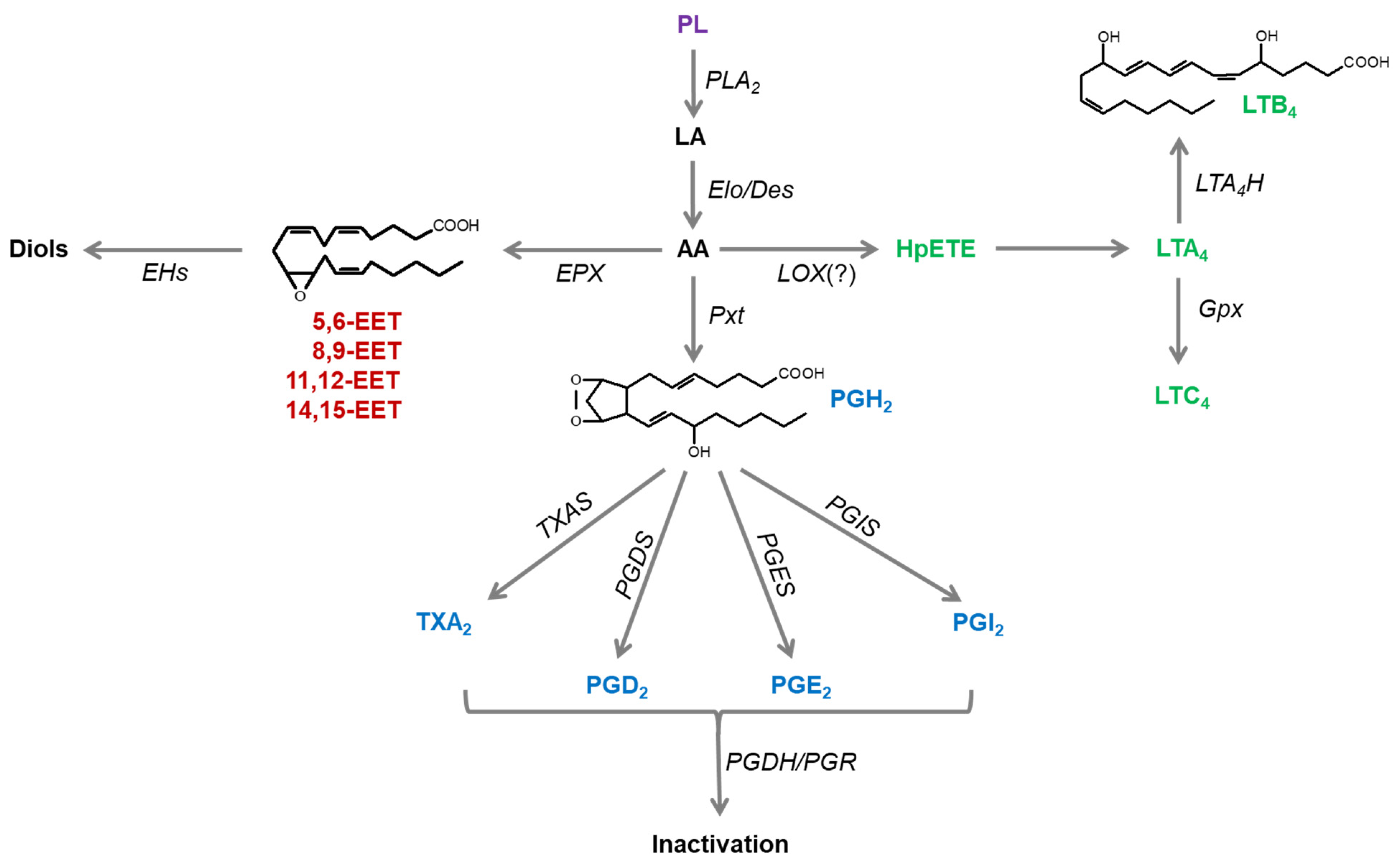
2. Eicosanoid Biosynthesis
2.1. Phospholipases A2 (PLA2)
2.2. Biosynthesis of AA
2.3. Mammalian Cyclooxygenases and Insect Peroxynectins (Pxts)
2.4. PG Biosynthesis
2.5. New Elements of Insect Oxylipins
2.6. PG Catabolism
2.7. EET Biosynthesis
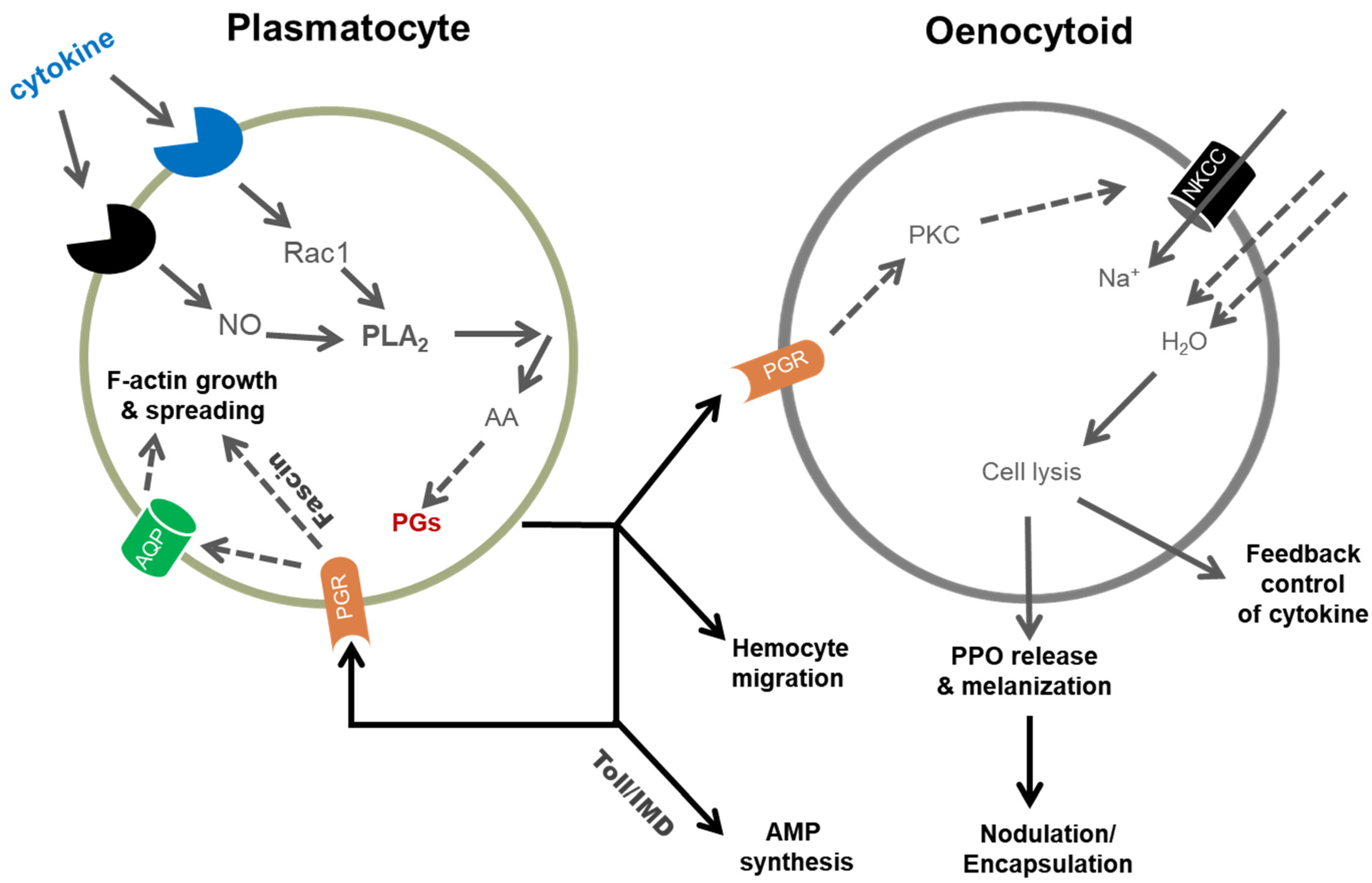
3. Eicosanoid Actions in Insect Immunity
3.1. Clearing Bacteria from Hemolymph
3.2. Nodulation
3.3. Cell Spreading
3.4. Releasing Prophenoloxidase (PPO) from Oenocytoids
3.5. Hemocyte Migration
3.6. PG Actions in Gut Immunity
3.7. Eicosanoid Actions in Humoral Immunity
3.8. Eicosanoid Actions in Mosquito Immunity
4. Prospectus
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Westra, E.R.; Levin, B.R. It is unclear how important CRISPR-Cas systems are for protecting natural populations of bacteria against infections by mobile genetic elements. Proc. Natl. Acad. Sci. USA 2020, 117, 27777–27785. [Google Scholar] [CrossRef] [PubMed]
- Pita, L.; Rix, L.; Slaby, B.M.; Franke, A.; He ntschel, U. The sponge holobiont in a changing ocean: From microbes to ecosystems. Microbiome 2018, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Stokes, B.A.; Yadav, S.; Shokal, U.; Smith, L.C.; Eleftherianos, I. Bacterial and fungal pattern recognition receptors in homologous innate signaling pathways of insects and mammals. Front. Microbiol. 2015, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Albright, J.O.; Barton, A.E.; Hashimoto, S. Chemical and enzymic syntheses of 5-HPETE, a key biological precursor of slow-reacting substance of anaphylaxis (SRS), and 5-HETE. J. Am. Chem. Soc. 1980, 102, 1435–1436. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.W.; Kim, Y. Eicosanoid signaling in insects: From discovery to plant protection. Crit. Rev. Plant. Sci. 2014, 33, 20–63. [Google Scholar] [CrossRef]
- Stanley, D.; Kim, Y. Insect prostaglandins and other eicosanoids: From molecular to physiological actions. Adv. Insect Physiol. 2019, 56, 283–343. [Google Scholar] [CrossRef]
- Kim, Y.; Ahmed, S.; Stanley, D.; An, C. Eicosanoid-mediated immunity in insects. Dev. Comp. Immunol. 2018, 83, 130–143. [Google Scholar] [CrossRef]
- Stanley, D. Eicosanoids in Invertebrate Signal. Transduction Systems; Princeton University Press: Princeton, NJ, USA, 2000; p. 292. ISBN 0691006601. [Google Scholar]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, 237–242. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y. Eicosanoids rescue Spodoptera exigua infected with Xenorhabdus nematophilus, the symbiotic bacteria to the entomopathogenic nematode Steinernema carpocapsae. J. Insect Physiol. 2000, 46, 1469–1476. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y. Xenorhabdus nematophila inhibits p-bromophenacyl bromide (BPB)-sensitive PLA2 of Spodoptera exigua. Arch. Insect Biochem. Physiol. 2003, 54, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, Y.; Stanley, D.W. The bacterium Xenorhabdus nematophila inhibits phospholipases A2 from insect, prokaryote, and vertebrate sources. Naturwissenschaften 2004, 91, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Al Baki, M.A.; Lee, D.W.; Jung, J.; Kim, Y. Deletion mutant of sPLA2 using CRISPR/Cas9 exhibits immunosuppression, developmental retardation, and failure of oocyte development in legume pod borer, Maruca vitrata. Dev. Comp. Immunol. 2020, 103, 103500. [Google Scholar] [CrossRef]
- Stanley, D.; Kim, Y. Why most insects have very low proportions of C20 polyunsaturated fatty acids: The oxidative damage hypothesis. Arch. Insect Biochem. Physiol. 2020, 103, e21622. [Google Scholar] [CrossRef]
- Stanley-Samuelson, D.W.; Jurenka, R.A.; Cripps, C.; Blomquist, G.J.; de Renobales, M. Fatty acids in insects: Composition, metabolism and biological significance. Arch. Insect Biochem. Physiol. 1988, 9, 1–33. [Google Scholar] [CrossRef]
- Hasan, M.A.; Ahmed, S.; Kim, Y. Biosynthetic pathway of arachidonic acid in Spodoptera exigua in response to bacterial challenge. Insect Biochem. Mol. Biol. 2019, 111, 103179. [Google Scholar] [CrossRef]
- Stanley, D.; Goodman, C.L.; Ringbauer, J.A.; Song, Q. Prostaglandins influence protein phosphorylation in established insect cell line. Arch. Insect Biochem. Physiol. 2020, 105, e21725. [Google Scholar] [CrossRef]
- Varvas, L.; Kurg, R.; Hansen, K.; Jarving, I.; Valmsen, K.; Lohelaid, H.; Samuel, N. Direct evidence of the cyclooxygenase pathway of prostaglandin synthesis in arthropods: Genetic and biochemical characterization of two crustacean cyclooxygenases. Insect Biochem. Mol. Biol. 2009, 39, 851–860. [Google Scholar] [CrossRef]
- Tootle, T.L.; Spradling, A.C. Drosophila pxt: A cyclooxygenase-like facilitator of follicle maturation. Development 2008, 135, 839–847. [Google Scholar] [CrossRef]
- Park, J.; Stanley, D.; Kim, Y. Roles of peroxinectin in PGE2-mediated cellular immunity in Spodoptera exigua. PLoS ONE 2014, 9, e105717–oi:10. [Google Scholar] [CrossRef]
- Barletta, A.B.F.; Trisnadi, N.; Ramirez, J.L.; Barillas-Mury, C. Mosquito midgut prostaglandin release establishes systemic immune priming. iScience 2019, 19, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Scarpati, M.; Qi, Y.; Govid, S.; Singh, S. A combined computational strategy of sequence and structural analysis predicts the existence of a functional eicosanoid pathway in Drosophila melanogaster. PLoS ONE 2019, 14, e0211897. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Stanley, D.; Kim, Y. An insect prostaglandin E2 synthase acts in immunity and reproduction. Front. Physiol. 2018, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Sajjadian, S.M.; Ahmed, S.; Al Baki, M.A.; Kim, Y. Prostaglandin D2 synthase and its functional association with immune and reproductive processes in a lepidopteran insect, Spodoptera exigua. Gen. Comp. Endocrinol. 2020, 287, 113352. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Gryglewski, R.; Bunting, S.; Vane, R.J. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature 1976, 263, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Kirkby, N.S. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br. J. Pharm. 2019, 176, 1038–1050. [Google Scholar] [CrossRef]
- Ahmed, S.; Al Baki, M.A.; Lee, J.; Seo, D.Y.; Lee, D.; Kim, Y. The first report of prostacyclin and its physiological roles in insects. Gen. Comp. Endocrinol. 2021, 301, 113659. [Google Scholar] [CrossRef]
- Vatanperast, M.; Ahmed, S.; Lee, D.-H.; Hwang, S.H.; Hammock, B.; Kim, Y. EpOMEs act as immune suppressors in a lepidopteran insect, Spodoptera exigua. Sci. Rep. 2020, 10, 20183. [Google Scholar] [CrossRef]
- Ahmed, S.; Kim, Y. Prostaglandin catabolism in Spodoptera exigua, a lepidopteran insect. J. Exp. Biol. 2020, 223, 233221. [Google Scholar] [CrossRef]
- McGiff, J.C. Cytochrome P-450 metabolism of arachidonic acid. Annu. Rev. Pharmacol. Toxicol. 1991, 31, 339–369. [Google Scholar] [CrossRef]
- Dadd, R.H. Essential fatty acids for mosquitoes, other insects and vertebrates. In Current Topics in Insect Endocrinology and Nutrition: A Tribute to Gottfried S. Fraenkel; Bhaskaran, G., Friedman, S., Rodriguez, J.G., Eds.; Springer: Boston, MA, USA, 1981; pp. 189–214. [Google Scholar]
- Dadd, R.H.; Kleinjan, J.E. Prostaglandin synthetase inhibitors modulate the effect of essential dietary arachidonic acid in the mosquito Culex pipiens. J. Insect Physiol. 1984, 30, 721–728. [Google Scholar] [CrossRef]
- Zeldin, D.C. Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem. 2001, 276, 36059–36062. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Morisseau, C.; Yang, J.; Mamatha, D.M.; Hammock, B.D. Epoxide hydrolase activities and epoxy fatty acids in the mosquito Culex quinquefasciatus. Insect Biochem. Mol. Biol. 2015, 59, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511. [Google Scholar] [CrossRef]
- Junier, M.P.; Dray, F.; Blair, I.A.N.; Capdevila, J.; Dishman, E.; Falck, J.R.; Ojeda, S.R. Epoxygenase products of arachidonic acid are endogenous constituents of the hypothalamus involved in D2 receptor-mediated, dopamine-induced release of somatostatin. Endocrinology 1990, 126, 1534–1540. [Google Scholar] [CrossRef]
- Falck, J.R.; Manna, S.; Moltz, J.; Chacos, N.; Capdevila, J. Epoxyeicosatrienoic acids stimulate glucagon and insulin release from isolated rat pancreatic islets. Biochem. Biophys. Res. Commun. 1983, 114, 743–749. [Google Scholar] [CrossRef]
- Satoh, T.; Cohen, H.T.; Katz, A.I. Intracellular signaling in the regulation of renal Na-K-ATPase. II. Role of eicosanoids. J. Clin. Investig. 1993, 91, 409–415. [Google Scholar] [CrossRef]
- Node, K.; Huo, Y.; Ruan, X.; Yang, B.; Spiecker, M.; Ley, K.; Zeldin, D.C.; Liao, J.K. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 1999, 285, 1276–1279. [Google Scholar] [CrossRef]
- Ishizuka, T.; Cheng, J.; Singh, H.; Vitto, M.D.; Manthati, V.L.; Falck, J.R. Laniado-Schwartzman, M. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-κB activation and the production of inflammatory cytokines in human endothelial cells. J. Pharmacol. Exp. Ther. 2008, 324, 103–110. [Google Scholar] [CrossRef]
- Xu, J.; Morisseau, C.; Yang, J.; Lee, K.S.S.; Karmita, S.G.; Hammock, B.D. Ingestion of the epoxide hydrolase inhibitor AUDA modulates immune responses of the mosquito, Culex quinquefasciatus. Insect Biochem. Mol. Biol. 2016, 76, 62–69. [Google Scholar] [CrossRef]
- Vatanparast, M.; Lee, D.H.; Kim, Y. Biosynthesis and immunity of epoxyeicosatrienoic acids in a lepidopteran insect, Spodoptera exigua. Dev. Comp. Immunol. 2020, 107, 103643. [Google Scholar] [CrossRef] [PubMed]
- Diani-Moore, S.; Ma, Y.; Gross, S.S.; Riflaud, A.B. Increases in levels of epoxyeicosatrienoic and dihydroxyeicosatrienoic acids (EETs and DHETs) in liver and heart in vivo by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and in hepatic EET:DHET ratios by cotreatment with TCDD and the soluble epoxide hydrolase inhibitor AUDA. Drug Metab. Dispos. 2014, 42, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Stanley-Samuelson, D.W.; Jensen, E.; Nickerson, K.W.; Tiebel, K.; Ogg, C.L.; Howard, R.W. Insect immune response to bacterial infection is mediated by eicosanoids. Proc. Natl. Acad. Sci. USA 1991, 88, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Nguyen, T.; Stanley-Samuelson, D.W. Eicosanoids mediate insect nodulation responses to bacterial infections. Proc. Natl. Acad. Sci. USA 1997, 91, 12418–12422. [Google Scholar] [CrossRef]
- Stanley, D. Prostaglandins and other eicosanoids in insects: Biological significance. Annu. Rev. Entomol. 2006, 51, 25–44. [Google Scholar] [CrossRef]
- Clark, K.; Pech, L.L.; Strand, M.R. Isolation and identification of a plasmatocyte spreading peptide from hemolymph of the lepidopteran insect Pseudoplusia includens. J. Biol. Chem. 1997, 272, 23440–23447. [Google Scholar] [CrossRef]
- Miller, J.S. Eicosanoids influence in vitro elongation of plasmatocytes from the tobacco hornworm, Manduca sexta. Arch. Insect Biochem. Physiol. 2005, 59, 42–51. [Google Scholar] [CrossRef]
- Srikanth, K.; Park, J.; Stanley, D.W.; Kim, Y. Plasmatocyte-spreading peptide influences hemocyte behavior via eicosanoids. Arch. Insect Biochem. Physiol. 2011, 78, 145–160. [Google Scholar] [CrossRef]
- Takai, Y.; Sasaki, T.; Matozaki, T. Small GTP-binding proteins. Physiol. Rev. 2001, 81, 153–208. [Google Scholar] [CrossRef]
- Groen, C.M.; Spracklen, A.J.; Fagan, T.N.; Tootle, T.L. Drosophila Fascin is a novel downstream target of prostaglandin signaling during actin remodeling. Mol. Biol. Cell 2012, 23, 4567–4578. [Google Scholar] [CrossRef]
- Park, J.; Stanley, D.; Kim, Y. Rac1 mediates cytokine-stimulated hemocyte spreading via prostaglandin biosynthesis in the beet armyworm, Spodoptera exigua. J. Insect Physiol. 2013, 59, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Rämet, M.; Manfruelli, P.; Pearson, A.; Mathey-Prevot, B.; Ezekowitz, R.A. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 2002, 416, 644–648. [Google Scholar] [CrossRef]
- Nobes, C.D.; Hall, A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995, 81, 53–62. [Google Scholar] [CrossRef]
- Hao, Y.; Yu, S.; Luo, F.; Jin, L.H. Jumu is required for circulating hemocyte differentiation and phagocytosis in Drosophila. Cell Commun. Signal. 2018, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J.; Millard, T.H.; Evans, I.R.; Wood, W. Ena orchestrates remodelling within the actin cytoskeleton to drive robust Drosophila macrophage chemotaxis. J. Cell Sci. 2019, 132, 224618. [Google Scholar] [CrossRef] [PubMed]
- Maciver, S.K.; Hussey, P.J. The ADF/cofilin family: Actin-remodeling proteins. Genome Biol. 2002, 3, 3007. [Google Scholar] [CrossRef]
- Ahmed, S.; Kim, Y. PGE2 mediates cytoskeletal rearrangement of hemocytes via Cdc42, a small G protein, to activate actin-remodeling factors in Spodoptera exigua (Lepidoptera: Noctuidae). Arch. Insect Biochem. Physiol. 2019, 102, e21607. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Cao, X.; Zou, Z.; Lu, Z.; Kanost, M.R.; Jiang, H. Hemolymph protease-5 links the melanization and Toll immune pathways in the tobacco hornworm, Manduca sexta. Proc. Natl. Acad. Sci. USA 2020, 117, 23581–23587. [Google Scholar] [CrossRef]
- Bidla, G.; Lindgren, M.; Theopold, U.; Dushay, M.S. Hemolymph coagulation and phenoloxidase in Drosophila larvae. Dev. Comp. Immunol. 2005, 29, 669–679. [Google Scholar] [CrossRef]
- Dudzic, J.P.; Hanson, M.A.; Iatsenko, I.; Kondo, S.; Lemaitre, B. More than black or white: Melanization and Toll share regulatory serine proteases in Drosophila. Cell Rep. 2019, 27, 1050–1061. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, Y. Eicosanoids mediate prophenoloxidase release from oenocytoids in the beet armyworm, Spodoptera exigua. Insect Biochem. Mol. Biol. 2008, 38, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Stanley, D.; Kim, Y. PGE2 induces oenocytoid cell lysis via a G protein-coupled receptor in the beet armyworm, Spodoptera exigua. J. Insect Physiol. 2011, 57, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Park, J.; Ahn, S.-J.; Kim, Y. PGE2 mediates oenocytdoid cell lysis via a sodium-potassium-chloride cotransporter. Arch. Insect Biochem. Physiol. 2015, 89, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Yang, Y.; Kumar, S.; Lee, D.W.; Bajracharya, P.; Calkins, T.L.; Kim, Y.; Pietrantonio, P.V. Characterization of the first insect prostaglandin (PGE2) receptor: MansePGE2R is expressed in oenocytoids and lipoteichoic acid (LTA) increases transcript expression. Insect Biochem. Mol. Biol. 2020, 117, 1–14. [Google Scholar] [CrossRef]
- Kim, Y.; Ahmed, S.; Al Baki, M.A.; Kumar, S.; Kim, K.; Park, Y.; Stanley, D. Deletion mutant of PGE2 receptor using CRISPR-Cas9 exhibits larval immunosuppression and adult infertility in a lepidopteran insect, Spodoptera exigua. Dev. Comp. Immunol. 2020, 111, 103743. [Google Scholar] [CrossRef]
- Kwon, H.; Hall, D.R.; Smith, R.C. Identification of a prostaglandin E2 receptor that regulates mosquito oenocytoid immune cell function in limiting bacteria and parasite infection. bioRxiv 2021. [Google Scholar] [CrossRef]
- Merchant, D.; Ertl, R.L.; Rennard, S.I.; Stanley, D.W.; Miller, J.S. Eicosanoids mediate insect hemocyte migration. J. Insect Physiol. 2008, 54, 215–221. [Google Scholar] [CrossRef]
- Marasco, W.A.; Phan, S.H.; Krutzsch, H.; Showell, H.J.; Feltner, D.E.; Nairn, R.; Becker, E.L.; Ward, P.A. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J. Biol. Chem. 1984, 259, 5430–5439. [Google Scholar] [CrossRef]
- Lee, K.A.; Cho, K.C.; Kim, B.; Jang, I.H.; Nam, K.; Kwon, Y.E.; Kim, M.; Hyeon, D.Y.; Hwang, D.; Seol, J.H.; et al. Inflammation-modulated metabolic reprogramming is required for DUOX-Dependent gut immunity in Drosophila. Cell Host Microbe 2018, 23, 338–352. [Google Scholar] [CrossRef]
- Sajjadian, S.M.; Kim, Y. PGE2 upregulates gene expression of dual oxidase in a lepidopteran insect midgut via cAMP signalling pathway. Open Biol. 2020, 10, 200197. [Google Scholar] [CrossRef]
- Yajima, M.; Tanaka, M.; Tanahashi, N.; Kikuchi, H.; Natori, S.; Oshima, Y.; Kurata, S. A newly established in vitro culture using transgenic Drosophila reveals functional coupling between the phospholipase A2-generated fatty acid cascade and lipopolysaccharide-dependent activation of the immune deficiency (imd) pathway in insect immunity. Biochem. J. 2003, 371, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Kim, Y. Activation of immune-associated phospholipase A2 is functionally linked to Toll/Imd signal pathways in the red flour beetle, Tribolium castaneum. Dev. Comp. Immunol. 2010, 34, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Shafeeq, T.; Ahmed, S.; Kim, Y. Toll immune signal activates cellular immune response via eicosanoids. Dev. Comp. Immunol. 2018, 84, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Horng, T.; Medzhitov, R. Drosophila MyD88 is an adapter in the Toll signaling pathway. Proc. Natl. Acad. Sci. USA 2001, 98, 12654–12658. [Google Scholar] [CrossRef] [PubMed]
- Moncrieffe, M.C.; Grossmann, J.G.; Gay, N.J. Assembly of oligomeric death domain complexes during Toll receptor signaling. J. Biol. Chem. 2008, 283, 33447–33454. [Google Scholar] [CrossRef]
- Wu, L.P.; Anderson, K.V. Regulated nuclear import of Rel proteins in the Drosophila immune response. Nature 1998, 392, 93–97. [Google Scholar] [CrossRef]
- Kwon, H.; Smith, R.C. Inhibitors of eicosanoid biosynthesis reveal that multiple lipid signaling pathways influence malaria parasite survival in Anopheles gambiae. Insects 2019, 10, 307. [Google Scholar] [CrossRef]
- Hildreth, K.; Kodani, S.D.; Hammock, B.D.; Zhao, L. Cytochrome P40-derived linoleic acid metabolites EpOMEs and DiHOMEs: A review of recent studies. J. Nutri Biochem 2020, 86, 108484. [Google Scholar] [CrossRef]
- Barletta, A.B.F.; Silva, T.L.A.; Talyuli, O.A.C.; Luna-Gomes, T.; Sim, S.; Anglero-Rodriguez, Y.; Dimopoulos, G.; Bandeira-Melo, C.; Sorgine, M.H.F. Prostaglandins regulate humoral immune responses in Aedes aegypti. PLoS Negl. Trop. Dis. 2020, 14, e0008706. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Stanley, D. Eicosanoid Signaling in Insect Immunology: New Genes and Unresolved Issues. Genes 2021, 12, 211. https://doi.org/10.3390/genes12020211
Kim Y, Stanley D. Eicosanoid Signaling in Insect Immunology: New Genes and Unresolved Issues. Genes. 2021; 12(2):211. https://doi.org/10.3390/genes12020211
Chicago/Turabian StyleKim, Yonggyun, and David Stanley. 2021. "Eicosanoid Signaling in Insect Immunology: New Genes and Unresolved Issues" Genes 12, no. 2: 211. https://doi.org/10.3390/genes12020211
APA StyleKim, Y., & Stanley, D. (2021). Eicosanoid Signaling in Insect Immunology: New Genes and Unresolved Issues. Genes, 12(2), 211. https://doi.org/10.3390/genes12020211





