Molecular Pharming of the Recombinant Protein hEGF-hEGF Concatenated with Oleosin Using Transgenic Arabidopsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. hEGF Gene Cloning and Vector Construction
2.3. Transformation of Arabidopsis
2.4. Extraction of Oil Bodies
2.5. Validation of Transgenic Arabidopsis Expressing Recombinant Oleosin–-hEGF–hEGF
2.6. Western Blot Analysis of the Oil Body-Expressed Oleosin-hEGF–hEGF
2.7. Measuring of the Oil Body Particle Size
2.8. Microstructure Detection of the Oil Body
2.9. Transdermal Absorption of the Transgenic Oil Body
2.10. Resistant to Proteolysis of Oil body-Expressed Oleosin-hEGF–hEGF
2.11. Proliferation Assay of the Oil body-Expressed Oleosin–hEGF–hEGF
2.12. Statistical Analysis
3. Results
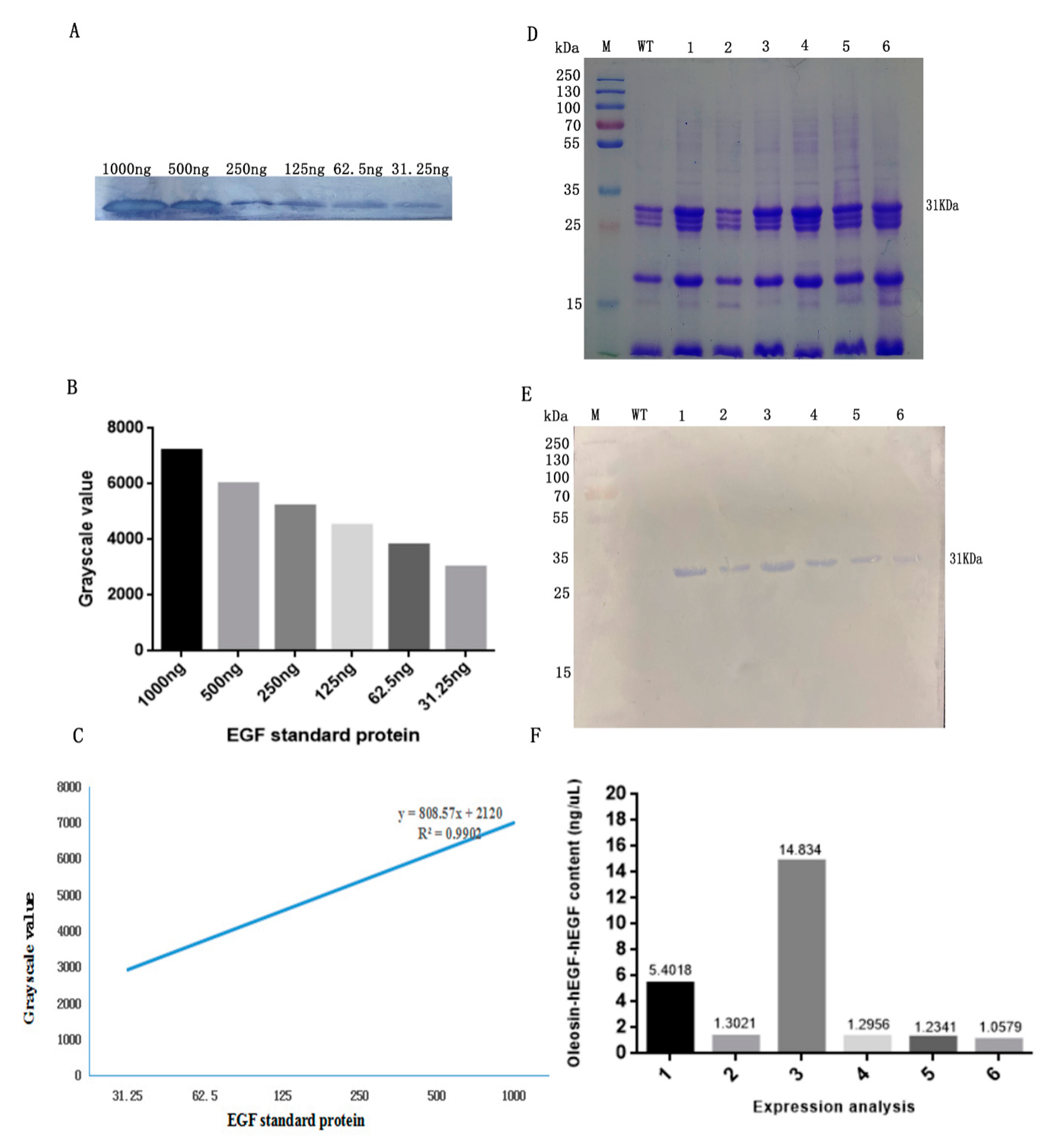
3.1. Transformation and Detection of Arabidopsis
3.2. Expression Analysis of Fusion Protein in Transgenic Arabidopsis
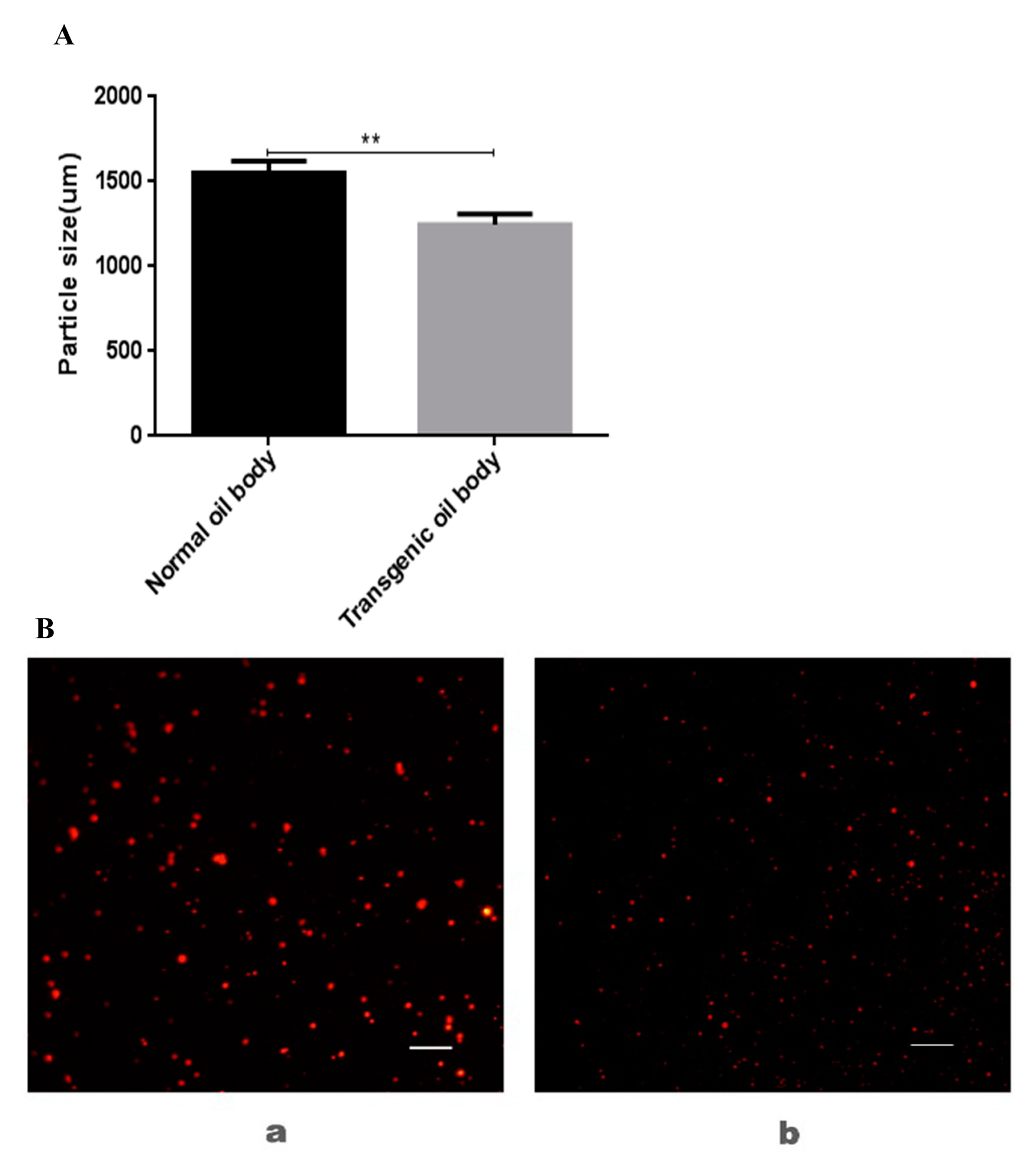
3.3. Analysis of Particle Size and Microstructure of Transgenic Oil Body
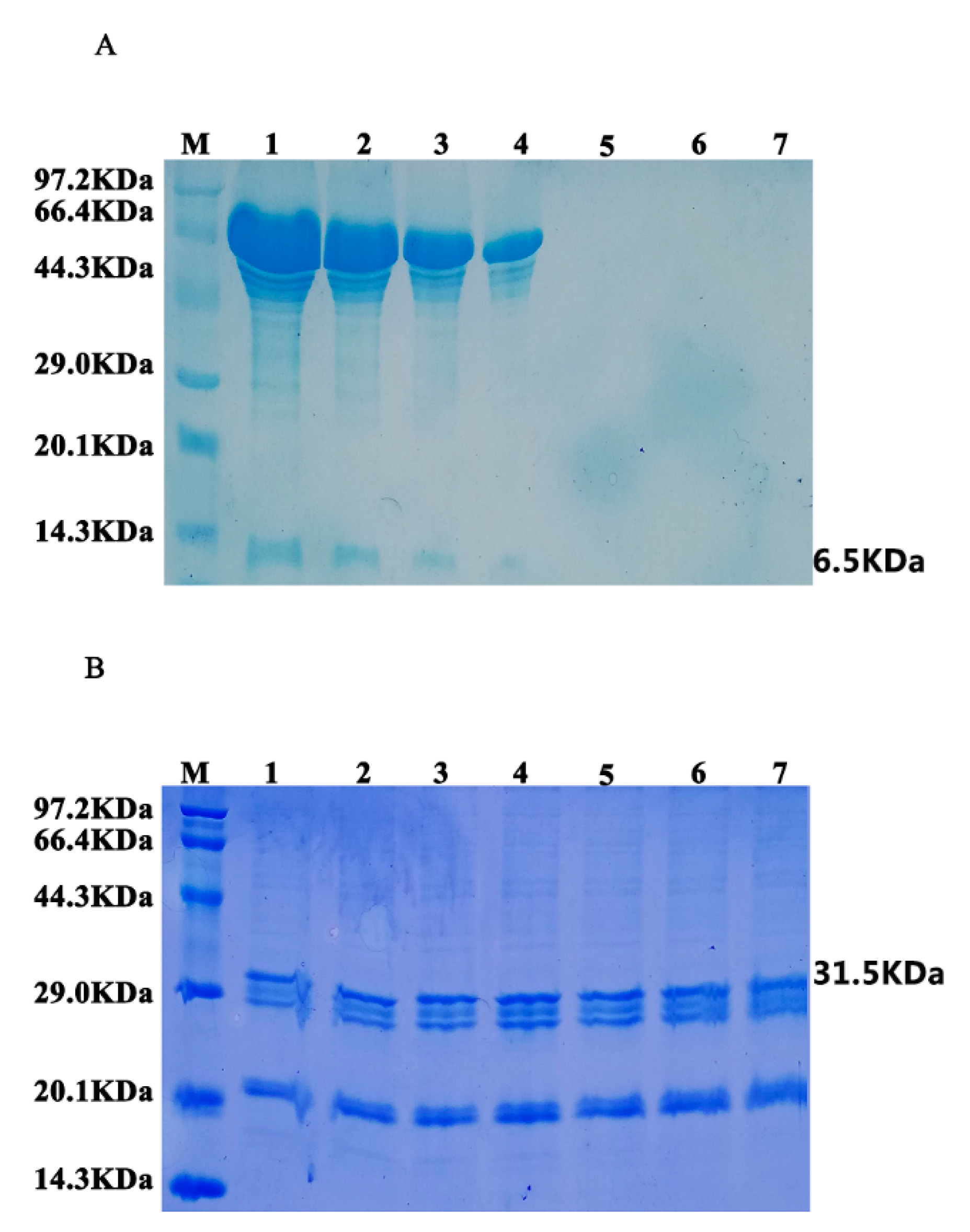
3.4. Stability Analysis of Oil Bodies-Expressed Oleosin–hEGF–hEGF
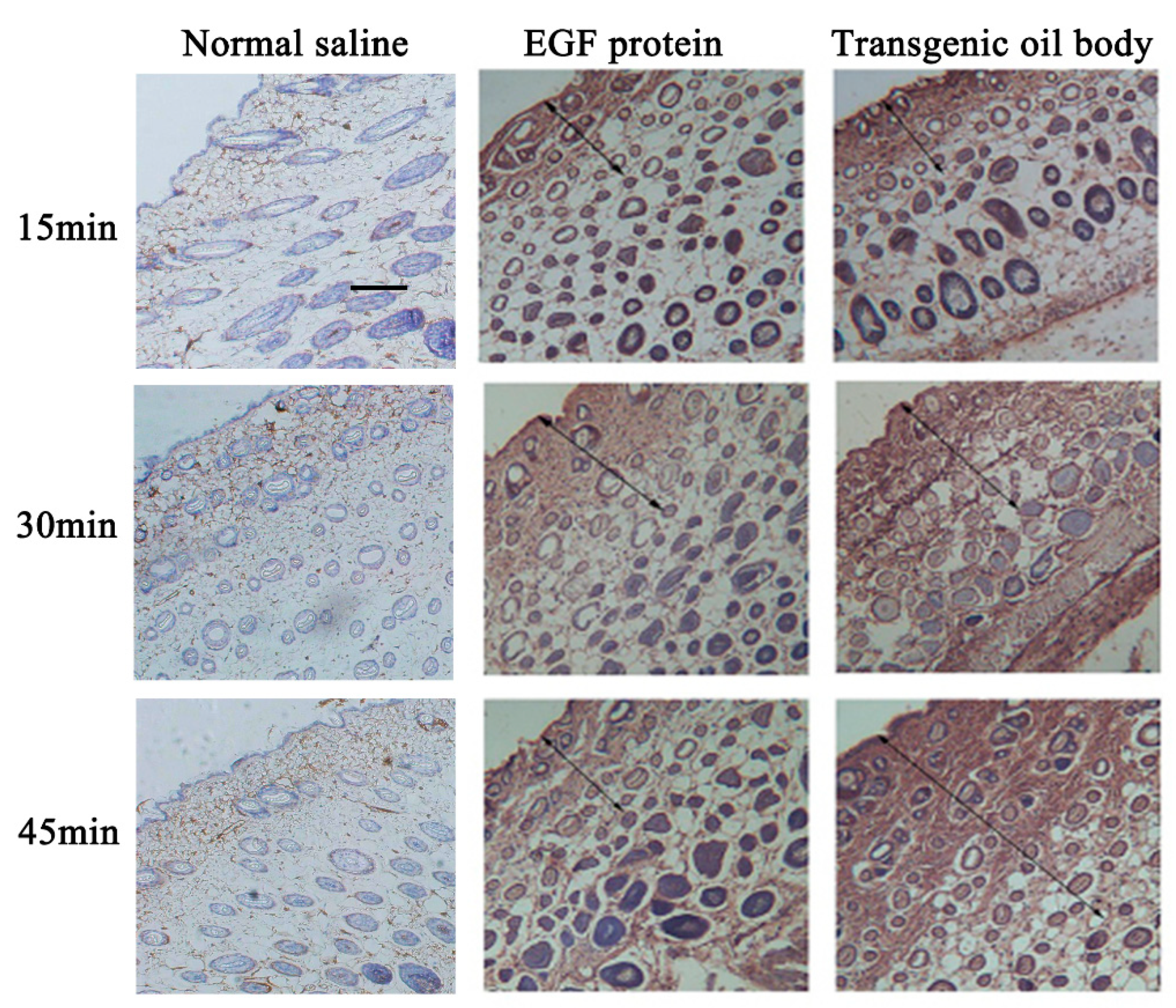
3.5. Transdermal Absorption of the Transgenic Oil Body
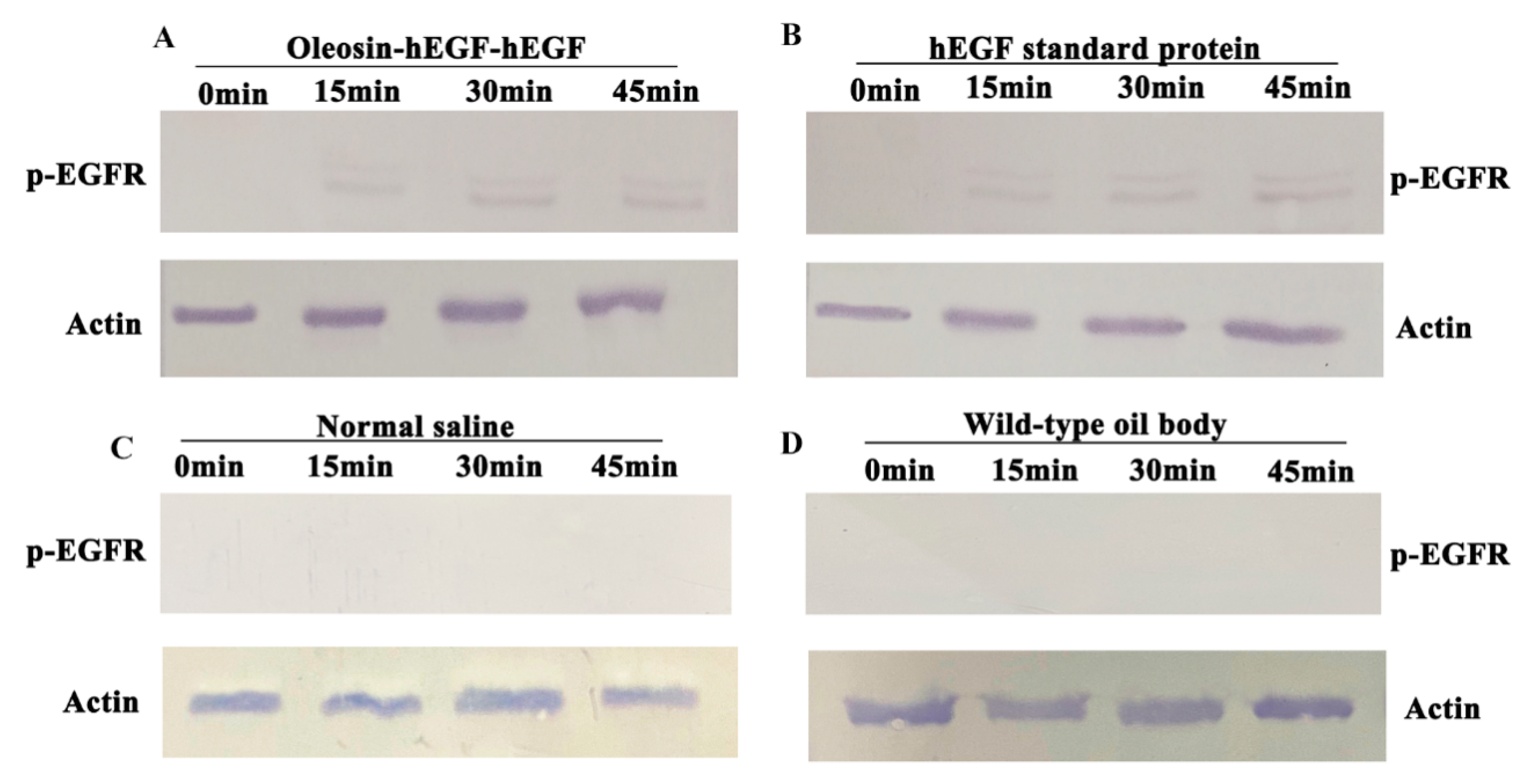
3.6. Analysis of p-EGFR Receptor Activation
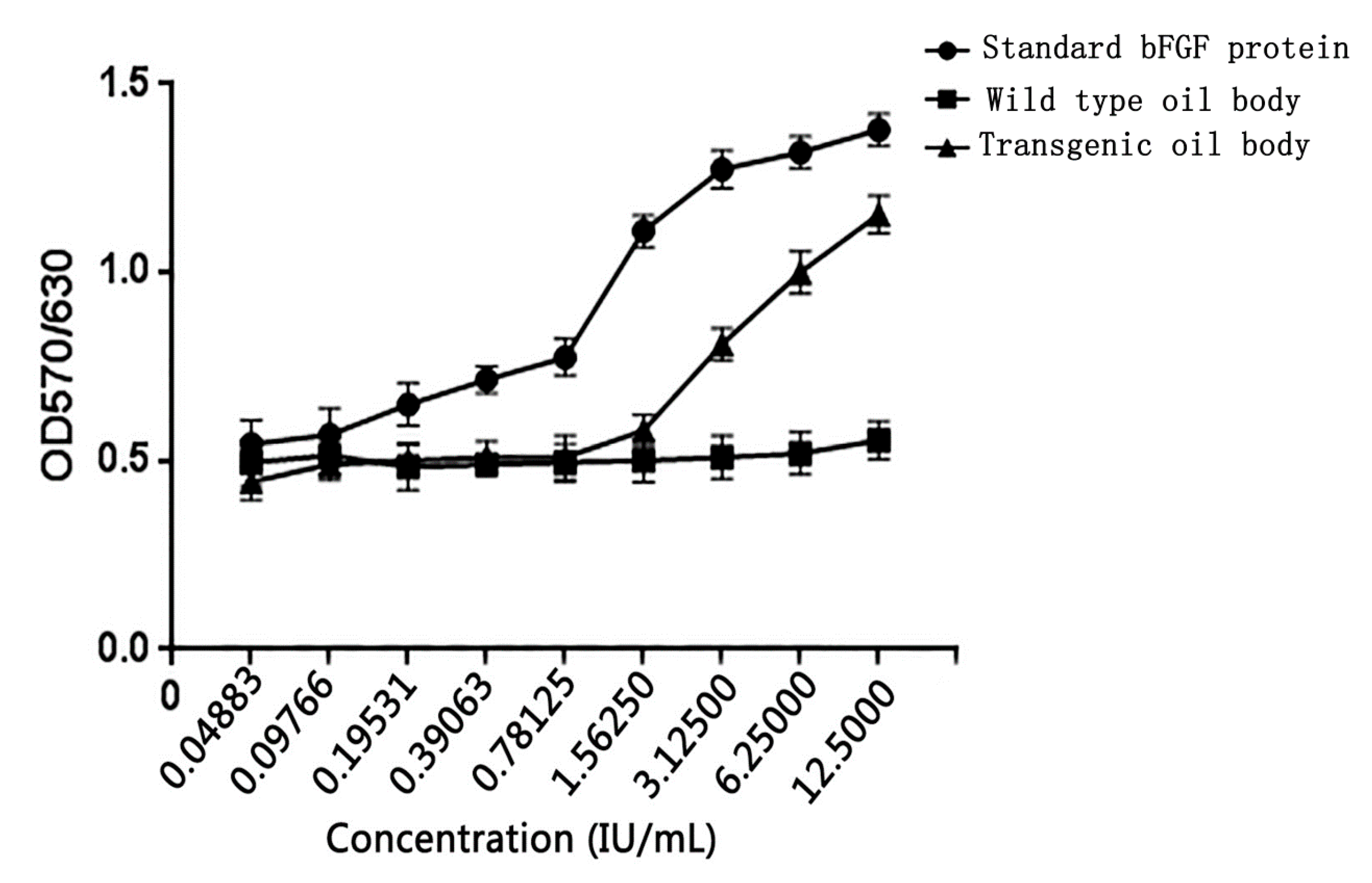
3.7. Oil Body-Expressed Fusion Protein Activity Analysis in NIH/3T3 cell
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Le, P.U.; Lenferink, A.E.G.; Pinard, M.; Baardsnes, J.; Massie, B.; O’Connor-McCourt, M.D. Escherichia coli expression and refolding of E/K-coil-tagged EGF generates fully bioactive EGF for diverse applications. Protein Expr. Purif. 2009, 64, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Mroczkowski, B.; Ball, R. Epidermal Growth Factor: Biology and Properties of its Gene and Protein Precursor BT—Growth Factors, Differentiation Factors, and Cytokines; Habenicht, A., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 18–30. ISBN 978-3-642-74856-1. [Google Scholar]
- Jahovic, N.; Güzel, E.; Arbak, S.; Yeğen, B.Ç. The healing-promoting effect of saliva on skin burn is mediated by epidermal growth factor (EGF): Role of the neutrophils. Burns 2004, 30, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Hee Na, D.; Seok Youn, Y.; Bok Lee, I.; Ji Park, E.; Jeon Park, C.; Choon Lee, K. Effect of Molecular Size of PEGylated Recombinant Human Epidermal Growth Factor on the Biological Activity and Stability in Rat Wound Tissue. Pharm. Dev. Technol. 2006, 11, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.C. Oil Bodies and Oleosins in Seeds. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 177–200. [Google Scholar] [CrossRef]
- Siloto, R.M.P.; Findlay, K.; Lopez-Villalobos, A.; Yeung, E.C.; Nykiforuk, C.L.; Moloney, M.M. The Accumulation of Oleosins Determines the Size of Seed Oilbodies in Arabidopsis. Plant Cell 2006, 18, 1961–1974. [Google Scholar] [CrossRef]
- Beaudoin, F.; Wilkinson, B.M.; Stirling, C.J.; Napier, J.A. In vivo targeting of a sunflower oil body protein in yeast secretory (sec) mutants. Plant J. 2000, 23, 159–170. [Google Scholar] [CrossRef]
- Chapman, K.D.; Dyer, J.M.; Mullen, R.T. Biogenesis and functions of lipid droplets in plants: Thematic Review Series: Lipid Droplet Synthesis and Metabolism: From Yeast to Man. J. Lipid Res. 2012, 53, 215–226. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Vandana, S.; Kaushik, V. Recent developments in the localization of oil body-associated signaling molecules during lipolysis in oilseeds. Plant Signal. Behav. 2009, 4, 176–182. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Kaushik, V.; Yadav, M.K. Use of oil bodies and oleosins in recombinant protein production and other biotechnological applications. Biotechnol. Adv. 2010, 28, 293–300. [Google Scholar] [CrossRef]
- Yang, J.; Guan, L.; Guo, Y.; Du, L.; Wang, F.; Wang, Y.; Zhen, L.; Wang, Q.; Zou, D.; Chen, W.; et al. Expression of biologically recombinant human acidic fibroblast growth factor in Arabidopsis thaliana seeds via oleosin fusion technology. Gene 2015, 566, 89–94. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Kiosseoglou, V. Physicochemical stability of maize germ oil body emulsions as influenced by oil body surface-xanthan gum interactions. J. Agric. Food Chem. 2010, 58, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Giddings, G.; Allison, G.; Brooks, D.; Carter, A. Transgenic plants as factories for biopharmaceuticals. Nat. Biotechnol. 2000, 18, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Nikiforidis, C.V.; Kiosseoglou, V.; Scholten, E. Oil bodies: An insight on their microstructure—Maize germ vs sunflower seed. Food Res. Int. 2013, 52, 136–141. [Google Scholar] [CrossRef]
- Tzen, J.T.; Peng, C.C.; Cheng, D.J.; Chen, E.C.; Chiu, J.M. A new method for seed oil body purification and examination of oil body integrity following germination. J. Biochem. 1997, 121, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Yang, J.; Huang, J.; Guan, L.; Du, L.; Guo, Y.; Zhai, F.; Wang, Y.; Lu, Z.; Wang, L.; et al. Expression of bioactive recombinant human fibroblast growth factor 9 in oil bodies of Arabidopsis thaliana. Protein Expr. Purif. 2015, 116, 127–132. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Chen, Y.; Guan, L.; Du, L.; Guo, Y.; Wang, W.; Wang, L.; Li, H.; Jiang, C.; et al. Expression of a functional recombinant oleosin-human hyaluronidase hPH-20 fusion in Arabidopsis thaliana. Protein Expr. Purif. 2014, 103, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Zhou, T.; Lan, X.; Zhang, X.; Guo, Y.; Noman, M.; Du, L.; Zheng, J.; Li, W.; Li, H.; et al. A new nanoscale transdermal drug delivery system: Oil body-linked oleosin-hEGF improves skin regeneration to accelerate wound healing. J. Nanobiotechnology 2018, 16, 62. [Google Scholar] [CrossRef]
- Gospodarowicz, D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature 1974, 249, 123–127. [Google Scholar] [CrossRef]
- Nykiforuk, C.L.; Boothe, J.G.; Murray, E.W.; Keon, R.G.; Goren, H.J.; Markley, N.A.; Moloney, M.M. Transgenic expression and recovery of biologically active recombinant human insulin from Arabidopsis thaliana seeds. Plant Biotechnol. J. 2006, 4, 77–85. [Google Scholar] [CrossRef]
- An, N.; Ou, J.; Jiang, D.; Zhang, L.; Liu, J.; Fu, K.; Dai, Y.; Yang, D. Expression of a Functional Recombinant Human Basic Fibroblast Growth Factor from Transgenic Rice Seeds. Int. J. Mol. Sci. 2013, 14, 3556–3567. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Cai, J.; Wang, H.; Tian, H.; Huang, J.; Qiang, W.; Zhang, L.; Li, H.; Li, X.; et al. Oil Body-Bound Oleosin-rhFGF-10: A Novel Drug Delivery System that Improves Skin Penetration to Accelerate Wound Healing and Hair Growth in Mice. Int. J. Mol. Sci. 2017, 18, 2177. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiang, W.; Gao, T.; Lan, X.; Guo, J.; Noman, M.; Li, Y.; Guo, Y.; Kong, J.; Li, H.; Du, L.; et al. Molecular Pharming of the Recombinant Protein hEGF-hEGF Concatenated with Oleosin Using Transgenic Arabidopsis. Genes 2020, 11, 959. https://doi.org/10.3390/genes11090959
Qiang W, Gao T, Lan X, Guo J, Noman M, Li Y, Guo Y, Kong J, Li H, Du L, et al. Molecular Pharming of the Recombinant Protein hEGF-hEGF Concatenated with Oleosin Using Transgenic Arabidopsis. Genes. 2020; 11(9):959. https://doi.org/10.3390/genes11090959
Chicago/Turabian StyleQiang, Weidong, Tingting Gao, Xinxin Lan, Jinnan Guo, Muhammad Noman, Yaying Li, Yongxin Guo, Jie Kong, Haiyan Li, Linna Du, and et al. 2020. "Molecular Pharming of the Recombinant Protein hEGF-hEGF Concatenated with Oleosin Using Transgenic Arabidopsis" Genes 11, no. 9: 959. https://doi.org/10.3390/genes11090959
APA StyleQiang, W., Gao, T., Lan, X., Guo, J., Noman, M., Li, Y., Guo, Y., Kong, J., Li, H., Du, L., & Yang, J. (2020). Molecular Pharming of the Recombinant Protein hEGF-hEGF Concatenated with Oleosin Using Transgenic Arabidopsis. Genes, 11(9), 959. https://doi.org/10.3390/genes11090959





