WNT-FRIZZLED-LRP5/6 Signaling Mediates Posterior Fate and Proliferation during Planarian Regeneration
Abstract
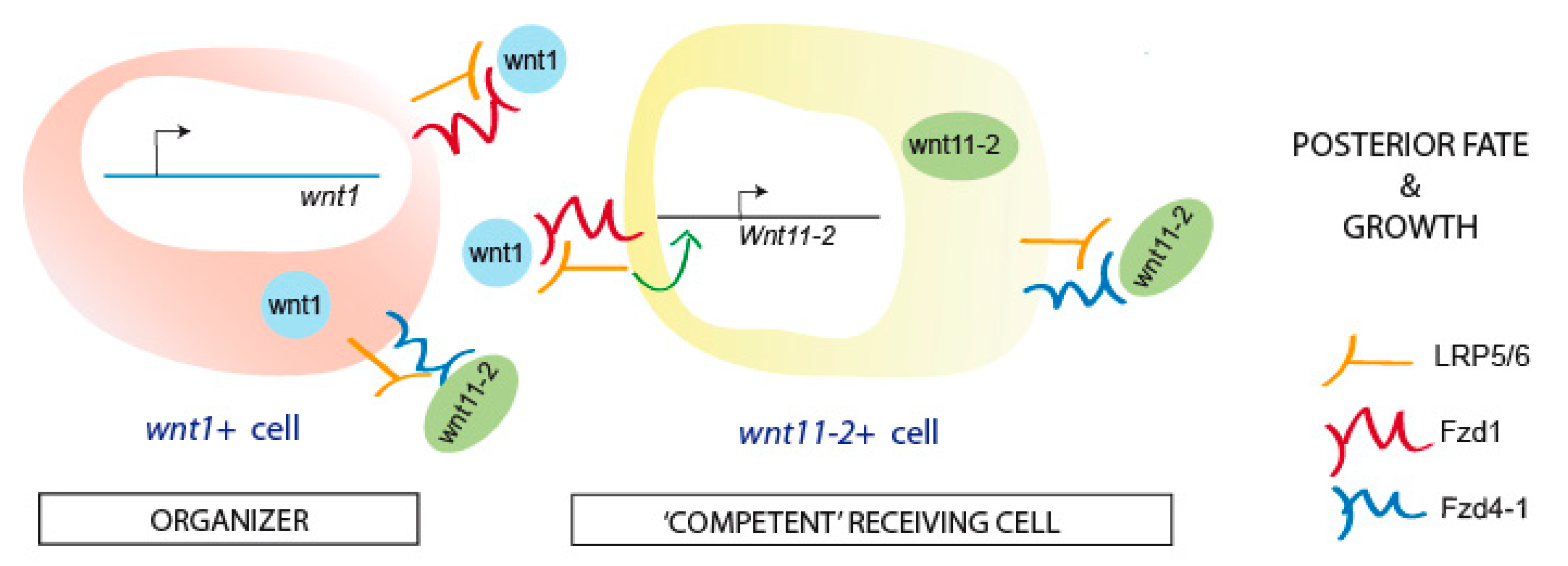
1. Introduction
2. Material and Methods
2.1. Phylogenetic and Sequence Analysis
2.2. Gene Cloning
2.3. Animal Care
2.4. RNAi Experiments
2.5. Whole-Mount In Situ Hybridization
2.6. Immunohistochemistry Staining
2.7. Imagining and Quantification
2.8. Single-Cell Visualization
2.9. Statistical Analysis and Visualization
3. Results
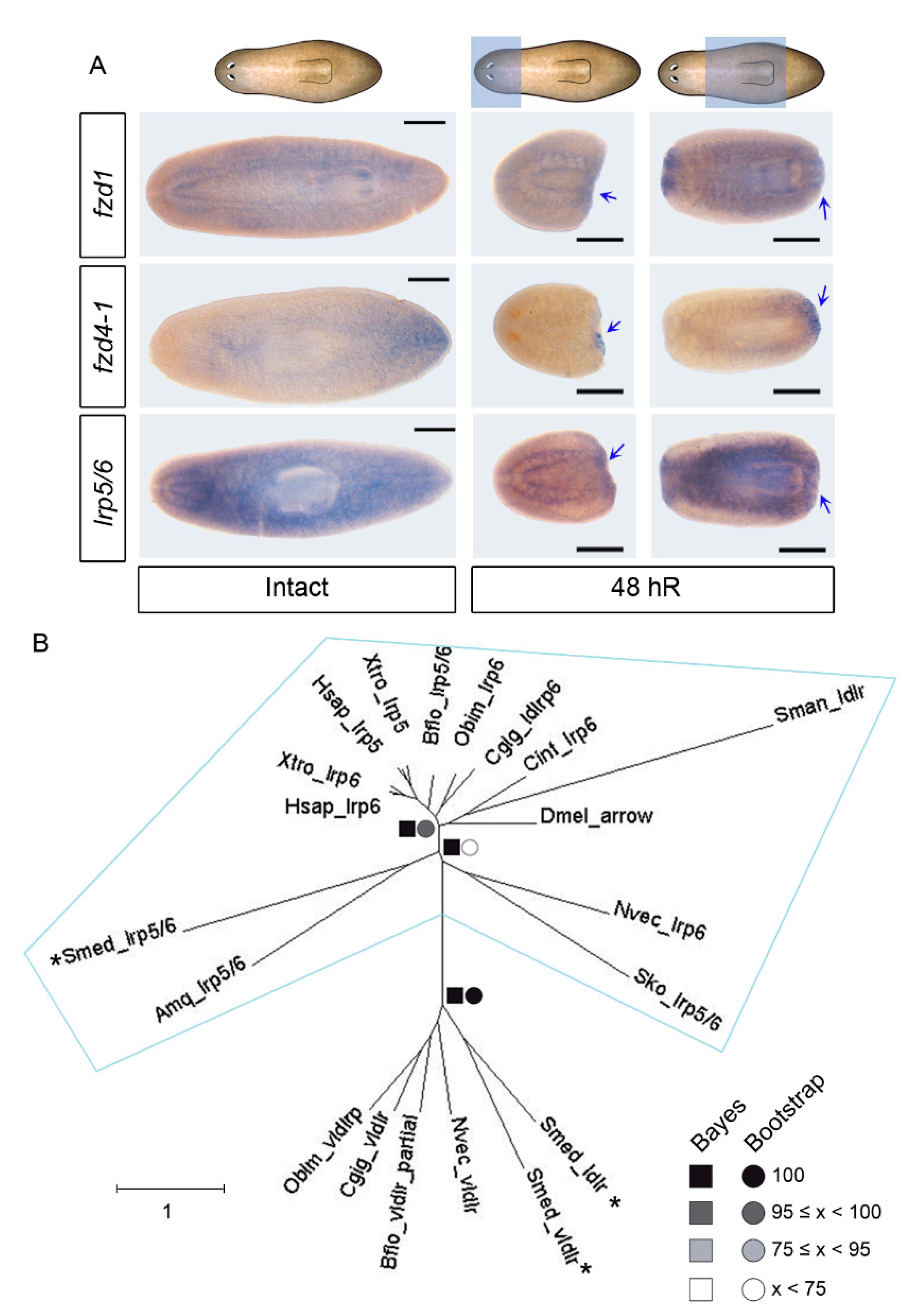
3.1. Identification of an lrp5/6 Homolog That, Together with fzd1 and fzd4-2, Is Expressed in Posterior Blastemas during Regeneration
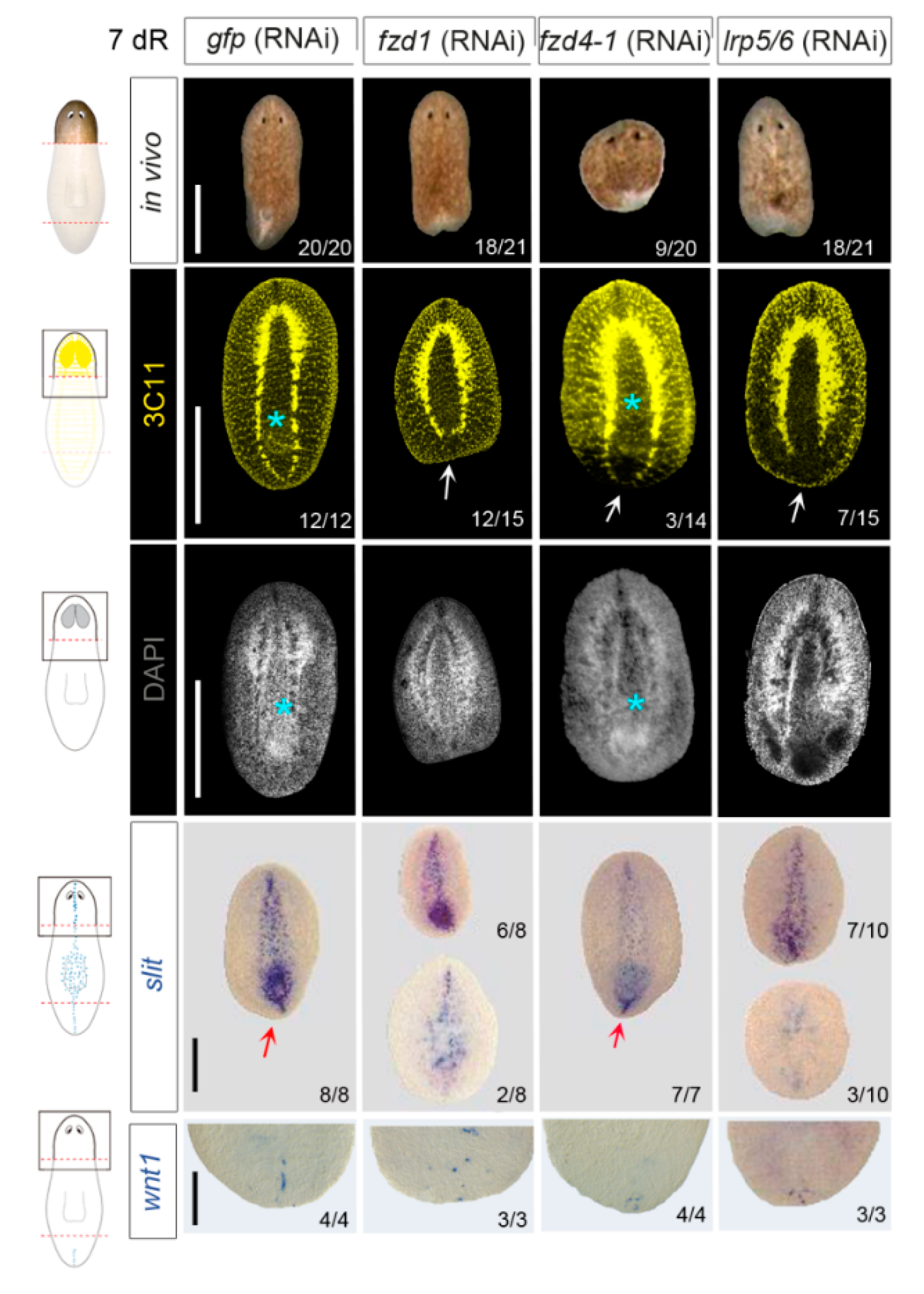
3.2. fzd1, fzd4-1, and Smed-lrp5/6 Specify Posterior Identity
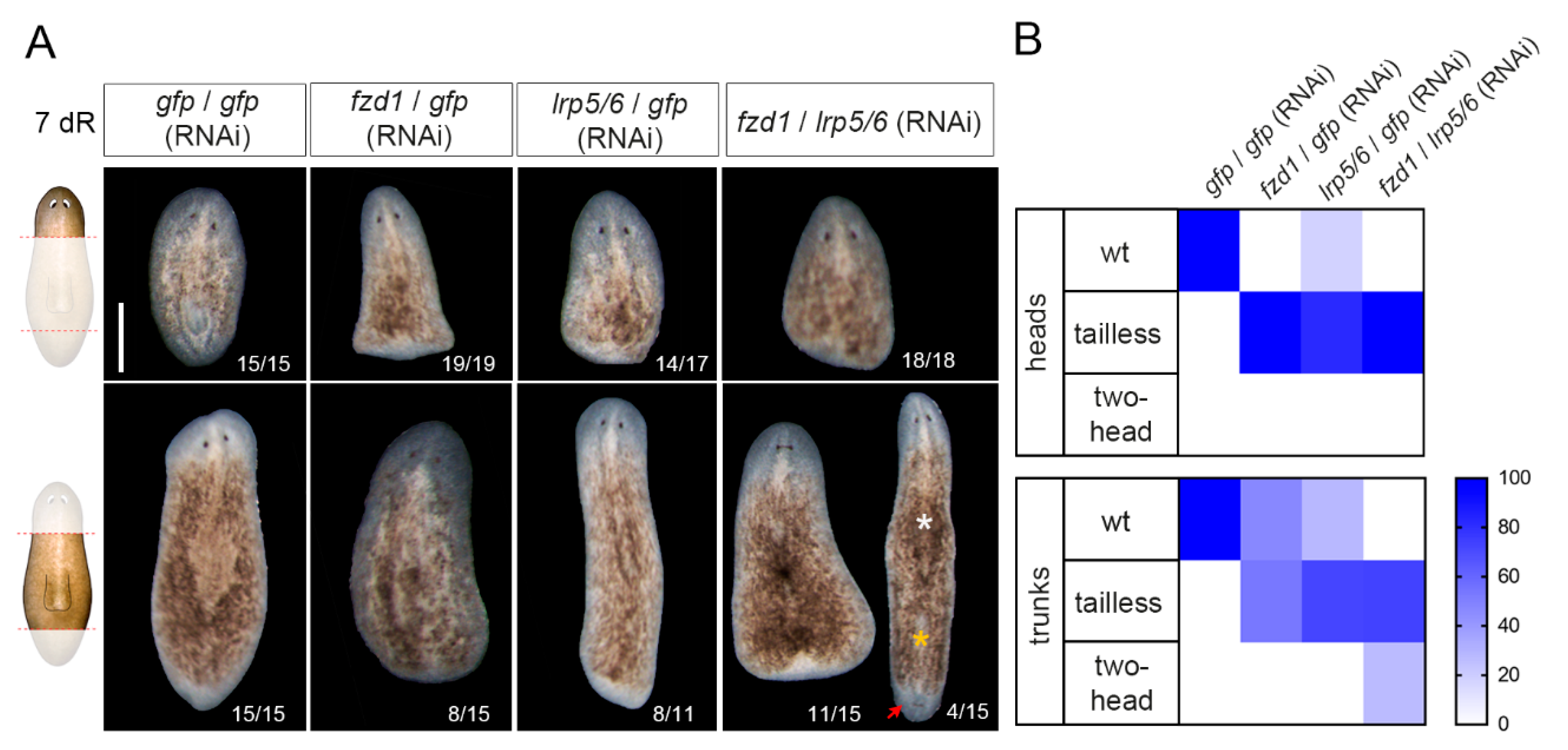
3.3. Smed-fzd1 and Smed-lrp5/6 Cooperate in Specifying Posterior Identity
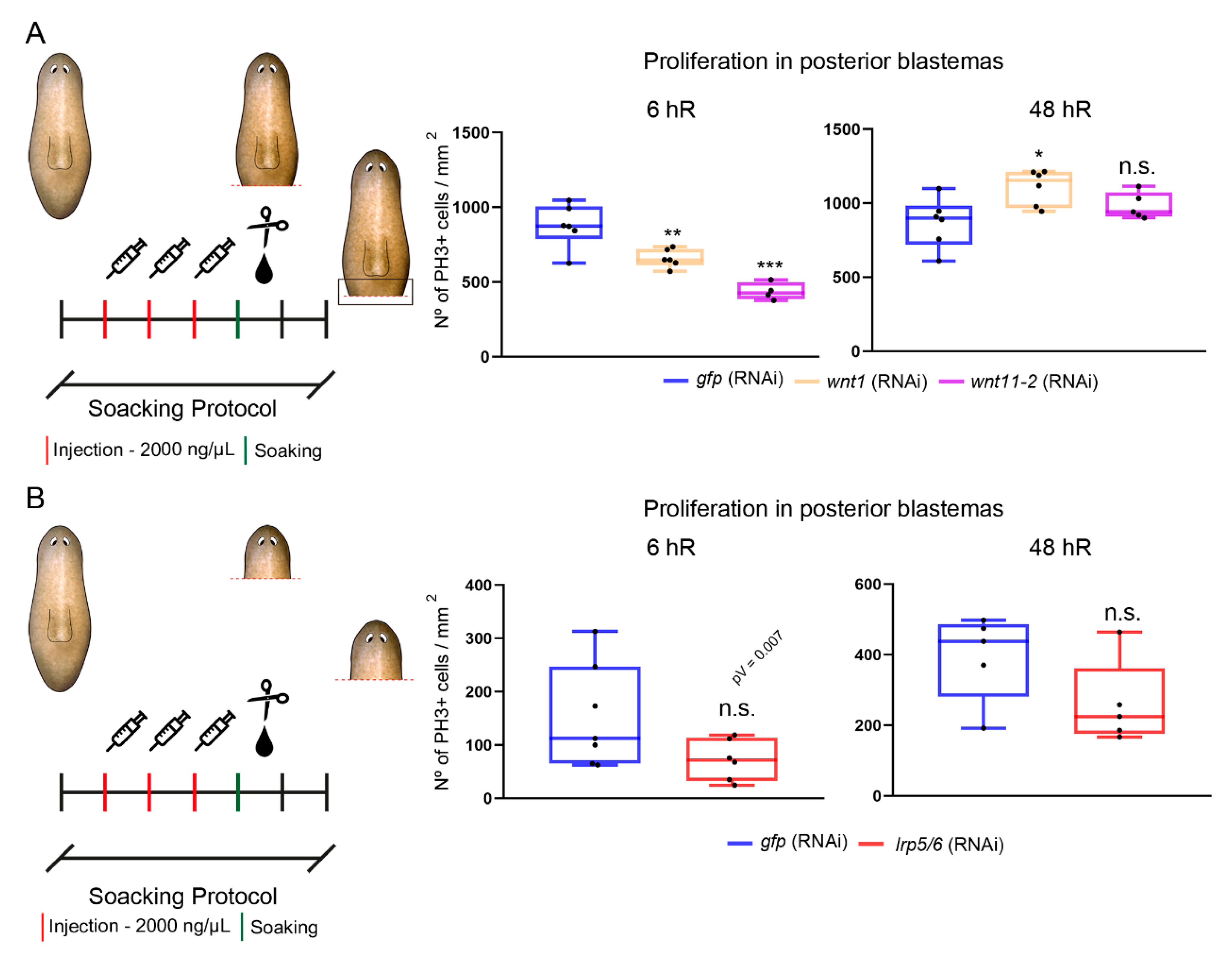
3.4. The cWNT Pathway Triggers Cell Proliferation during Regeneration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Arias, A.M.; Steventon, B. On the nature and function of organizers. Development 2018, 145, dev159525. [Google Scholar] [CrossRef] [PubMed]
- Vogg, M.C.; Wenger, Y.; Galliot, B. How Somatic Adult Tissues Develop Organizer Activity. In Current Topics in Developmental Biology, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 116, pp. 391–414. [Google Scholar]
- Anderson, C.; Stern, C.D. Organizers in Development. In Current Topics in Developmental Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 117, pp. 435–454. [Google Scholar]
- De Robertis, E.M.; Larraín, J.; Oelgeschläger, M.; Wessely, O. The establishment of spemann’s organizer and patterning of the vertebrate embryo. Nat. Rev. Genet. 2000, 1, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Spemann, H.; Mangold, H. Über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Dev. Genes Evol. 1924, 100, 599–638. [Google Scholar]
- Inagaki, T.; Schoenwolf, G. Axis development in avian embryos: The ability of Hensen’s node to self-differentiate, as analyzed with heterochronic grafting experiments. Brain Struct. Funct. 1993, 188, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, A. Next generation limb development and evolution: Old questions, new perspectives. Development 2015, 142, 3810–3820. [Google Scholar] [CrossRef]
- Anderson, C.; Khan, M.A.F.; Wong, F.; Solovieva, T.; Oliveira, N.M.M.; Baldock, R.A.; Tickle, C.; Burt, D.W.; Stern, C.D. A strategy to discover new organizers identifies a putative heart organizer. Nat. Commun. 2016, 7, 12656. [Google Scholar] [CrossRef]
- Browne, E.N. The production of new hydranths in Hydra by the insertion of small grafts. J. Exp. Zool. 2005, 7, 1–23. [Google Scholar] [CrossRef]
- Vogg, M.C.; Beccari, L.; Ollé, L.I.; Rampon, C.; Vriz, S.; Perruchoud, C.; Wenger, Y.; Galliot, B. An evolutionarily-conserved Wnt3/β-catenin/Sp5 feedback loop restricts head organizer activity in Hydra. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Wehner, D.; Cizelsky, W.; Vasudevaro, M.D.; Ozhan, G.; Haase, C.; Kagermeier-Schenk, B.; Röder, A.; Dorsky, R.I.; Moro, E.; Argenton, F.; et al. Wnt/β-Catenin Signaling Defines Organizing Centers that Orchestrate Growth and Differentiation of the Regenerating Zebrafish Caudal Fin. Cell Rep. 2014, 6, 467–481. [Google Scholar] [CrossRef]
- Lengfeld, T.; Watanabe, H.; Simakov, O.; Lindgens, D.; Gee, L.; Law, L.; Schmidt, H.A.; Özbek, S.; Bode, H.R.; Holstein, T.W. Multiple Wnts are involved in Hydra organizer formation and regeneration. Dev. Biol. 2009, 330, 186–199. [Google Scholar] [CrossRef]
- Holstein, T.W. The Evolution of the Wnt Pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a007922. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.P.; Reddien, P.W. Wnt Signaling and the Polarity of the Primary Body Axis. Cell 2009, 139, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.E.; Wang, I.E.; Reddien, P. Clonogenic Neoblasts Are Pluripotent Adult Stem Cells That Underlie Planarian Regeneration. Science 2011, 332, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Baguñà, J. Regeneration and pattern formation in planarians. J. Embryol. Exp. Morphol. 1984, 80, 63–80. [Google Scholar]
- Riutort, M.; Álvarez-Presas, M.; Lázaro, E.; Saló, E.; Paps, J. Evolutionary history of the Tricladida and the Platyhelminthes: An up-to-date phylogenetic and systematic account. Int. J. Dev. Biol. 2012, 56, 5–17. [Google Scholar] [CrossRef]
- Wenemoser, D.; Reddien, P. Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Dev. Biol. 2010, 344, 979–991. [Google Scholar] [CrossRef]
- Pellettieri, J.; Fitzgerald, P.; Watanabe, S.; Mancuso, J.; Green, D.R.; Alvarado, A.S. Cell death and tissue remodeling in planarian regeneration. Dev. Biol. 2010, 338, 76–85. [Google Scholar] [CrossRef]
- Witchley, J.N.; Mayer, M.; Wagner, D.E.; Owen, J.H.; Reddien, P. Muscle Cells Provide Instructions for Planarian Regeneration. Cell Rep. 2013, 4, 633–641. [Google Scholar] [CrossRef]
- Scimone, M.L.; Cote, L.E.; Rogers, T.; Reddien, P. Two FGFRL-Wnt circuits organize the planarian anteroposterior axis. eLife 2016, 5. [Google Scholar] [CrossRef]
- Petersen, C.P.; Reddien, P. Polarized notum Activation at Wounds Inhibits Wnt Function to Promote Planarian Head Regeneration. Science 2011, 332, 852–855. [Google Scholar] [CrossRef]
- Gurley, K.A.; Elliott, S.A.; Simakov, O.; Schmidt, H.A.; Holstein, T.W.; Alvarado, A.S. Expression of secreted Wnt pathway components reveals unexpected complexity of the planarian amputation response. Dev. Biol. 2010, 347, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.P.; Reddien, P. A wound-induced Wnt expression program controls planarian regeneration polarity. Proc. Natl. Acad. Sci. USA 2009, 106, 17061–17066. [Google Scholar] [CrossRef] [PubMed]
- Adell, T.; Salò, E.; Boutros, M.; Bartscherer, K. Smed-Evi/Wntless is required for β-catenin-dependent and -independent processes during planarian regeneration. Development 2009, 136, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Sureda-Gómez, M.; Pascual-Carreras, E.; Adell, T. Posterior Wnts Have Distinct Roles in Specification and Patterning of the Planarian Posterior Region. Int. J. Mol. Sci. 2015, 16, 26543–26554. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; Von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Huson, D.H.; Scornavacca, C. Dendroscope 3: An Interactive Tool for Rooted Phylogenetic Trees and Networks. Syst. Biol. 2012, 61, 1061–1067. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Fernandéz-Taboada, E.; Moritz, S.; Zeuschner, D.; Stehling, M.; Schöler, H.R.; Saló, E.; Gentile, L. Smed-SmB, a member of the LSm protein superfamily, is essential for chromatoid body organization and planarian stem cell proliferation. Development 2010, 137, 1055–1065. [Google Scholar] [CrossRef]
- Alvarado, A.S.; Newmark, P.A. Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proc. Natl. Acad. Sci. USA 1999, 96, 5049–5054. [Google Scholar] [CrossRef]
- Currie, K.W.; Brown, D.D.R.; Zhu, S.; Xu, C.; Voisin, V.; Bader, G.D.; Pearson, B.J. HOX gene complement and expression in the planarian Schmidtea mediterranea. EvoDevo 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- King, R.S.; Newmark, P.A. In Situ hybridization protocol for enhanced detection of gene expression in the planarian Schmidtea mediterranea. BMC Dev. Biol. 2013, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.G.; Omuro, K.C.; Taylor, M.R.; Munday, R.K.; Hubert, A.; King, R.S.; Zayas, R.M. Novel monoclonal antibodies to study tissue regeneration in planarians. BMC Dev. Biol. 2015, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Lara, S.; Pascual-Carreras, E.; Abril, J. PlanExp: Intuitive integration of complex RNA-seq datasets with planarian omics resources. Bioinformatics 2019, 36, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Lara, S.; Abril, J.F. PlanNET: Homology-based predicted interactome for multiple planarian transcriptomes. Bioinformatics 2017, 34, 1016–1023. [Google Scholar] [CrossRef]
- Fincher, C.T.; Wurtzel, O.; De Hoog, T.; Kravarik, K.M.; Reddien, P. Cell type transcriptome atlas for the planarian Schmidtea mediterranea. Science 2018, 360, eaaq1736. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Selck, C.; Friedrich, B.; Lutz, R.; Vila-Farré, M.; Dahl, A.; Brandl, H.; Lakshmanaperumal, N.; Henry, I.; Rink, J.C. Reactivating head regrowth in a regeneration-deficient planarian species. Nat. Cell Biol. 2013, 500, 81–84. [Google Scholar] [CrossRef]
- Stückemann, T.; Cleland, J.P.; Werner, S.; Vu, H.T.-K.; Bayersdorf, R.; Liu, S.-Y.; Friedrich, B.; Jülicher, F.; Rink, J.C. Antagonistic Self-Organizing Patterning Systems Control Maintenance and Regeneration of the Anteroposterior Axis in Planarians. Dev. Cell 2017, 40, 248–263.e4. [Google Scholar] [CrossRef]
- Currie, K.W.; Molinaro, A.M.; Pearson, B.J. Neuronal sources of hedgehog modulate neurogenesis in the adult planarian brain. eLife 2016, 5, e19735. [Google Scholar] [CrossRef]
- Dieckmann, M.; Dietrich, M.F.; Herz, J. Lipoprotein receptors—An evolutionarily ancient multifunctional receptor family. Biol. Chem. 2010, 391, 1341–1363. [Google Scholar] [CrossRef]
- Gordon, M.D.; Nusse, R. Wnt Signaling: Multiple Pathways, Multiple Receptors, and Multiple Transcription Factors. J. Biol. Chem. 2006, 281, 22429–22433. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.M.; Petersen, C.P. Wnt/Notum spatial feedback inhibition controls neoblast differentiation to regulate reversible growth of the planarian brain. Development 2015, 142, 4217–4229. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.; Gomez-Skarmeta, J.L.; Saló, E.; Adell, T. Silencing of Smed- catenin1 generates radial-like hypercephalized planarians. Development 2008, 135, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, B.T.; He, X. Frizzled and LRP5/6 Receptors for Wnt/-Catenin Signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a007880. [Google Scholar] [CrossRef]
- Hendzel, M.J.; Wei, Y.; Mancini, M.A.; Van Hooser, A.; Ranalli, T.; Brinkley, B.R.; Bazett-Jones, D.P.; Allis, C.D. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 1997, 106, 348–360. [Google Scholar] [CrossRef]
- Pascual-Carreras, E.; Marin-Barba, M.; Herrera-Úbeda, C.; Font-Martín, D.; Eckelt, K.; De Sousa, N.; García-Fernández, J.; Saló, E.; Adell, T. Planarian cell number depends on blitzschnell, a novel gene family that balances cell proliferation and cell death. Development 2020, 147, dev184044. [Google Scholar] [CrossRef]
- Hayashi, T.; Motoishi, M.; Itomi, K.; Tanegashima, C.; Nishimura, O.; Agata, K.; Tarui, H. A LIM-homeobox gene is required for differentiation of Wnt-expressing cells at the posterior end of the planarian body. Development 2011, 138, 3679–3688. [Google Scholar] [CrossRef]
- Guder, C.; Philipp, I.; Lengfeld, T.; Watanabe, H.; Hobmayer, B.; Holstein, T.W. The Wnt code: Cnidarians signal the way. Oncogene 2006, 25, 7450–7460. [Google Scholar] [CrossRef]
- Minobe, S.; Fei, K.; Yan, L.; Sarras, M.P., Jr.; Werle, M.J. Identification and characterization of the epithelial polarity receptor ”Frizzled” in Hydra vulgaris. Dev. Genes Evol. 2000, 210, 258–262. [Google Scholar] [CrossRef]
- Adler, P.N. Planar signaling and morphogenesis in Drosophila. Dev. Cell 2002, 2, 525–535. [Google Scholar] [CrossRef]
- Adell, T.; Nefkens, I.; Müller, W.E. Polarity factor ‘Frizzled’ in the demosponge Suberites domuncula: Identification, expression and localization of the receptor in the epithelium/pinacoderm1. FEBS Lett. 2003, 554, 363–368. [Google Scholar] [CrossRef]
- Gehart, H.; Clevers, H. Tales from the crypt: New insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, X.; Zhang, H.; Wang, Z.; Nan, G.; Li, Y.; Zhang, F.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; et al. The evolving roles of canonical WNT signaling in stem cells and tumorigenesis: Implications in targeted cancer therapies. Lab. Investig. 2016, 96, 116–136. [Google Scholar] [CrossRef] [PubMed]
- Raz, A.A.; Srivastava, M.; Salvamoser, R.; Reddien, P. Acoel regeneration mechanisms indicate an ancient role for muscle in regenerative patterning. Nat. Commun. 2017, 8, 1260. [Google Scholar] [CrossRef]
- Ramirez, A.N.; Loubet-Senear, K.; Srivastava, M. A Regulatory Program for Initiation of Wnt Signaling during Posterior Regeneration. Cell Rep. 2020, 32, 108098. [Google Scholar] [CrossRef]
- Chera, S.; Ghila, L.M.; Dobretz, K.; Wenger, Y.; Bauer, C.R.; Buzgariu, W.C.; Martinou, J.-C.; Galliot, B. Apoptotic Cells Provide an Unexpected Source of Wnt3 Signaling to Drive Hydra Head Regeneration. Dev. Cell 2009, 17, 279–289. [Google Scholar] [CrossRef]
- Brock, C.K.; Wallin, S.T.; Ruiz, O.E.; Samms, K.M.; Mandal, A.; Sumner, E.A.; Eisenhoffer, G.T. Stem cell proliferation is induced by apoptotic bodies from dying cells during epithelial tissue maintenance. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Bergantiños, C.; Corominas, M.; Serras, F. Cell death-induced regeneration in wing imaginal discs requires JNK signalling. Development 2010, 137, 1169–1179. [Google Scholar] [CrossRef]
- Santabárbara-Ruiz, P.; López-Santillán, M.; Martínez-Rodríguez, I.; Binagui-Casas, A.; Pérez, L.; Milán, M.; Corominas, M.; Serras, F. ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Drosophila Regeneration. PLoS Genet. 2015, 11, e1005595. [Google Scholar] [CrossRef]
- Pérez-Garijo, A.; Steller, H. Spreading the word: Non-autonomous effects of apoptosis during development, regeneration and disease. Development 2015, 142, 3253–3262. [Google Scholar] [CrossRef]
- Warner, J.F.; Amiel, A.R.; Johnston, H.; Röttinger, E. Regeneration is a partial redeployment of the embryonic gene network. bioRxiv 2019. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual-Carreras, E.; Sureda-Gómez, M.; Barrull-Mascaró, R.; Jordà, N.; Gelabert, M.; Coronel-Córdoba, P.; Saló, E.; Adell, T. WNT-FRIZZLED-LRP5/6 Signaling Mediates Posterior Fate and Proliferation during Planarian Regeneration. Genes 2021, 12, 101. https://doi.org/10.3390/genes12010101
Pascual-Carreras E, Sureda-Gómez M, Barrull-Mascaró R, Jordà N, Gelabert M, Coronel-Córdoba P, Saló E, Adell T. WNT-FRIZZLED-LRP5/6 Signaling Mediates Posterior Fate and Proliferation during Planarian Regeneration. Genes. 2021; 12(1):101. https://doi.org/10.3390/genes12010101
Chicago/Turabian StylePascual-Carreras, Eudald, Miquel Sureda-Gómez, Ramon Barrull-Mascaró, Natàlia Jordà, Maria Gelabert, Pablo Coronel-Córdoba, Emili Saló, and Teresa Adell. 2021. "WNT-FRIZZLED-LRP5/6 Signaling Mediates Posterior Fate and Proliferation during Planarian Regeneration" Genes 12, no. 1: 101. https://doi.org/10.3390/genes12010101
APA StylePascual-Carreras, E., Sureda-Gómez, M., Barrull-Mascaró, R., Jordà, N., Gelabert, M., Coronel-Córdoba, P., Saló, E., & Adell, T. (2021). WNT-FRIZZLED-LRP5/6 Signaling Mediates Posterior Fate and Proliferation during Planarian Regeneration. Genes, 12(1), 101. https://doi.org/10.3390/genes12010101




