Phenotypic and Genomic Analysis of Cystic Hygroma in Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and DNA Samples
2.3. Clinical and Further Examination
2.4. Postmortem Examination, Histology, and Bacteriology
2.5. Genetic Analyses
2.6. Whole-Genome Sequencing
2.7. Availability of Data and Material
3. Results
3.1. Clinical and Further Examination
3.2. Postmortem Examination, Histology, and Bacteriology
3.3. Genetic Analyses
3.4. Whole-Genome Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirza, B.; Ijaz, L.; Saleem, M.; Sharif, M.; Sheikh, A. Cystic hygroma: An overview. J. Cutan. Aesthet. Surg. 2010, 3, 139. [Google Scholar] [CrossRef]
- Noia, G.; Maltese, P.E.; Zampino, G.; D’Errico, M.; Cammalleri, V.; Convertini, P.; Marceddu, G.; Mueller, M.; Guerri, G.; Bertelli, M. Cystic hygroma: A preliminary genetic study and a short review from the literature. Lymphat. Res. Biol. 2019, 17, 30–39. [Google Scholar] [CrossRef]
- Chen, Y.N.; Chen, C.P.; Lin, C.J.; Chen, S.W. Prenatal Ultrasound Evaluation and Outcome of Pregnancy with Fetal Cystic Hygromas and Lymphangiomas. J. Med. Ultrasound 2017, 25, 12–15. [Google Scholar] [CrossRef]
- Levy, A.T.; Berghella, V.; Al-Kouatly, H.B. Outcome of 45, X fetuses with cystic hygroma: A systematic review. Am. J. Med. Genet. Part A 2020, 185, 26–32. [Google Scholar] [CrossRef]
- Fisher, R.; Partington, A.; Dykes, E. Cystic hygroma: Comparison between prenatal and postnatal diagnosis. J. Pediatr. Surg. 1996, 31, 473–476. [Google Scholar] [CrossRef]
- Chervenak, F.A.; Isaacson, G.; Blakemore, K.J.; Breg, W.R.; Hobbins, J.C.; Berkowitz, R.L.; Marge, T.; Mayden, K.; Mahoney, M.J. Fetal Cystic Hygroma—Cause and Natural History. N. Engl. J. Med. 1983, 309, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H. Lymphangiosarcoma of dogs: A review. J. S. Afr. Vet. Assoc. 2005, 76, 127–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thornton, H. Hygromas in cattle. Cent. Afr. J. Med. 1970, 16, 66–67. [Google Scholar] [PubMed]
- Mauldin, E.A.; Peters-Kennedy, J. Integumentary System. In Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed.; Grant Maxie, M., Ed.; Elsevier: St. Louis, MO, USA, 2016. [Google Scholar]
- Savage, V.L.; Cudmore, L.A.; Russell, C.M.; Railton, D.I.; Begg, A.P.; Collins, N.M.; Adkins, A.R. Intra-abdominal cystic lymphangiomatosis in a Thoroughbred foal. Equine Vet. Educ. 2018, 30, 403–408. [Google Scholar] [CrossRef]
- Gehlen, H.; Wohlsein, P. Cutaneous lymphangioma in a young Standardbred mare. Equine Vet. J. 2000, 32, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Liu, F.F.; Jan, S.W.; Lee, C.C.; Town, D.D.; Lan, C.C. Cytogenetic evaluation of cystic hygroma associated with hydrops fetalis, oligohydramnios or intrauterine fetal death: The roles of amniocentesis, postmortem chorionic villus sampling and cystic hygroma paracentesis. Acta Obstet. Gynecol. Scand. 1996, 75, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kohli, M.; Pandey, S.; Tulsi, S.P.S. Cystic hygroma. Natl. J. Maxillofac. Surg. 2001, 1, 528. [Google Scholar] [CrossRef] [PubMed]
- Shulman, L.P.; Emerson, D.S.; Felke, R.E.; Phillips, P.O.; Simpson, L.J.; Elias, S. High frequency of cytogenetic abnormalities in fetuses with cystic hygroma diagnosed in the first trimester. Obstet. Gynecol. 1992, 80, 80–82. [Google Scholar] [PubMed]
- van Zalen-Sprock, R.M.; van Vugt, J.M.G.; van Geijn, H.P. First-trimester diagnosis of cystic hygroma—Course and outcome. Am. J. Obstet. Gynecol. 1992, 167, 94–98. [Google Scholar] [CrossRef]
- Baxi, L.; Brown, S.; Desai, K.; Thaker, H. Recurrent cystic hygroma with hydrops. Fetal Diagn. Ther. 2009, 25, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Rotmensch, S.; Celentano, C.; Sadan, O.; Liberati, M.; Lev, D.; Glezerman, M. Familial occurrence of isolated nonseptated nuchal cystic hygromata in midtrimester of pregnancy. Prenat. Diagn. 2004, 24, 260–264. [Google Scholar] [CrossRef]
- Teague, K.E.; Eggleston, M.K.; Muffley, P.E.; Gherman, R.B. Recurrent fetal cystic hygroma with normal chromosomes: Case report and review of the literature. J. Matern. Fetal Med. 2000, 9, 366–369. [Google Scholar]
- Maltese, P.E.; Rakhmanov, Y.; Zulian, A.; Notarangelo, A.; Bertelli, M. Genetic testing for cystic hygroma. Eur. Biotechnol. J. 2018, 2, 22–25. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.A.M.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Abecasis, G.R.; Cherny, S.S.; Cookson, W.O.; Cardon, L.R. Merlin—Rapid analysis of dense genetic maps using sparse gene flow trees. Nat. Genet. 2002, 30, 97–101. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Broad Institute. “Picard Toolkit.” GitHub Repository. 2019. Available online: http://broadinstitute.github.io/picard/ (accessed on 1 October 2020).
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118, iso-2; iso-3. Fly (Austin) 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Derks, M.F.L.; Gjuvsland, A.B.; Bosse, M.; Lopes, M.S.; Van Son, M.; Harlizius, B.; Tan, B.F.; Hamland, H.; Grindflek, E.; Groenen, M.A.M.; et al. Loss of function mutations in essential genes cause embryonic lethality in pigs. PLoS Genet. 2019, 15, 1008055. [Google Scholar] [CrossRef]
- Garrison, E.; Marth, G. Haplotype-based variant detection from short-read sequencing. arXiv 2012, arXiv:1207.3907. [Google Scholar]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef]
- Turner, S. qqman: An R package for visualizing GWAS results using Q-Q and manhattan plots. J. Open Source Softw. 2018, 3, 731. [Google Scholar] [CrossRef]
- Čandek-Potokar, M.; Škrlep, M. Factors in pig production that impact the quality of dry-cured ham: A review. Animal 2012, 6, 327–338. [Google Scholar] [CrossRef]
- Knox, R.V. Artificial insemination in pigs today. Theriogenology 2016, 85, 83–93. [Google Scholar] [CrossRef]
- Grahofer, A.; Letko, A.; Häfliger, I.M.; Jagannathan, V.; Ducos, A.; Richard, O.; Peter, V.; Nathues, H.; Drögemüller, C. Chromosomal imbalance in pigs showing a syndromic form of cleft palate. BMC Genom. 2019, 20, 349. [Google Scholar] [CrossRef] [PubMed]
- Grahofer, A.; Nathues, H.; Gurtner, C. Multicystic degeneration of the Cowper’s gland in a Large White boar. Reprod. Domest. Anim. 2016, 51, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Pejaver, V.; Urresti, J.; Lugo-Martinez, J.; Pagel, K.A.; Lin, G.N.; Nam, H.-J.; Mort, M.; Cooper, D.N.; Sebat, J.; Iakoucheva, L.M.; et al. MutPred2: Inferring the molecular and phenotypic impact of amino acid variants. Nat. Commun. 2020, 11, 5918. [Google Scholar] [CrossRef] [PubMed]
- Alfares, A.; Alsubaie, L.; Aloraini, T.; Alaskar, A.; Althagafi, A.; Alahmad, A.; Rashid, M.; Alswaid, A.; Alothaim, A.; Eyaid, W.; et al. What is the right sequencing approach? Solo VS extended family analysis in consanguineous populations. BMC Med. Genom. 2020, 13, 103. [Google Scholar] [CrossRef] [PubMed]

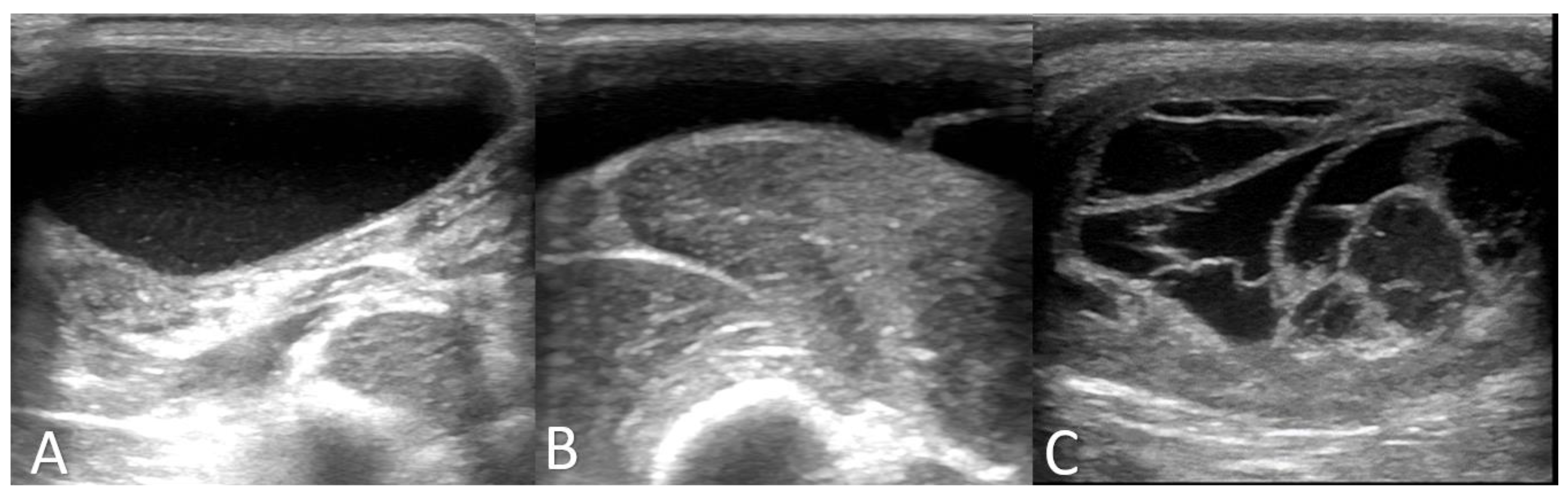
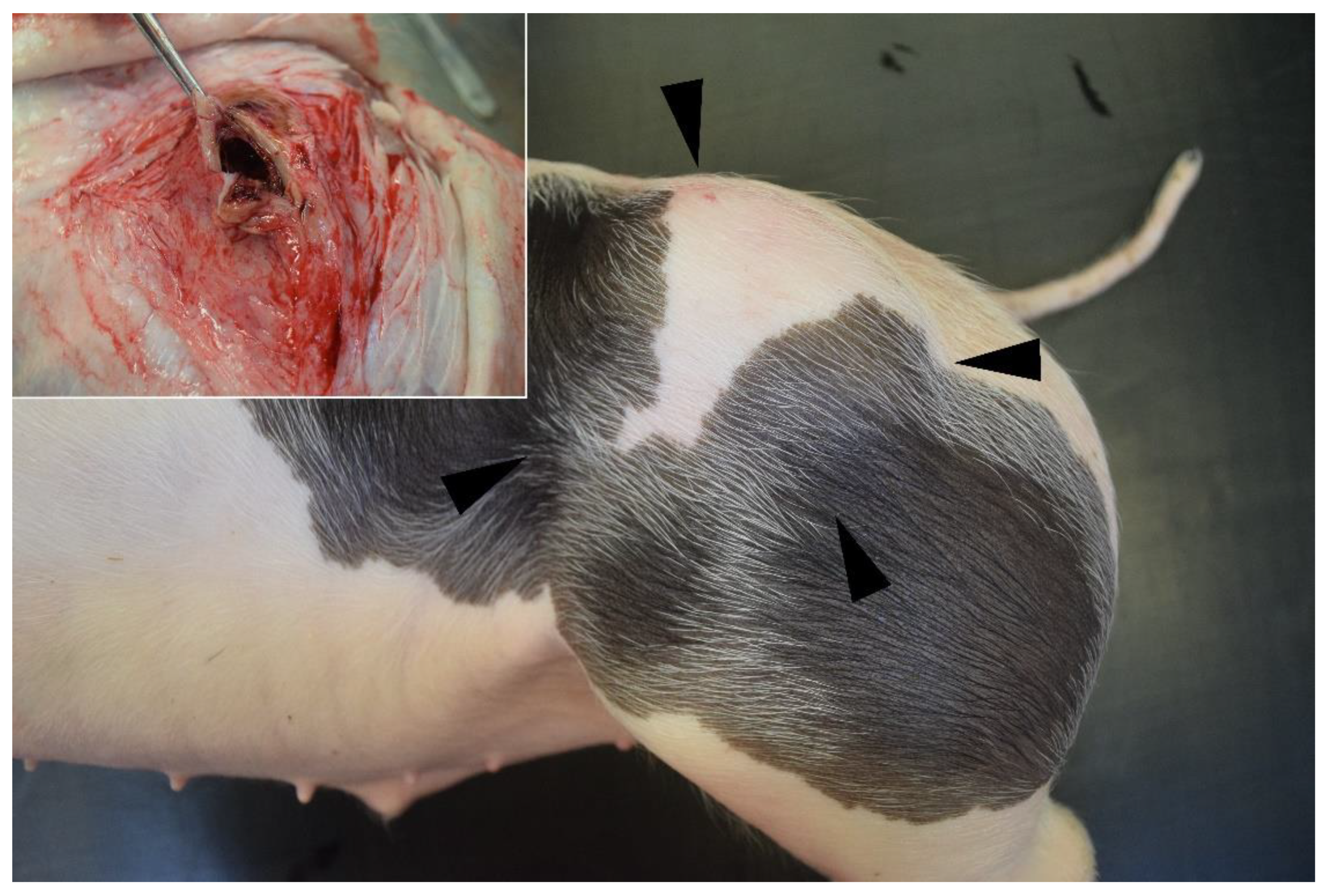
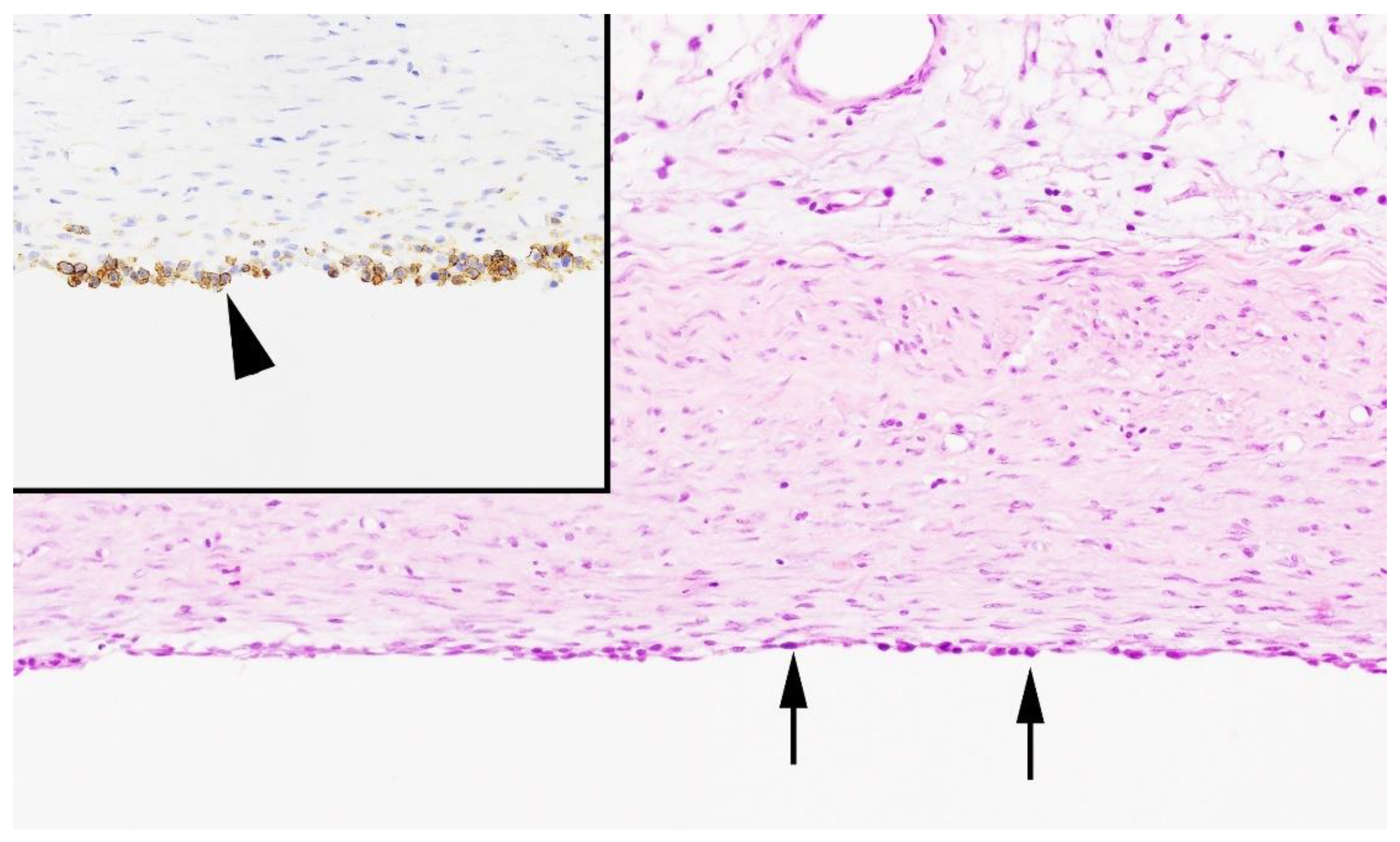
| Parameters | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Colour | Light red | Red | Red |
| Transparency | Cloudy | Cloudy | Cloudy |
| Protein (g/l) | 16 | 26 | 44 |
| Specific weight | 1.016 | 1.022 | 1.032 |
| Cell count (10 × 109/l) | 4.50 | 2.70 | 13.03 |
| Conclusion | Hygroma or seroma | Hygroma or seroma and blood | Hygroma or seroma and mild inflammation |
| Strategy 1 | Purebred Piètrain | Crossbred | 10 Unrelated Controls | No. of Variants with Different Predicted Impact | after Filtering against Validation Cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Dam/Sire of Case 1 | Full-sib Of Case 1 | Case 3 | High | Moderate | Low | High | Moderate | Low | ||
| Piètrain-specific simple AR | 1/1 | 0/1 | 0/1 or 0/0 | 0/0 | 0/0 | 1 | 33 | 37 | 0 | 2 | 5 |
| Piètrain-specific compound heterozygous AR | 0/1 | 0/1 or 0/0 | 0/1 or 0/0 | 0/0 | 0/0 | 0 | 4 | 9 | 0 | 1 | 1 |
| Piètrain-specific XR | 1/- | 0/1/0/- | 0/- | 0/0 | 0/0 | 0 | 2 | 5 | 0 | 2 | 5 |
| Piètrain-specific AD (de novo) | 0/1 | 0/0 | 0/0 | 0/0 | 0/0 | 0 | 6 | 7 | 0 | 3 | 3 |
| Case 3-specific AR/AD | 0/0 | 0/0 | 0/0 | 0/1 or 1/1 | 0/0 | 50 | 872 | 1663 | 12 | 189 | 299 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letko, A.; Schauer, A.M.; Derks, M.F.L.; Grau-Roma, L.; Drögemüller, C.; Grahofer, A. Phenotypic and Genomic Analysis of Cystic Hygroma in Pigs. Genes 2021, 12, 207. https://doi.org/10.3390/genes12020207
Letko A, Schauer AM, Derks MFL, Grau-Roma L, Drögemüller C, Grahofer A. Phenotypic and Genomic Analysis of Cystic Hygroma in Pigs. Genes. 2021; 12(2):207. https://doi.org/10.3390/genes12020207
Chicago/Turabian StyleLetko, Anna, Alexandria Marie Schauer, Martijn F. L. Derks, Llorenç Grau-Roma, Cord Drögemüller, and Alexander Grahofer. 2021. "Phenotypic and Genomic Analysis of Cystic Hygroma in Pigs" Genes 12, no. 2: 207. https://doi.org/10.3390/genes12020207
APA StyleLetko, A., Schauer, A. M., Derks, M. F. L., Grau-Roma, L., Drögemüller, C., & Grahofer, A. (2021). Phenotypic and Genomic Analysis of Cystic Hygroma in Pigs. Genes, 12(2), 207. https://doi.org/10.3390/genes12020207







