Expression Signatures of microRNAs and Their Targeted Pathways in the Adipose Tissue of Chickens during the Transition from Embryonic to Post-Hatch Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Birds
2.3. Next Generation Sequencing Library Construction
2.4. Next Generation Sequencing Analysis
2.5. Real-Time Quantitive PCR (RT-qPCR)
3. Results
3.1. Small RNA-Seq and RNA-Seq Library Characteristics
3.2. Expression Dynamics of miRNAs in Adipose Tissue during the Peri-Hatching Period in Chickens
3.3. Modulation of the Adipocytic Transcriptome during the Metabolic Switch in Chickens
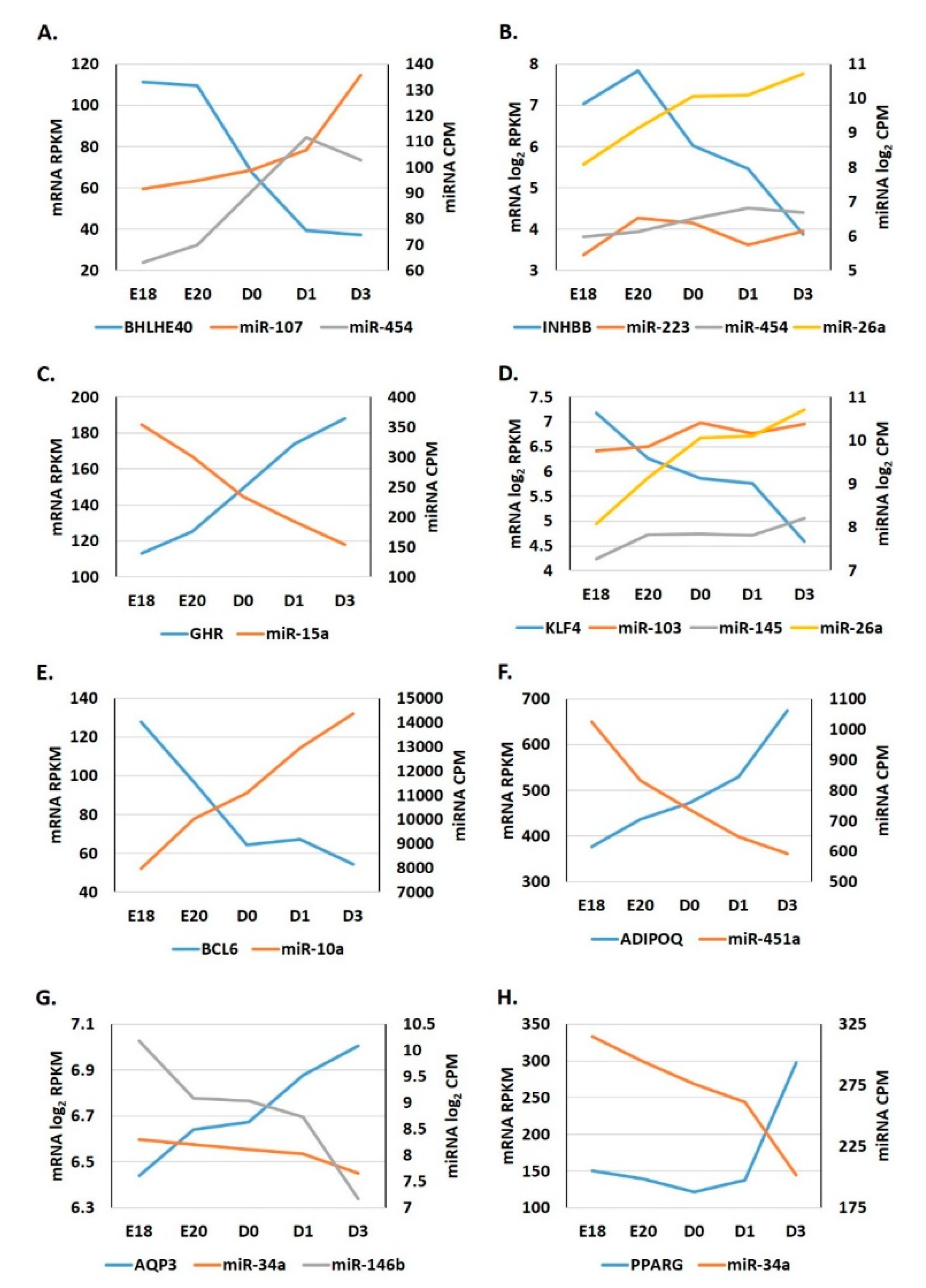
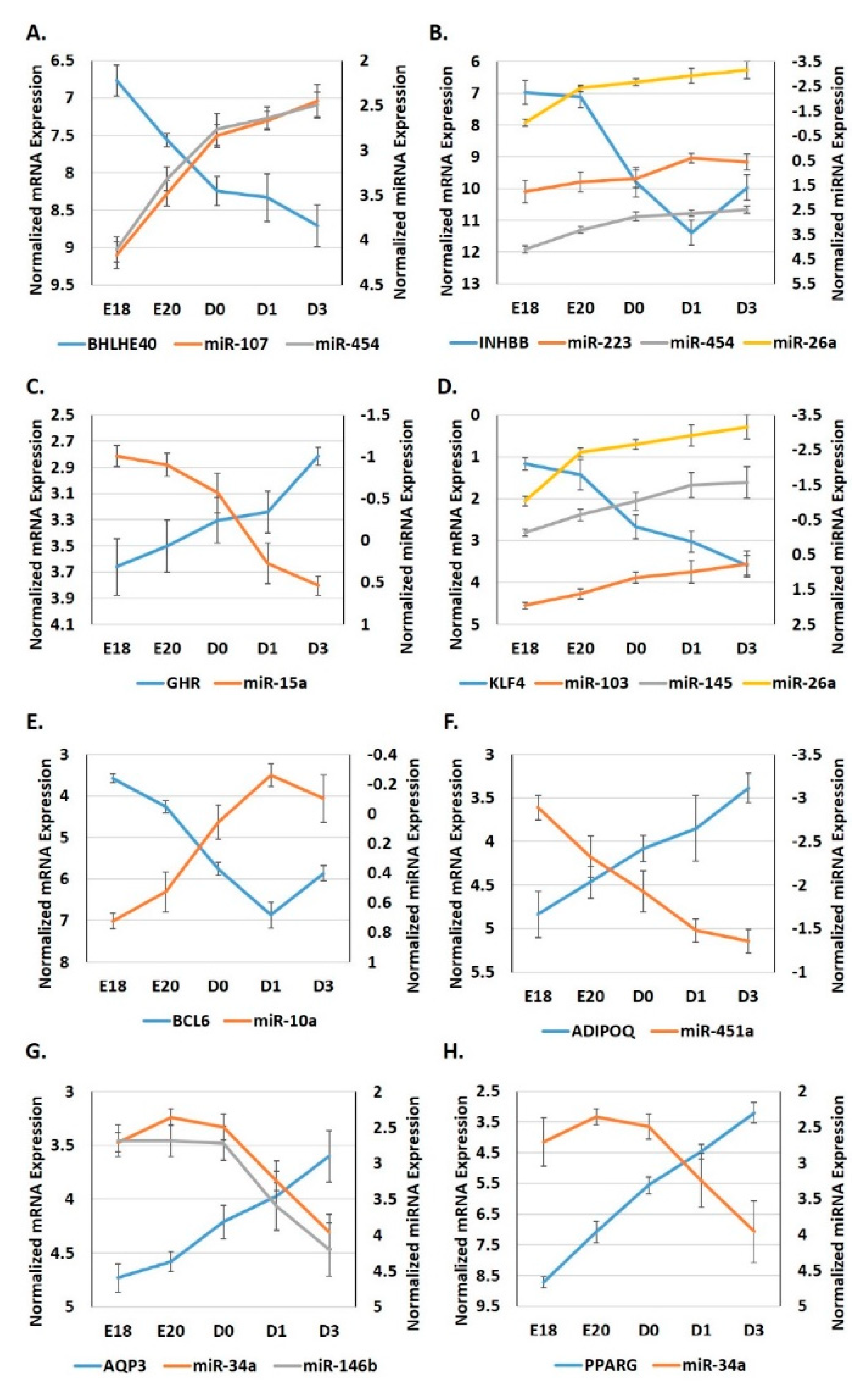
3.4. Expression Patterns of miRNA: Target Gene Pairings in Adipose Tissue during the Metabolic Switch
3.5. Expression Patterns of miRNA: Target Pairings in the Adipose Tissue of Ross 708 Broilers
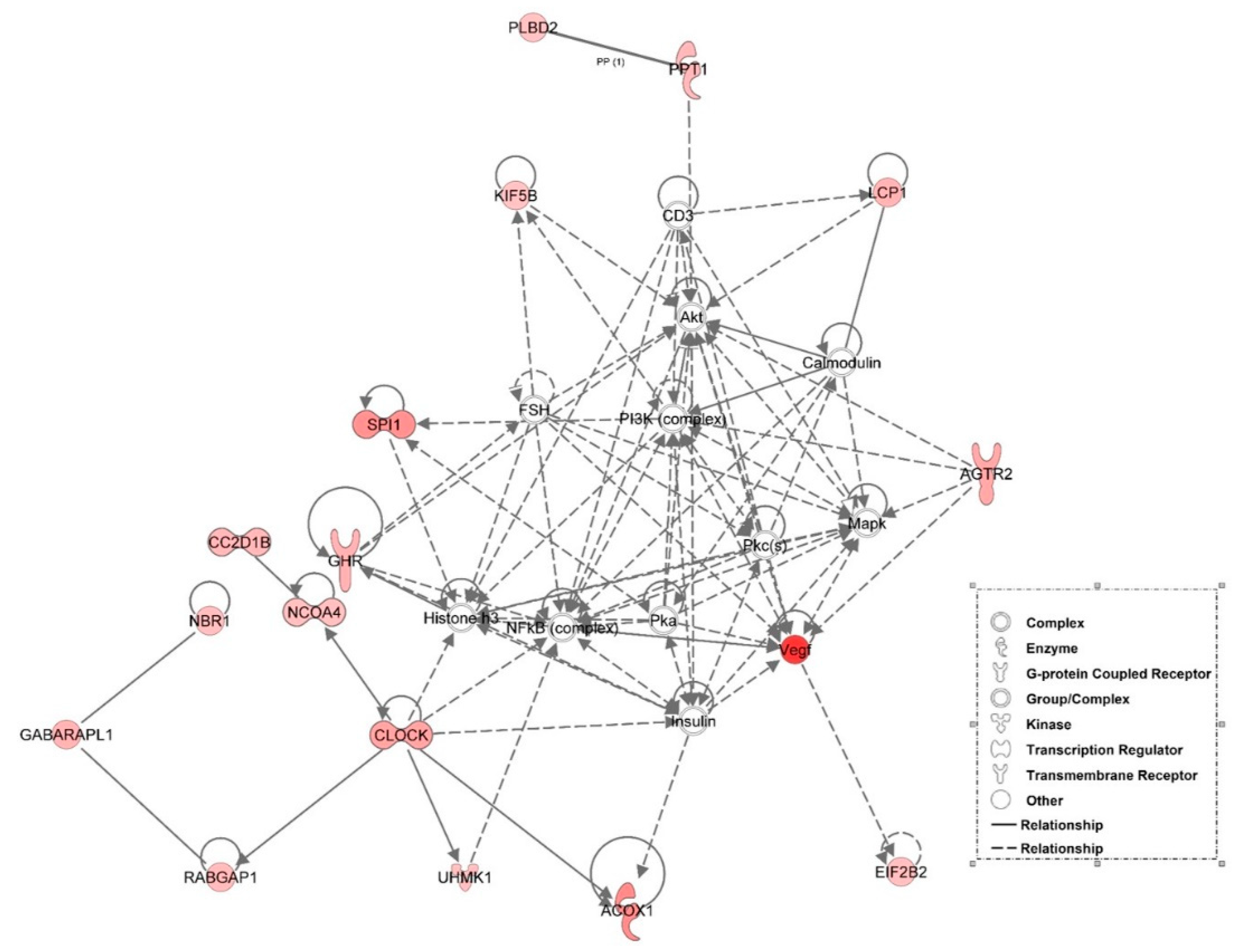
3.6. Examination of miRNA-Regulated Networks in Adipose Tissue during the Metabolic Switch
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angel, R. Metabolic Disorders: Limitations to Growth of and Mineral Deposition into the Broiler Skeleton after Hatch and Potential Implications for Leg Problems. J. Appl. Poult. Res. 2007, 16, 138–149. [Google Scholar] [CrossRef]
- Chen, P.; Suh, Y.; Choi, Y.M.; Shin, S.; Lee, K. Developmental regulation of adipose tissue growth through hyperplasia and hypertrophy in the embryonic Leghorn and broiler. Poult. Sci. 2014, 93, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Tůmová, E.; Teimouri, A.E. Fat deposition in the broiler chicken: A review. Sci. Agri. Bohem. 2010, 41, 121–128. [Google Scholar]
- Wang, G.; Kim, W.K.; Cline, M.A.; Gilbert, E.R. Factors affecting adipose tissue development in chickens: A review. Poult. Sci. 2017, 96, 3687–3699. [Google Scholar] [PubMed]
- Daković, N.; Térézol, M.; Pitel, F.; Maillard, V.; Elis, S.; Leroux, S.; Lagarrigue, S.; Gondret, F.; Klopp, C.; Baeza, E.; et al. The loss of adipokine genes in the chicken genome and implications for insulin metabolism. Mol. Biol Evol. 2014, 31, 2637–2646. [Google Scholar] [CrossRef]
- Buyse, J.; Decuypere, E. Adipose Tissue and Lipid Metabolism. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Academic Press: London, UK, 2015; pp. 443–453. [Google Scholar]
- Sims, W.; Yi, J.; Cline, M.A.; Gilbert, E.R. Central injection of a synthetic chicken partial leptin peptide does not affect food intake in chicks. Neurosci. Lett. 2017, 656, 165–168. [Google Scholar] [CrossRef]
- Dallinga-Thie, G.M.; Franssen, R.; Mooij, H.L.; Visser, M.E.; Hassing, H.C.; Peelman, F.; Kastelein, J.J.; Péterfy, M.; Nieuwdorp, M. The metabolism of triglyceride-rich lipoproteins revisited: New players, new insight. Atherosclerosis. 2010, 211, 1–8. [Google Scholar] [CrossRef]
- Ji, B.; Middleton, J.L.; Ernest, B.; Saxton, A.M.; Lamont, S.J.; Campagna, S.R.; Voy, B.H. Molecular and metabolic profiles suggest that increased lipid catabolism in adipose tissue contributes to leanness in domestic chickens. Physiol. Genom. 2014, 46, 315–327. [Google Scholar] [CrossRef]
- Tomin, T.; Fritz, K.; Gindlhuber, J.; Waldherr, L.; Pucher, B.; Thallinger, G.G.; Nomura, D.K.; Schittmayer, M.; Birner-Gruenberger, R. Deletion of Adipose Triglyceride Lipase Links Triacylglycerol Accumulation to a More-Aggressive Phenotype in A549 Lung Carcinoma Cells. J. Proteome Res. 2018, 17, 1415–1425. [Google Scholar]
- Schoiswohl, G.; Stefanovic-Racic, M.; Menke, M.N.; Wills, R.C.; Surlow, B.A.; Basantani, M.K.; Sitnick, M.T.; Cai, L.; Yazbeck, C.F.; Stolz, D.B.; et al. Impact of Reduced ATGL-Mediated Adipocyte Lipolysis on Obesity-Associated Insulin Resistance and Inflammation in Male Mice. Endocrinology. 2015, 156, 3610–3624. [Google Scholar] [CrossRef]
- List, E.O.; Berryman, D.E.; Funk, K.; Gosney, E.S.; Jara, A.; Kelder, B.; Wang, X.; Kutz, L.; Troike, K.; Lozier, N.; et al. The role of GH in adipose tissue: Lessons from adipose-specific GH receptor gene-disrupted mice. Mol. Endocrinol. 2013, 27, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Asada, N.; Takahashi, Y.; Wada, M.; Naito, N.; Uchida, H.; Ikeda, M.; Honjo, M. GH induced lipolysis stimulation in 3T3-L1 adipocytes stably expressing hGHR: Analysis on signaling pathway and activity of 20K hGH. Mol. Cell Endocrinol. 2000, 162, 121–129. [Google Scholar] [CrossRef]
- Fain, J.N.; Ihle, J.H.; Bahouth, S.W. Stimulation of lipolysis but not of leptin release by growth hormone is abolished in adipose tissue from Stat5a and b knockout mice. Biochem Biophys Res. Commun. 1999, 263, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Jiang, X.; Liu, Y.P.; Du, H.R.; Zhu, Q. Identification of Avian polymorphisms in the third intron of GH gene and their associations with abdominal fat in chickens. Poult. Sci. 2007, 86, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, G.; Babini, L.; Della Guardia, L. Potential role of microRNAs in the regulation of adipocytes liposecretion and adipose tissue physiology. J. Cell Physiol. 2018, 233, 9077–9086. [Google Scholar]
- Price, N.L.; Fernández-Hernando, C. miRNA regulation of white and brown adipose tissue differentiation and function. Biochim Biophys Acta. 2016, 1861, 2104–2110. [Google Scholar] [CrossRef]
- Martin, E.C.; Qureshi, A.T.; Dasa, V.; Freitas, M.A.; Gimble, J.M.; Davis, T.A. MicroRNA regulation of stem cell differentiation and diseases of the bone and adipose tissue: Perspectives on miRNA biogenesis and cellular transcriptome. Biochimie 2016, 124, 98–111. [Google Scholar] [CrossRef]
- Pek, S.L.; Sum, C.F.; Lin, M.X.; Cheng, A.K.; Wong, M.T.; Lim, S.C.; Tavintharan, S. Circulating and visceral adipose miR-100 is down-regulated in patients with obesity and Type 2 diabetes. Mol. Cell Endocrinol. 2016, 427, 112–123. [Google Scholar] [CrossRef]
- Landrier, J.F.; Derghal, A.; Mounien, L. MicroRNAs in Obesity and Related Metabolic Disorders. Cells 2019, 8, E859. [Google Scholar]
- Ahn, J.; Lee, H.; Jung, C.H.; Jeon, T.I.; Ha, T.Y. MicroRNA-146b promotes adipogenesis by suppressing the SIRT1-FOXO1 cascade. EMBO Mol. Med. 2013, 5, 1602–1612. [Google Scholar] [CrossRef]
- Irani, S.; Pan, X.; Peck, B.C.; Iqbal, J.; Sethupathy, P.; Hussain, M.M. MicroRNA-30c Mimic Mitigates Hypercholesterolemia and Atherosclerosis in Mice. J. Biol. Chem. 2016, 291, 18397–18409. [Google Scholar] [CrossRef] [PubMed]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar]
- Kuryłowicz, A.; Wicik, Z.; Owczarz, M.; Jonas, M.I.; Kotlarek, M.; Świerniak, M.; Lisik, W.; Jonas, M.; Noszczyk, B.; Puzianowska-Kuźnicka, M. NGS Reveals Molecular Pathways Affected by Obesity and Weight Loss-Related Changes in miRNA Levels in Adipose Tissue. Int. J. Mol. Sci. 2017, 19, E66. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.A.; Porter, T.E.; Liu, H.C. Identification of microRNAs controlling hepatic mRNA levels for metabolic genes during the metabolic transition from embryonic to posthatch development in the chicken. BMC Genom. 2017, 18, 687. [Google Scholar]
- Hicks, J.A.; Porter, T.E.; Sunny, N.E.; Liu, H.C. Delayed Feeding Alters Transcriptional and Post-Transcriptional Regulation of Hepatic Metabolic Pathways in Peri-Hatch Broiler Chicks. Genes 2019, 10, E272. [Google Scholar] [CrossRef] [PubMed]
- Kane, H.; Lynch, L. Innate Immune Control of Adipose Tissue Homeostasis. Trends Immunol. 2019, 40, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S.; Thumkeo, D. Rho signaling research: History, current status and future directions. FEBS Lett. 2018, 592, 1763–1776. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Wakino, S.; Tanabe, Y.; Saito, M.; Tokuyama, H.; Washida, N.; Tatematsu, S.; Yoshioka, K.; Homma, K.; Hasegawa, K.; et al. Rho and Rho-kinase activity in adipocytes contributes to a vicious cycle in obesity that may involve mechanical stretch. Sci. Signal. 2011, 4, ra3. [Google Scholar] [CrossRef]
- Amri, E.Z.; Scheideler, M. Small non coding RNAs in adipocyte biology and obesity. Mol. Cell Endocrinol. 2017, 456, 87–94. [Google Scholar] [CrossRef]
- Magnusson, B.; Svensson, P.A.; Carlsson, L.M.; Sjöholm, K. Activin B inhibits lipolysis in 3T3-L1 adipocytes. Biochem Biophys Res. Commun. 2010, 395, 373–376. [Google Scholar] [CrossRef]
- Sahu, S.K.; Kumar, M.; Chakraborty, S.; Banerjee, S.K.; Kumar, R.; Gupta, P.; Jana, K.; Gupta, U.D.; Ghosh, Z.; Kundu, M.; et al. MicroRNA 26a (miR-26a)/KLF4 and CREB-C/EBPβ regulate innate immune signaling, the polarization of macrophages and the trafficking of Mycobacterium tuberculosis to lysosomes during infection. PLoS Pathog. 2017, 13, e1006410. [Google Scholar] [CrossRef] [PubMed]
- Birsoy, K.; Chen, Z.; Friedman, J. Transcriptional regulation of adipogenesis by KLF4. Cell Metab. 2008, 7, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Torrens, J.I.; Anand, A.; Spiegelman, B.M.; Friedman, J.M. Krox20 stimulates adipogenesis via C/EBPbeta-dependent and -independent mechanisms. Cell Metab. 2005, 1, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Matson, R.I.; Perry, R.; Hunt, L.P.; Chong, A.H.; Beynon, R.; Hamilton-Shield, J.; Birch, L. Change in obesity-related metabolic abnormalities associated with body mass index improvement through life-style intervention: A meta-regression. Pediatr. Diabetes 2020, 21, 173–193. [Google Scholar] [CrossRef]
- Ying, W.; Fu, W.; Lee, Y.S.; Olefsky, J.M. The role of macrophages in obesity-associated islet inflammation and β-cell abnormalities. Nat. Rev. Endocrinol. 2020, 16, 81–90. [Google Scholar] [CrossRef]
- Parida, S.; Siddharth, S.; Sharma, D. Adiponectin, Obesity, and Cancer: Clash of the Bigwigs in Health and Disease. Int. J. Mol. Sci. 2019, 20, 2519. [Google Scholar]
- Yan, J.; Yang, H.; Gan, L.; Sun, C. Adiponectin-impaired adipocyte differentiation negatively regulates fat deposition in chicken. J. Anim. Physiol. Anim. Nutr. 2014, 98, 530–537. [Google Scholar] [CrossRef]
- Saha, A.; Ahn, S.; Blando, J.; Su, F.; Kolonin, M.G.; DiGiovanni, J. Proinflammatory CXCL12-CXCR4/CXCR7 Signaling Axis Drives Myc-Induced Prostate Cancer in Obese Mice. Cancer Res. 2017, 77, 5158–5168. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.G.; Healey, G.D.; Gabler, C.; Heuwieser, W.; Streyl, D.; Bromfield, J.J.; Miyamoto, A.; Fergani, C.; Dobson, H. Innate immunity and inflammation of the bovine female reproductive tract in health and disease. Reproduction 2014, 148, R41–R51. [Google Scholar] [CrossRef]
- Lekkas, D.; Paschos, G.K. The circadian clock control of adipose tissue physiology and metabolism. Auton. Neurosci. 2019, 219, 66–70. [Google Scholar] [CrossRef]
- Gimble, J.M.; Sutton, G.M.; Ptitsyn, A.A.; Floyd, Z.E.; Bunnell, B.A. Circadian rhythms in adipose tissue: An update. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Kohsaka, A.; Bhawal, U.K.; Muragaki, Y. Potential Roles of Dec and Bmal1 Genes in Interconnecting Circadian Clock and Energy Metabolism. Int. J. Mol. Sci. 2018, 19, 781. [Google Scholar] [CrossRef]
- Barnea, M.; Chapnik, N.; Genzer, Y.; Froy, O. The circadian clock machinery controls adiponectin expression. Mol. Cell Endocrinol. 2015, 399, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Feldman, B.J. A context-specific circadian clock in adipocyte precursor cells modulates adipogenesis. Adipocyte 2018, 7, 273–276. [Google Scholar] [CrossRef]
- Yang, Y.F.; Jin, S.F.; Zhong, Z.T.; Yu, Y.H.; Yang, B.; Yuan, H.B.; Pan, J.M. Growth responses of broiler chickens to different periods of artificial light. J. Anim. Sci. 2015, 93, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, G.; Huo, J.S.; Barney, D.; Wang, Z.; Livshiz, T.; States, D.J.; Qin, Z.S.; Schwartz, J. Computational and functional analysis of growth hormone (GH)-regulated genes identifies the transcriptional repressor B-cell lymphoma 6 (Bc16) as a participant in GH-regulated transcription. Endocrinology 2009, 150, 3645–3654. [Google Scholar] [CrossRef]
- LaPensee, C.R.; Lin, G.; Dent, A.L.; Schwartz, J. Deficiency of the transcriptional repressor B cell lymphoma 6 (Bcl6) is accompanied by dysregulated lipid metabolism. PLoS ONE 2014, 9, e97090. [Google Scholar] [CrossRef]
- Slootweg, M.C.; Ohlsson, C.; van Elk, E.J.; Netelenbos, J.C.; Andress, D.L. Growth hormone receptor activity is stimulated by insulin-like growth factor binding protein 5 in rat osteosarcoma cells. Growth Regul. 1996, 6, 238–346. [Google Scholar]
- Xiang, A.; Chu, G.; Zhu, Y.; Ma, G.; Yang, G.; Sun, S. IGFBP5 suppresses oleate-induced intramyocellular lipids deposition and enhances insulin signaling. J. Cell Physiol. 2019, 234, 15288–15298. [Google Scholar] [CrossRef]
- Vienberg, S.; Geiger, J.; Madsen, S.; Dalgaard, L.T. MicroRNAs in metabolism. Acta Physiol. 2017, 219, 346–361. [Google Scholar] [CrossRef]
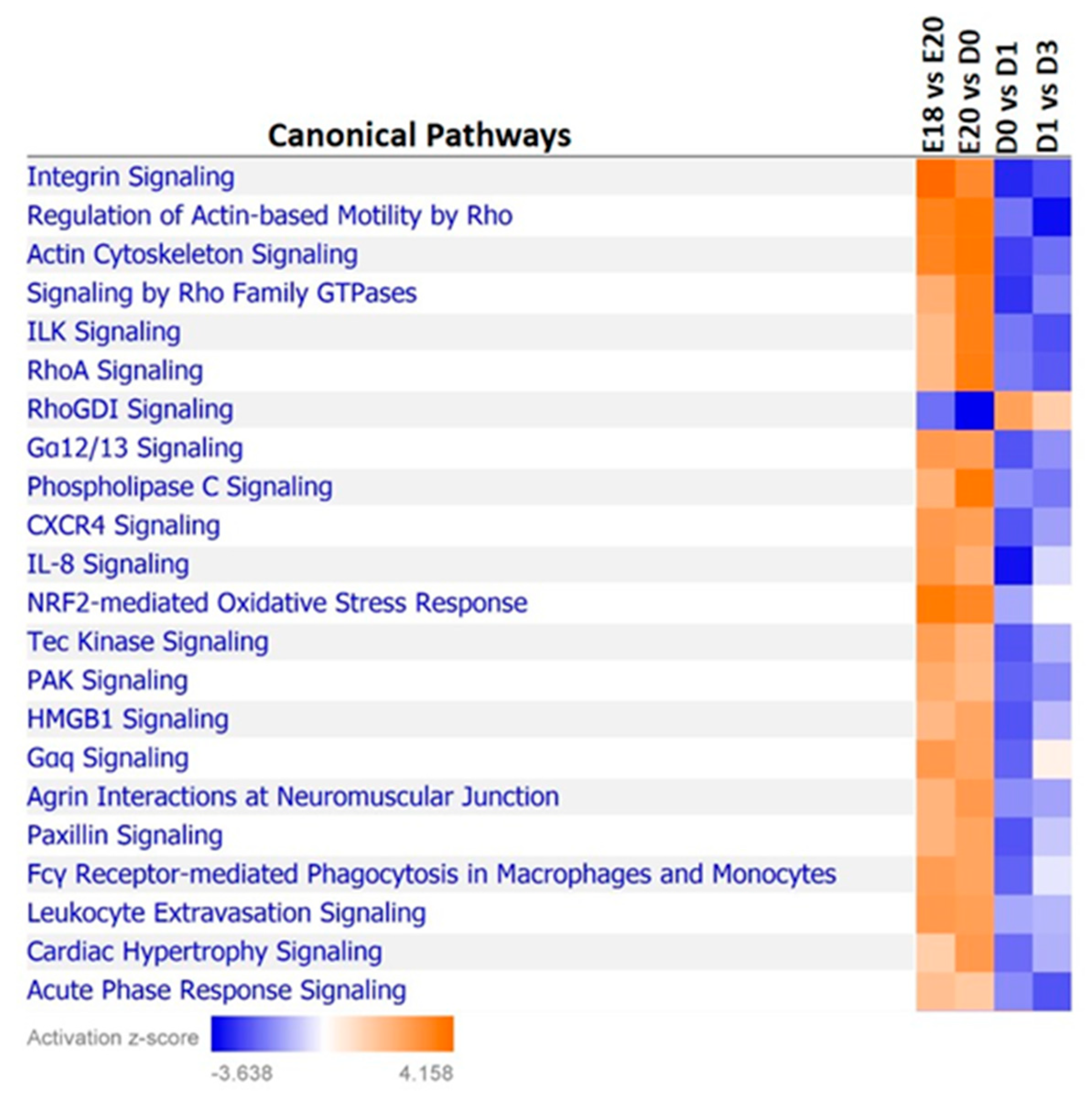

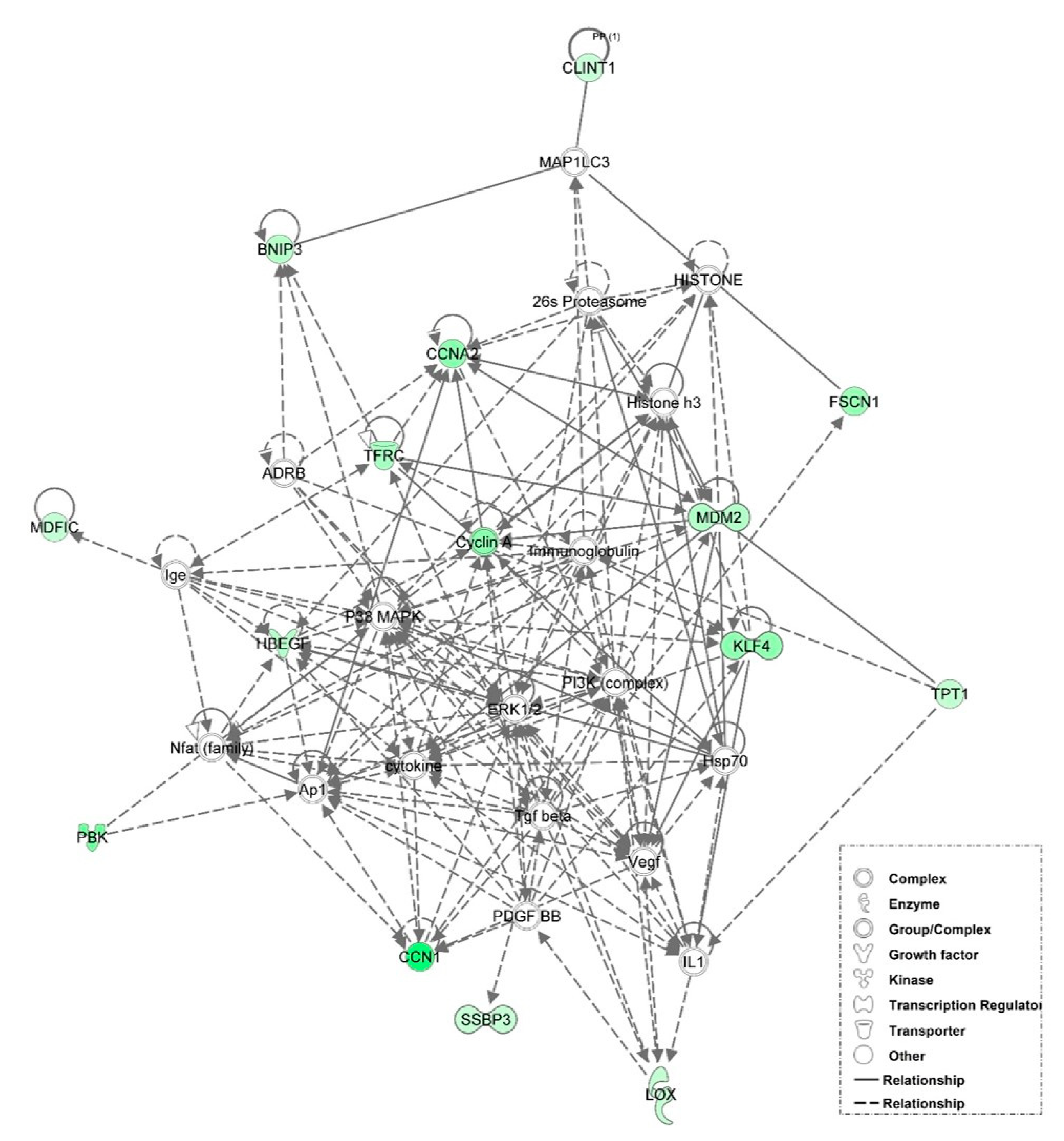
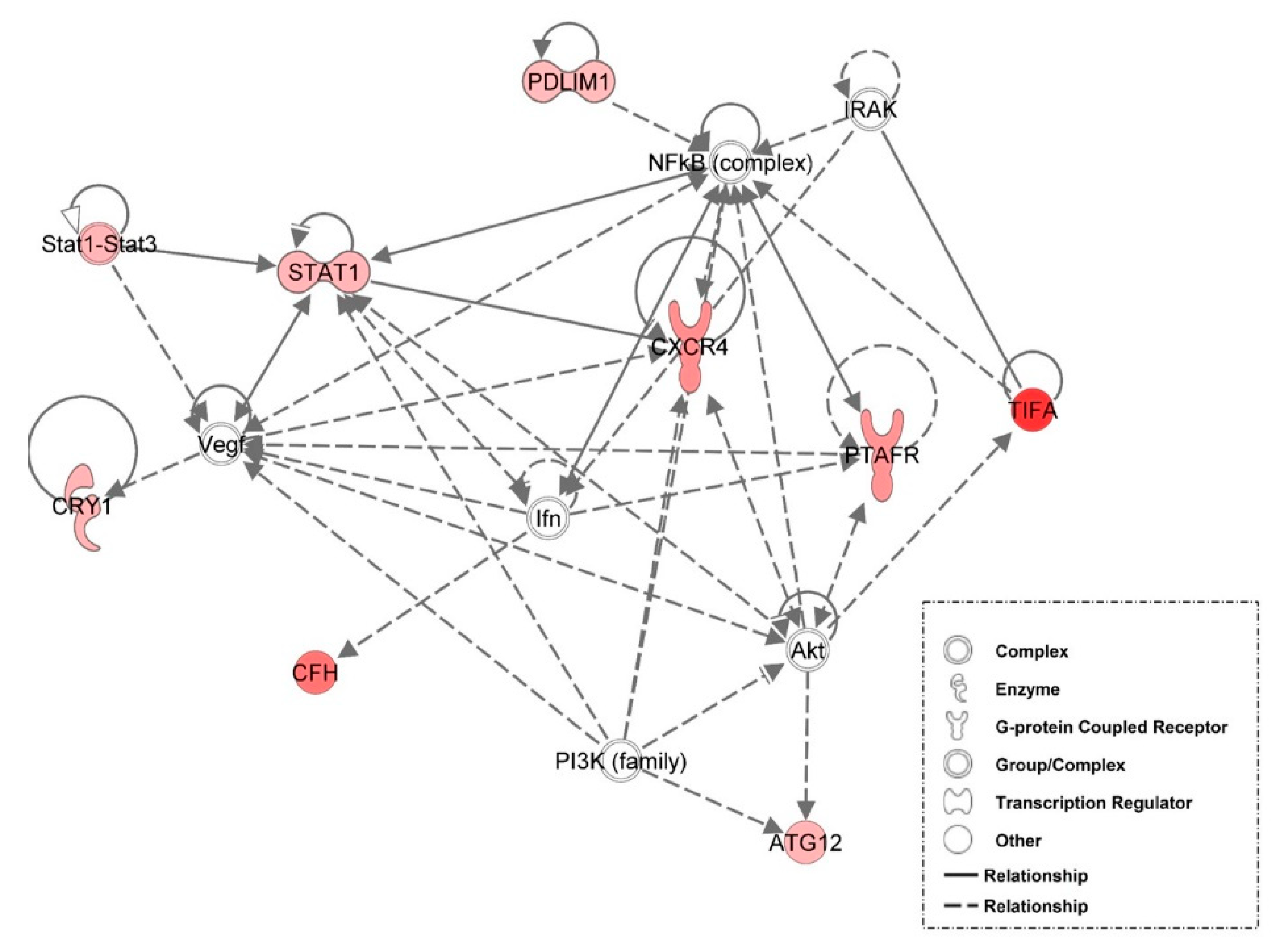

| Time Point Comparison | Top Canonical Pathways | Top Upstream Regulators | Molecular and Cellular Functions | Physiological System Development and Function |
|---|---|---|---|---|
| E18-E20 | EIF2 signaling; NRF2-mediated oxidative stress response; VEGF signaling; PDGF signaling; IGF-1 signaling | TP53; TGFB1; dexamethasone; β-estradiol; sirolimus | cell death and survival; cellular assembly and organization; cellular function and maintenance; cellular movement; cellular compromise | organismal survival; skeletal and muscular system development and function; organismal development; tissue development |
| E20-D0 | NRF2-mediated oxidative stress response; EIF2 signaling; ILK signaling; tight junction signaling | KRAS; TGFB1; β-estradiol; dexamethasone; MYC | cell death and survival; cellular compromise; lipid metabolism; small molecular biochemistry; molecular transport | organismal survival; organismal development; skeletal and muscular system development and function; tissue morphology |
| D0-D1 | NRF2-mediated oxidative stress response; PI3K/AKT signaling; IGF-1 signaling; 14-3-3-mediated signaling; cell cycle: G2/M DNA damage checkpoint regulation | TGFB1; β-estradiol; TP53; ERBB2; dexamethasone | cell death and survival; cellular movement; cellular growth and proliferation; cellular development; protein synthesis | organismal survival; organismal development; tissue development |
| D1-D3 | calcium signaling; RhoA signaling; signaling by Rho family GTPases; epithelial adherens junction signaling; regulation of actin-based motility by Rho | dexamethasone; TP53; β-estradiol; TNF; MEF2C | cell death and survival; cellular assembly and organization; cellular movement; lipid metabolism; molecular transport | organismal development; organismal survival; skeletal and muscular system development and function; tissue development |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hicks, J.A.; Liu, H.-C. Expression Signatures of microRNAs and Their Targeted Pathways in the Adipose Tissue of Chickens during the Transition from Embryonic to Post-Hatch Development. Genes 2021, 12, 196. https://doi.org/10.3390/genes12020196
Hicks JA, Liu H-C. Expression Signatures of microRNAs and Their Targeted Pathways in the Adipose Tissue of Chickens during the Transition from Embryonic to Post-Hatch Development. Genes. 2021; 12(2):196. https://doi.org/10.3390/genes12020196
Chicago/Turabian StyleHicks, Julie A., and Hsiao-Ching Liu. 2021. "Expression Signatures of microRNAs and Their Targeted Pathways in the Adipose Tissue of Chickens during the Transition from Embryonic to Post-Hatch Development" Genes 12, no. 2: 196. https://doi.org/10.3390/genes12020196
APA StyleHicks, J. A., & Liu, H.-C. (2021). Expression Signatures of microRNAs and Their Targeted Pathways in the Adipose Tissue of Chickens during the Transition from Embryonic to Post-Hatch Development. Genes, 12(2), 196. https://doi.org/10.3390/genes12020196





