Strong Association of the rs4986790 Single Nucleotide Polymorphism (SNP) of the Toll-Like Receptor 4 (TLR4) Gene with Human Immunodeficiency Virus (HIV) Infection: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Association Analysis
2.3. Meta-Analysis
3. Results
3.1. Strong Association between the rs4986790 SNP (D299G) of the TLR4 Gene and Susceptibility to HIV Infection in Three Caucasian Populations
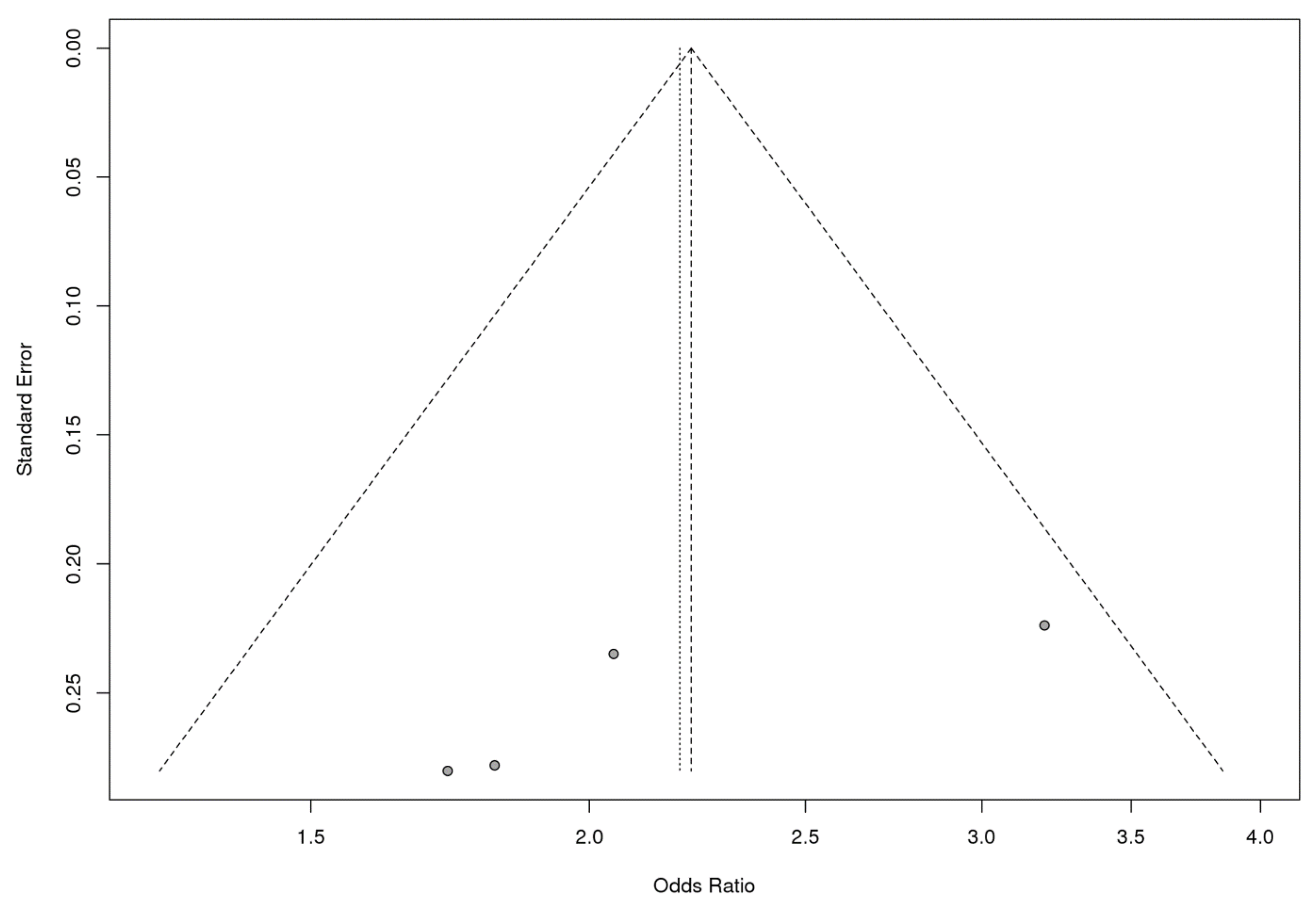
3.2. Strong Association between the rs4986790 SNP of the TLR4 Gene and Susceptibility to HIV Infection Based on a Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HIV | Human immunodeficiency virus |
| SNP | Single nucleotide polymorphism |
| TLR4 | Toll-like receptor 4 |
| AIDS | Acquired immune deficiency syndrome |
| CCR | CC-chemokine receptor 5 |
| CXCR4 | CXC-chemokine receptor 4 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| TNF-α | Tumor necrosis factor-α |
References
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV infection. Nat. Rev. Dis. Primers 2015, 1, 15035. [Google Scholar] [CrossRef] [PubMed]
- Barre-Sinoussi, F.; Ross, A.L.; Delfraissy, J.F. Past, present and future: 30 years of HIV research. Nat. Rev. Microbiol. 2013, 11, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Chun, T.W.; Fauci, A.S. Pathogenic mechanisms of HIV disease. Annu Rev. Pathol 2011, 6, 223–248. [Google Scholar] [CrossRef]
- Ghosn, J.; Taiwo, B.; Seedat, S.; Autran, B.; Katlama, C. Hiv. Lancet 2018, 392, 685–697. [Google Scholar] [CrossRef]
- Beutler, B.A. TLRs and innate immunity. Blood 2009, 113, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Vidya, M.K.; Kumar, V.G.; Sejian, V.; Bagath, M.; Krishnan, G.; Bhatta, R. Toll-like receptors: Significance, ligands, signaling pathways, and functions in mammals. Int. Rev. Immunol. 2018, 37, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate immune pattern recognition: A cell biological perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.C.; Stevenson, M.; Latz, E.; Urcuqui-Inchima, S. HIV type 1 infection up-regulates TLR2 and TLR4 expression and function in vivo and in vitro. AIDS Res. Hum. Retrovir. 2012, 28, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Ben Haij, N.; Leghmari, K.; Planes, R.; Thieblemont, N.; Bahraoui, E. HIV-1 Tat protein binds to TLR4-MD2 and signals to induce TNF-α and IL-10. Retrovirology 2013, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Cromarty, R.; Sigal, A.; Liebenberg, L.J.P.; McKinnon, L.R.; Abdool Karim, S.S.; Passmore, J.S.; Archary, D. Diminished HIV Infection of Target CD4+ T Cells in a Toll-Like Receptor 4 Stimulated in vitro Model. Front. Immunol. 2019, 10, 1705. [Google Scholar] [CrossRef] [PubMed]
- Vidyant, S.; Chatterjee, A.; Dhole, T.N. A single-nucleotide polymorphism in TLR4 is linked with the risk of HIV-1 infection. Br. J. Biomed. Sci. 2019, 76, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Pine, S.O.; McElrath, M.J.; Bochud, P.Y. Polymorphisms in toll-like receptor 4 and toll-like receptor 9 influence viral load in a seroincident cohort of HIV-1-infected individuals. AIDS 2009, 23, 2387–2395. [Google Scholar] [CrossRef]
- Pulido, I.; Leal, M.; Genebat, M.; Pacheco, Y.M.; Saez, M.E.; Soriano-Sarabia, N. The TLR4 ASP299GLY polymorphism is a risk factor for active tuberculosis in Caucasian HIV-infected patients. Curr. HIV Res. 2010, 8, 253–258. [Google Scholar] [CrossRef]
- Papadopoulos, A.I.; Ferwerda, B.; Antoniadou, A.; Sakka, V.; Galani, L.; Kavatha, D.; Panagopoulos, P.; Poulakou, G.; Kanellakopoulou, K.; van der Meer, J.W.; et al. Association of toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms with increased infection risk in patients with advanced HIV-1 infection. Clin. Infect. Dis. 2010, 51, 242–247. [Google Scholar] [CrossRef]
- 1000 Genomes Project Consortium; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Mukherjee, S.; Karmakar, S.; Babu, S.P. TLR2 and TLR4 mediated host immune responses in major infectious diseases: A review. Braz. J. Infect. Dis. 2016, 20, 193–204. [Google Scholar] [CrossRef]
- Janardhanan, J.; Joseph Martin, S.; Astrup, E.; Veeramanikandan, R.; Aukrust, P.; Abraham, O.C.; Varghese, G.M. Single-nucleotide polymorphisms in Toll-like receptor (TLR)-2, TLR4 and heat shock protein 70 genes and susceptibility to scrub typhus. J. Hum. Genet. 2013, 58, 707–710. [Google Scholar] [CrossRef]
- Ohto, U.; Yamakawa, N.; Akashi-Takamura, S.; Miyake, K.; Shimizu, T. Structural analyses of human Toll-like receptor 4 polymorphisms D299G and T399I. J. Biol. Chem. 2012, 287, 40611–40617. [Google Scholar] [CrossRef]
- Anwar, M.A.; Choi, S. Structure-Activity Relationship in TLR4 Mutations: Atomistic Molecular Dynamics Simulations and Residue Interaction Network Analysis. Sci. Rep. 2017, 7, 43807. [Google Scholar] [CrossRef]
- Kim, Y.C.; Won, S.Y.; Jeong, B.H. Identification of Prion Disease-Related Somatic Mutations in the Prion Protein Gene (PRNP) in Cancer Patients. Cells 2020, 9, 1480. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Jeong, M.J.; Jeong, B.H. Strong association of regulatory single nucleotide polymorphisms (SNPs) of the IFITM3 gene with influenza H1N1 2009 pandemic virus infection. Cell Mol. Immunol. 2020, 17, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Won, S.Y.; Kim, Y.C.; Jeong, B.H. First Report of the Potential Bovine Spongiform Encephalopathy (BSE)-Related Somatic Mutation E211K of the Prion Protein Gene (PRNP) in Cattle. Int. J. Mol. Sci. 2020, 21, 4246. [Google Scholar] [CrossRef] [PubMed]
- Ben Haij, N.; Planes, R.; Leghmari, K.; Serrero, M.; Delobel, P.; Izopet, J.; BenMohamed, L.; Bahraoui, E. HIV-1 Tat Protein Induces Production of Proinflammatory Cytokines by Human Dendritic Cells and Monocytes/Macrophages through Engagement of TLR4-MD2-CD14 Complex and Activation of NF-kappaB Pathway. PLoS ONE 2015, 10, e0129425. [Google Scholar] [CrossRef]
- Bahraoui, E.; Serrero, M.; Planes, R. HIV-1 Tat—TLR4/MD2 interaction drives the expression of IDO-1 in monocytes derived dendritic cells through NF-kappaB dependent pathway. Sci. Rep. 2020, 10, 8177. [Google Scholar] [CrossRef]
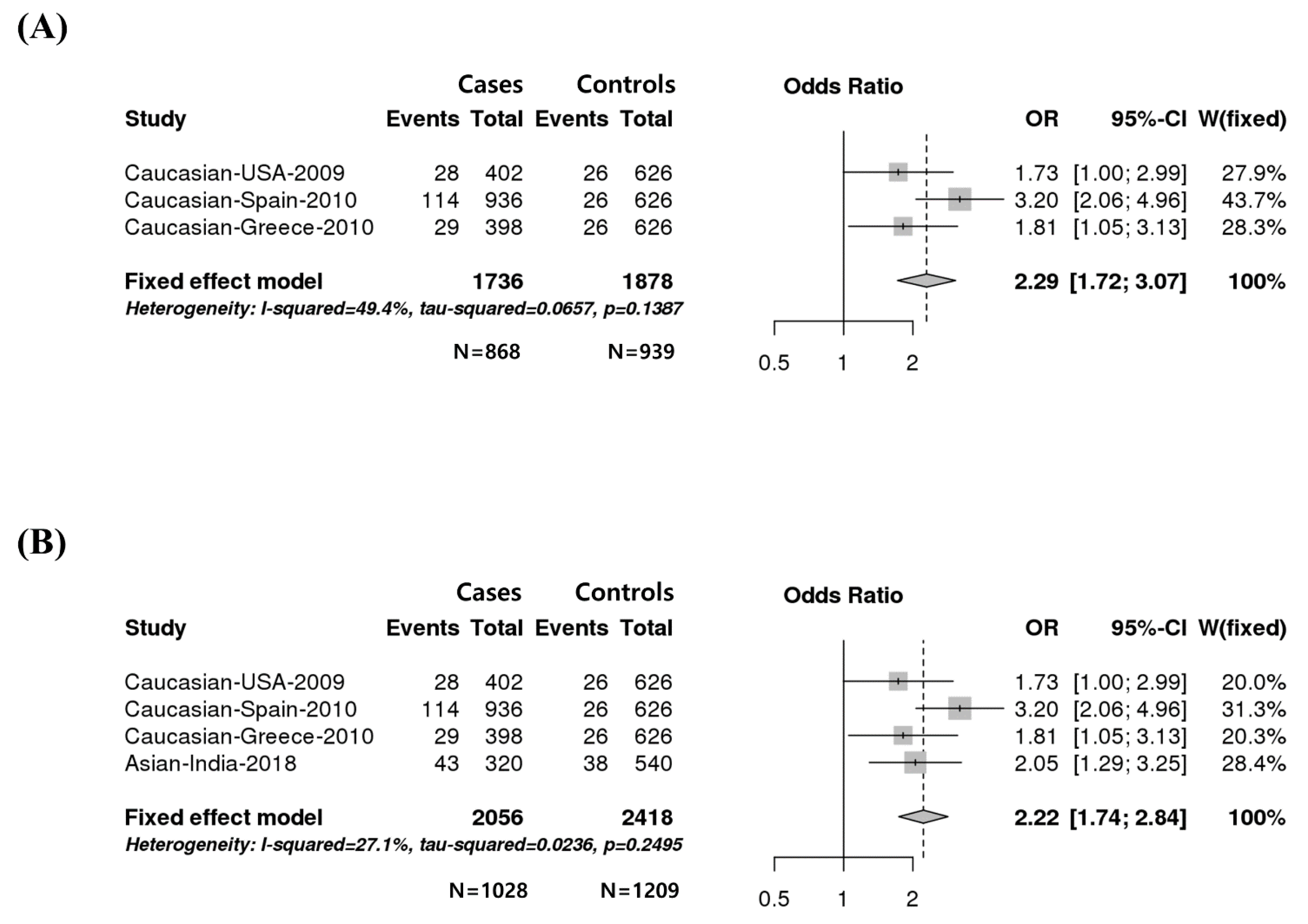
| Population | Country | Year of Publication | Authors | Cases | Controls | p-Value | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Allele Frequencies, n (%) | Total, n | Allele Frequencies, n (%) | Total, n | ||||||||
| A | G | A | G | ||||||||
| Caucasian | USA | 2009 | Pine | 374 (93.03) | 28 (6.97) | 201 | 600 (95.85) | 26 (4.15) | 313 | 0.0486 | 1000 Genomes Project, [14] |
| Caucasian | Spain | 2010 | Pulido | 822 (87.82) | 114 (12.18) | 468 | 600 (95.85) | 26 (4.15) | 313 | 5.2687 × 10−8 | 1000 Genomes Project, [15] |
| Caucasian | Greece | 2010 | Papadopoulos | 369 (92.71) | 29 (7.29) | 199 | 600 (95.85) | 26 (4.15) | 313 | 0.0302 | 1000 Genomes Project, [16] |
| Asian | India | 2018 | Vidyant | 277 (86.56) | 43 (13.44) | 160 | 502 (92.96) | 38 (7.04) | 270 | 0.0019 | [13] |
| Populations | Adjust p-Value | Heterogeneity | Egger’s Test | |
|---|---|---|---|---|
| p-Value | I2 Value | |||
| Caucasian | 1.438 × 10−7 | 0.1387 | 49.4% | 0.0208 |
| Total | 2 × 10−10 | 0.2495 | 27.1% | 0.1598 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-C.; Jeong, B.-H. Strong Association of the rs4986790 Single Nucleotide Polymorphism (SNP) of the Toll-Like Receptor 4 (TLR4) Gene with Human Immunodeficiency Virus (HIV) Infection: A Meta-Analysis. Genes 2021, 12, 36. https://doi.org/10.3390/genes12010036
Kim Y-C, Jeong B-H. Strong Association of the rs4986790 Single Nucleotide Polymorphism (SNP) of the Toll-Like Receptor 4 (TLR4) Gene with Human Immunodeficiency Virus (HIV) Infection: A Meta-Analysis. Genes. 2021; 12(1):36. https://doi.org/10.3390/genes12010036
Chicago/Turabian StyleKim, Yong-Chan, and Byung-Hoon Jeong. 2021. "Strong Association of the rs4986790 Single Nucleotide Polymorphism (SNP) of the Toll-Like Receptor 4 (TLR4) Gene with Human Immunodeficiency Virus (HIV) Infection: A Meta-Analysis" Genes 12, no. 1: 36. https://doi.org/10.3390/genes12010036
APA StyleKim, Y.-C., & Jeong, B.-H. (2021). Strong Association of the rs4986790 Single Nucleotide Polymorphism (SNP) of the Toll-Like Receptor 4 (TLR4) Gene with Human Immunodeficiency Virus (HIV) Infection: A Meta-Analysis. Genes, 12(1), 36. https://doi.org/10.3390/genes12010036






