Exploring Eimeria Genomes to Understand Population Biology: Recent Progress and Future Opportunities
Abstract
1. Introduction
2. Eimeria Genomes
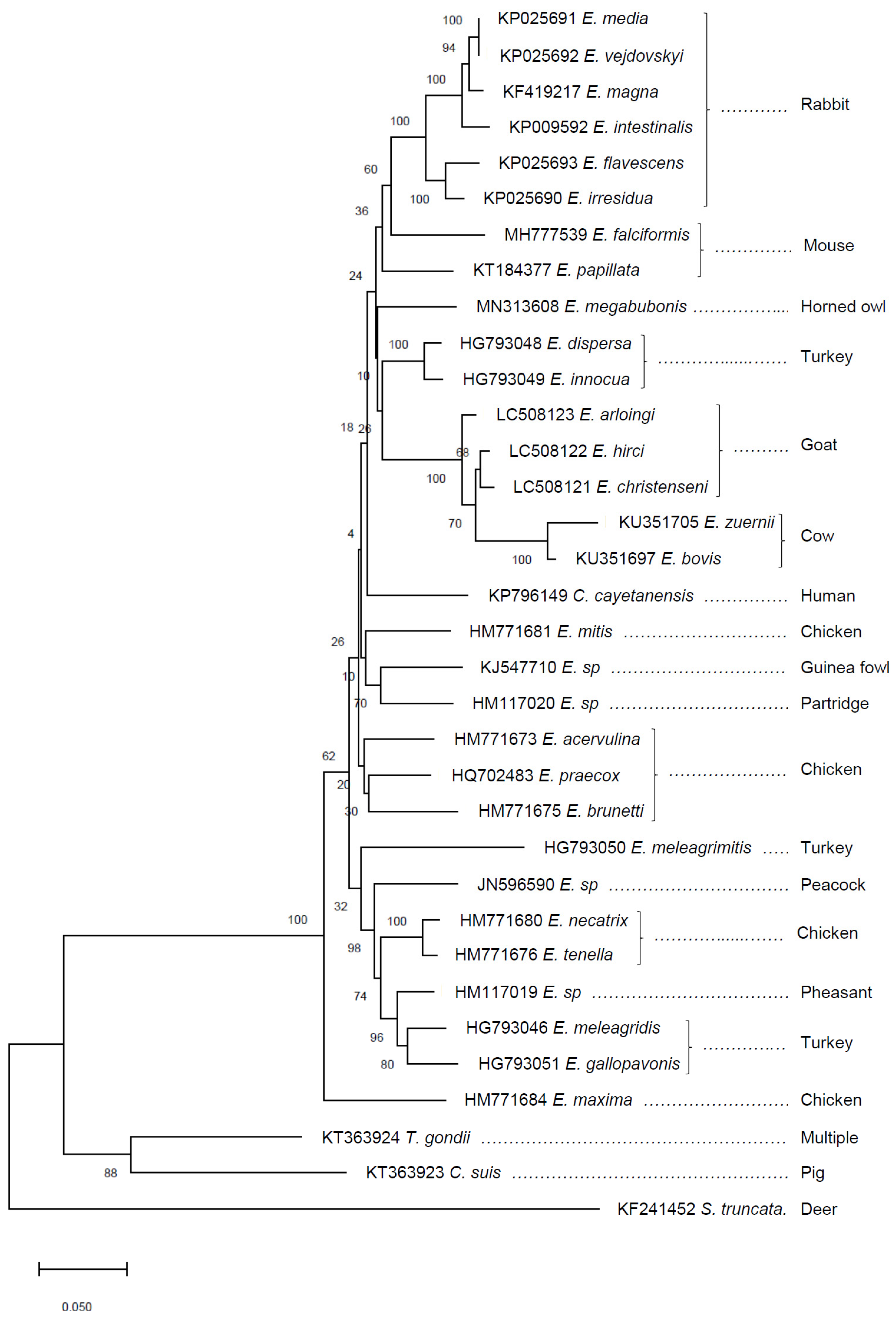
3. Comparative Genomics
4. Population Genetics
5. Challenges
6. Opportunities
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P. Coccidiosis in Livestock, Poultry, Companion Animals, and Humans; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020. [Google Scholar]
- Vrba, V.; Pakandl, M. Host specificity of turkey and chicken Eimeria: Controlled cross-transmission studies and a phylogenetic view. Vet. Parasitol. 2015, 208, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, S.; Erdbeer, A.; Volke, B.; Randel, S.; Kapplusch, F.; Hanig, S.; Kurth, M. Identification and analysis of Eimeria nieschulzi gametocyte genes reveal splicing events of gam genes and conserved motifs in the wall-forming proteins within the genus Eimeria (coccidia, apicomplexa). Parasite 2017, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Morrissette, N.S.; Sibley, L.D. Cytoskeleton of apicomplexan parasites. Microbiol. Mol. Biol. Rev. 2002, 66, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.; Jatau, I.; et al. Re-Calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.D.; Jeffers, T.K. Vaccination of chickens against coccidiosis ameliorates drug resistance in commercial poultry production. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 214–217. [Google Scholar] [CrossRef] [PubMed]
- USAHA. Report of the USAHA Committee on Poultry and Other Avian Species. United States Animal Health Association: 2019. Available online: https://www.Usaha.Org/transmissible-diseases-of-poultry-avian-species (accessed on 12 June 2020).
- FAOSTAT. Food and Agriculture Organization of the United Nations Faostat Database. Available online: http://faostat3.fao.org/home/E (accessed on 19 May 2020).
- Lopez-Osorio, S.; Silva, L.M.R.; Chaparro-Gutierrez, J.J.; Velasquez, Z.D.; Taubert, A.; Hermosilla, C. Optimized excystation protocol for ruminant Eimeria bovis- and Eimeria arloingi-sporulated oocysts and first 3d holotomographic microscopy analysis of differing sporozoite egress. Parasitol. Int. 2020, 76, 102068. [Google Scholar] [CrossRef]
- Reid, A.J.; Blake, D.P.; Ansari, H.R.; Billington, K.; Browne, H.P.; Bryant, J.; Dunn, M.; Hung, S.S.; Kawahara, F.; Miranda-Saavedra, D. Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Res. 2014, 24, 1676–1685. [Google Scholar] [CrossRef]
- Blake, D.P.; Billington, K.J.; Copestake, S.L.; Oakes, R.D.; Quail, M.A.; Wan, K.L.; Shirley, M.W.; Smith, A.L. Genetic mapping identifies novel highly protective antigens for an apicomplexan parasite. PLoS Pathog. 2011, 7, e1001279. [Google Scholar] [CrossRef]
- Shirley, M.W.; Harvey, D.A. Eimeria tenella: Infection with a single sporocyst gives a clonal population. Parasitology 1996, 112, 523–528. [Google Scholar] [CrossRef]
- Williams, R.B. Epidemiological studies of coccidiosis in the domesticated fowl (gallus gallus): II. Physical condition and survival of Eimeria acervulina oocysts in poultry-house litter. Appl. Parasitol. 1995, 36, 90–96. [Google Scholar]
- Jenkins, M.C.; Parker, C.C.; O’Brien, C.N.; Ritter, D. Viable Eimeria oocysts in poultry house litter at the time of chick placement. Poult. Sci. 2019, 98, 3176–3180. [Google Scholar] [CrossRef] [PubMed]
- Long, P.; Joyner, L.; Millard, B.; Norton, C. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet. Lat. 1976, 6, 201–217. [Google Scholar] [PubMed]
- Jeanes, C.; Vaughan-Higgins, R.; Green, R.E.; Sainsbury, A.W.; Marshall, R.N.; Blake, D.P. Two new Eimeria species parasitic in corncrakes (Crex crex) (gruiformes: Rallidae) in the United Kingdom. J. Parasitol. 2013, 99, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Novilla, M.N.; Carpenter, J.W.; Jeffers, T.K.; White, S.L. Pulmonary lesions in disseminated visceral coccidiosis of sandhill and whooping cranes. J. Wildl. Dis. 1989, 25, 527–533. [Google Scholar] [CrossRef]
- Matsubayashi, M.; Takami, K.; Abe, N.; Kimata, I.; Tani, H.; Sasai, K.; Baba, E. Molecular characterization of crane coccidia, Eimeria gruis and E. reichenowi, found in feces of migratory cranes. Parasitol. Res. 2005, 97, 80–83. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Zheng, H.; Xu, Z.; Roellig, D.M.; Li, N.; Frace, M.A.; Tang, K.; Arrowood, M.J.; Moss, D.M.; et al. Comparative genomics reveals Cyclospora cayetanensis possesses coccidia-like metabolism and invasion components but unique surface antigens. BMC Genom. 2016, 17, 316. [Google Scholar] [CrossRef]
- Palmieri, N.; Shrestha, A.; Ruttkowski, B.; Beck, T.; Vogl, C.; Tomley, F.; Blake, D.P.; Joachim, A. The genome of the protozoan parasite Cystoisospora suis and a reverse vaccinology approach to identify vaccine candidates. Int. J. Parasitol. 2017, 47, 189–202. [Google Scholar] [CrossRef]
- Vrba, V.; Poplstein, M.; Pakandl, M. The discovery of the two types of small subunit ribosomal RNA gene in Eimeria mitis contests the existence of E. mivati as an independent species. Vet. Parasitol. 2011, 183, 47–53. [Google Scholar] [CrossRef]
- Ogedengbe, M.E.; El-Sherry, S.; Ogedengbe, J.D.; Chapman, H.D.; Barta, J.R. Phylogenies based on combined mitochondrial and nuclear sequences conflict with morphologically defined genera in the eimeriid coccidia (apicomplexa). Int. J. Parasitol. 2018, 48, 59–69. [Google Scholar] [CrossRef]
- Beck, H.P.; Blake, D.; Darde, M.L.; Felger, I.; Pedraza-Diaz, S.; Regidor-Cerrillo, J.; Gomez-Bautista, M.; Ortega-Mora, L.M.; Putignani, L.; Shiels, B.; et al. Molecular approaches to diversity of populations of apicomplexan parasites. Int. J. Parasitol. 2009, 39, 175–189. [Google Scholar] [CrossRef]
- Cantacessi, C.; Riddell, S.; Morris, G.M.; Doran, T.; Woods, W.G.; Otranto, D.; Gasser, R.B. Genetic characterization of three unique operational taxonomic units of Eimeria from chickens in Australia based on nuclear spacer ribosomal DNA. Vet. Parasitol. 2008, 152, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.L.; Macdonald, S.E.; Thenmozhi, V.; Kundu, K.; Garg, R.; Kumar, S.; Ayoade, S.; Fornace, K.M.; Jatau, I.D.; Moftah, A.; et al. Cryptic Eimeria genotypes are common across the southern but not northern hemisphere. Int. J. Parasitol. 2016, 46, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.T.; Godwin, R.M. Mitochondrial genomes of australian chicken Eimeria support the presence of ten species with low genetic diversity among strains. Vet. Parasitol. 2017, 243, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Hauck, R.; Carrisosa, M.; McCrea, B.A.; Dormitorio, T.; Macklin, K.S. Evaluation of next-generation amplicon sequencing to identify Eimeria spp. of chickens. Avian Dis. 2019, 63, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. The genome of Eimeria tenella: Further studies on its molecular organisation. Parasitol. Res. 1994, 80, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Del Cacho, E.; Pages, M.; Gallego, M.; Monteagudo, L.; Sánchez-Acedo, C. Synaptonemal complex karyotype of Eimeria tenella. Int. J. Parasitol. 2005, 35, 1445–1451. [Google Scholar] [CrossRef]
- Cai, X.; Fuller, A.L.; McDougald, L.R.; Zhu, G. Apicoplast genome of the coccidian Eimeria tenella. Gene 2003, 321, 39–46. [Google Scholar] [CrossRef]
- Lee, S.; Fernando, M.A.; Nagy, E. dsRNA associated with virus-like particles in Eimeria spp. of the domestic fowl. Parasitol. Res. 1996, 82, 518–523. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, X.; Gong, P.; Li, M.; Ding, H.; Xin, C.; Zhao, N.; Li, J. Eimeria tenella: A novel dsRNA virus in E. tenella and its complete genome sequence analysis. Virus Genes 2016, 52, 244–252. [Google Scholar] [CrossRef]
- Shirley, M.W.; Harvey, D.A. A genetic linkage map of the apicomplexan protozoan parasite Eimeria tenella. Genome Res. 2000, 10, 1587–1593. [Google Scholar] [CrossRef][Green Version]
- Del Cacho, E.; Gallego, M.; Monteagudo, L.; Lopez-Bernad, F.; Quilez, J.; Sanchez-Acedo, C. A method for the sequential study of eimerian chromosomes by light and electron microscopy. Vet. Parasitol. 2001, 94, 221–226. [Google Scholar] [CrossRef]
- Xia, J.; Venkat, A.; Le Roch, K.; Ay, F.; Boyle, J. Third generation sequencing revises the molecular karyotype for Toxoplasma gondii and identifies emerging copy number variants in sexual recombinants. bioRxiv 2020. [Google Scholar] [CrossRef]
- Berna, L.; Marquez, P.; Cabrera, A.; Greif, G.; Francia, M.; Robello, C. Reevaluation of the Toxoplasma gondii and Neospora caninum genomes reveals misassembly, karyotype differences and chromosomal rearrangements. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ling, K.-H.; Rajandream, M.-A.; Rivailler, P.; Ivens, A.; Yap, S.-J.; Madeira, A.M.; Mungall, K.; Billington, K.; Yee, W.-Y.; Bankier, A.T. Sequencing and analysis of chromosome 1 of Eimeria tenella reveals a unique segmental organization. Genome Res. 2007, 17, 311–319. [Google Scholar] [CrossRef][Green Version]
- Heitlinger, E.; Spork, S.; Lucius, R.; Dieterich, C. The genome of Eimeria falciformis—Reduction and specialization in a single host apicomplexan parasite. BMC Genom. 2014, 15, 696. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.P.; Alias, H.; Billington, K.J.; Clark, E.L.; Mat-Isa, M.N.; Mohamad, A.F.; Mohd-Amin, M.R.; Tay, Y.L.; Smith, A.L.; Tomley, F.M.; et al. EmaxDB: Availability of a first draft genome sequence for the apicomplexan Eimeria maxima. Mol. Biochem. Parasitol. 2012, 184, 48–51. [Google Scholar] [CrossRef]
- Gajria, B.; Bahl, A.; Brestelli, J.; Dommer, J.; Fischer, S.; Gao, X.; Heiges, M.; Iodice, J.; Kissinger, J.C.; Mackey, A.J.; et al. ToxoDB: An integrated Toxoplasma gondii database resource. Nucleic Acids Res. 2008, 36, D553–D556. [Google Scholar] [CrossRef]
- Blake, D.P.; Clark, E.L.; Macdonald, S.E.; Thenmozhi, V.; Kundu, K.; Garg, R.; Jatau, I.D.; Ayoade, S.; Kawahara, F.; Moftah, A.; et al. Population, genetic, and antigenic diversity of the apicomplexan Eimeria tenella and their relevance to vaccine development. Proc. Natl. Acad. Sci. USA 2015, 112, e5343–e5350. [Google Scholar] [CrossRef]
- Gopinath, G.R.; Cinar, H.N.; Murphy, H.R.; Durigan, M.; Almeria, M.; Tall, B.D.; DaSilva, A.J. A hybrid reference-guided de novo assembly approach for generating Cyclospora mitochondrion genomes. Gut Pathog. 2018, 10, 15. [Google Scholar] [CrossRef]
- Jahn, D.; Matros, A.; Bakulina, A.Y.; Tiedemann, J.; Schubert, U.; Giersberg, M.; Haehnel, S.; Zoufal, K.; Mock, H.P.; Kipriyanov, S.M. Model structure of the immunodominant surface antigen of Eimeria tenella identified as a target for sporozoite-neutralizing monoclonal antibody. Parasitol. Res. 2009, 105, 655–668. [Google Scholar] [CrossRef]
- Chow, Y.P.; Wan, K.L.; Blake, D.P.; Tomley, F.; Nathan, S. Immunogenic Eimeria tenella glycosylphosphatidylinositol-anchored surface antigens (SAGS) induce inflammatory responses in avian macrophages. PLoS ONE 2011, 6, e25233. [Google Scholar] [CrossRef] [PubMed]
- Denny, P.W.; Preiser, P.R.; Rangachari, K.; Roberts, K.; Roy, A.; Whyte, A.; Strath, M.; Moore, D.J.; Moore, P.W.; Williamson, D.H. Complete gene map of the plastid-like DNA of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 1996, 261, 155–172. [Google Scholar]
- Köhler, S.; Delwiche, C.F.; Denny, P.W.; Tilney, L.G.; Webster, P.; Wilson, R.; Palmer, J.D.; Roos, D.S. A plastid of probable green algal origin in apicomplexan parasites. Science 1997, 275, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Docampo, M.D.; MacRae, J.I.; Pujol, F.M.; Brooks, C.F.; Van Dooren, G.G.; Hiltunen, J.K.; Kastaniotis, A.J.; McConville, M.J.; Striepen, B. Apicoplast and endoplasmic reticulum cooperate in fatty acid biosynthesis in apicomplexan parasite Toxoplasma gondii. J. Biol. Chem. 2012, 287, 4957–4971. [Google Scholar] [CrossRef]
- Van Dooren, G.G.; Striepen, B. The algal past and parasite present of the apicoplast. Annu. Rev. Microbiol. 2013, 67, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Van Dooren, G.G.; Kennedy, A.T.; McFadden, G.I. The use and abuse of heme in apicomplexan parasites. Antioxid. Redox Signal. 2012, 17, 634–656. [Google Scholar] [CrossRef]
- Tang, K.; Guo, Y.; Zhang, L.; Rowe, L.A.; Roellig, D.M.; Frace, M.A.; Li, N.; Liu, S.; Feng, Y.; Xiao, L. Genetic similarities between Cyclospora cayetanensis and cecum-infecting avian Eimeria spp. in apicoplast and mitochondrial genomes. Parasites Vectors 2015, 8, 358. [Google Scholar] [CrossRef]
- Ogedengbe, M.E.; El-Sherry, S.; Whale, J.; Barta, J.R. Complete mitochondrial genome sequences from five Eimeria species (apicomplexa; coccidia; eimeriidae) infecting domestic turkeys. Parasites Vectors 2014, 7, 335. [Google Scholar] [CrossRef]
- Liu, G.H.; Tian, S.Q.; Cui, P.; Fang, S.F.; Wang, C.R.; Zhu, X.Q. The complete mitochondrial genomes of five Eimeria species infecting domestic rabbits. Exp. Parasitol. 2015, 159, 67–71. [Google Scholar] [CrossRef]
- Mehlhorn, H. Coccidia. In Encyclopedia of Parasitology; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Jarquin-Diaz, V.H.; Balard, A.; Macova, A.; Jost, J.; Roth von Szepesbela, T.; Berktold, K.; Tank, S.; Kvicerova, J.; Heitlinger, E. Generalist Eimeria species in rodents: Multilocus analyses indicate inadequate resolution of established markers. Ecol. Evol. 2020, 10, 1378–1389. [Google Scholar] [CrossRef]
- Norton, C.C.; Joyner, L.P. The appearance of bisporocytic oocysts of Eimeria maxima in drug-treated chicks. Parasitology 1978, 77, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. Mega X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Carruthers, V.B.; Tomley, F.M. Microneme proteins in apicomplexans. Subcell. Biochem. 2008, 47, 33–45. [Google Scholar] [PubMed]
- Dubey, J.P.; Bauer, C. A review of Eimeria infections in horses and other equids. Vet. Parasitol. 2018, 256, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. A review of coccidiosis in south american camelids. Parasitol. Res. 2018, 117, 1999–2013. [Google Scholar] [CrossRef]
- Jing, J.; Liu, C.; Zhu, S.X.; Jiang, Y.M.; Wu, L.C.; Song, H.Y.; Shao, Y.X. Pathological and ultrastructural observations and liver function analysis of Eimeria stiedai-infected rabbits. Vet. Parasitol. 2016, 223, 165–172. [Google Scholar] [CrossRef]
- Gajadhar, A.A.; Stockdale, P.H.; Cawthorn, R.J. Ultrastructural studies of the zygote and oocyst wall formation of Eimeria truncata of the lesser snow goose. J. Protozool. 1986, 33, 341–344. [Google Scholar] [CrossRef]
- Jatau, I.D.; Lawal, I.A.; Kwaga, J.K.; Tomley, F.M.; Blake, D.P.; Nok, A.J. Three operational taxonomic units of Eimeria are common in nigerian chickens and may undermine effective molecular diagnosis of coccidiosis. BMC Vet. Res. 2016, 12, 86. [Google Scholar] [CrossRef]
- Smith, A.L.; Hesketh, P.; Archer, A.; Shirley, M.W. Antigenic diversity in Eimeria maxima and the influence of host genetics and immunization schedule on cross-protective immunity. Infect. Immun. 2002, 70, 2472–2479. [Google Scholar] [CrossRef]
- Boulton, K.; Nolan, M.J.; Wu, Z.; Psifidi, A.; Riggio, V.; Harman, K.; Bishop, S.C.; Kaiser, P.; Abrahamsen, M.S.; Hawken, R.; et al. Phenotypic and genetic variation in the response of chickens to Eimeria tenella induced coccidiosis. Genet. Sel. Evol. 2018, 50, 63. [Google Scholar] [CrossRef]
- Bumstead, N.; Millard, B. Variation in susceptibility of inbred lines of chickens to seven species of Eimeria. Parasitology 1992, 104, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Joyner, L.P. Immunological variation between two strains of Eimeria acervulina. Parasitology 1969, 59, 725–732. [Google Scholar] [CrossRef] [PubMed]
- McDonald, V.; Shirley, M.W.; Chapman, H.D. Attenuation of Eimeria species: Further characterisation of two lines of Eimeria mitis. Res. Vet. Sci. 1985, 39, 328–332. [Google Scholar] [CrossRef]
- Awad, A.M.; El-Nahas, A.F.; Abu-Akkada, S.S. Evaluation of the protective efficacy of the anticoccidial vaccine coccivac-b in broilers, when challenged with egyptian field isolates of E. tenella. Parasitol. Res. 2013, 112, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Abu-Akkada, S.S.; Awad, A.M. Isolation, propagation, identification and comparative pathogenicity of five Egyptian field strains of Eimeria tenella from broiler chickens in five different provinces in Egypt. Res. Vet. Sci. 2012, 92, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B.; Marshall, R.N.; Pages, M.; Dardi, M.; del Cacho, E. Pathogenesis of Eimeria praecox in chickens: Virulence of field strains compared with laboratory strains of e. Praecox and Eimeria acervulina. Avian Pathol. 2009, 38, 359–366. [Google Scholar] [CrossRef]
- Shirley, M.W. The genome of Eimeria spp., with special reference to eimeria tenella—A coccidium from the chicken. Int. J. Parasitol. 2000, 30, 485–493. [Google Scholar] [CrossRef]
- Kumar, S.; Garg, R.; Moftah, A.; Clark, E.L.; Macdonald, S.E.; Chaudhry, A.S.; Sparagano, O.; Banerjee, P.S.; Kundu, K.; Tomley, F.M.; et al. An optimised protocol for molecular identification of Eimeria from chickens. Vet. Parasitol. 2014, 199, 24–31. [Google Scholar] [CrossRef]
- Schwarz, R.S.; Jenkins, M.C.; Klopp, S.; Miska, K.B. Genomic analysis of Eimeria spp. Populations in relation to performance levels of broiler chicken farms in arkansas and north carolina. J. Parasitol. 2009, 95, 871–880. [Google Scholar] [CrossRef]
- Kundu, K.; Garg, R.; Kumar, S.; Mandal, M.; Tomley, F.M.; Blake, D.P.; Banerjee, P.S. Humoral and cytokine response elicited during immunisation with recombinant immune mapped protein-1 (etimp-1) and oocysts of Eimeria tenella. Vet. Parasitol. 2017, 244, 44–53. [Google Scholar] [CrossRef]
- Chengat Prakashbabu, B.; Thenmozhi, V.; Limon, G.; Kundu, K.; Kumar, S.; Garg, R.; Clark, E.L.; Srinivasa Rao, A.S.; Raj, D.G.; Raman, M.; et al. Eimeria species occurrence varies between geographic regions and poultry production systems and may influence parasite genetic diversity. Vet. Parasitol. 2017, 233, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Pegg, E.; Doyle, K.; Clark, E.L.; Jatau, I.D.; Tomley, F.M.; Blake, D.P. Application of a new PCR-RFLP panel suggests a restricted population structure for Eimeria tenella in UK and Irish chickens. Vet. Parasitol. 2016, 229, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, S.E.; Nolan, M.J.; Harman, K.; Boulton, K.; Hume, D.A.; Tomley, F.M.; Stabler, R.A.; Blake, D.P. Effects of Eimeria tenella infection on chicken caecal microbiome diversity, exploring variation associated with severity of pathology. PLoS ONE 2017, 12, e0184890. [Google Scholar] [CrossRef] [PubMed]
- Avramenko, R.W.; Redman, E.M.; Lewis, R.; Bichuette, M.A.; Palmeira, B.M.; Yazwinski, T.A.; Gilleard, J.S. The use of nemabiome metabarcoding to explore gastro-intestinal nematode species diversity and anthelmintic treatment effectiveness in beef calves. Int. J. Parasitol. 2017, 47, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Hemmink, J.D.; Weir, W.; MacHugh, N.D.; Graham, S.P.; Patel, E.; Paxton, E.; Shiels, B.; Toye, P.G.; Morrison, W.I.; Pelle, R. Limited genetic and antigenic diversity within parasite isolates used in a live vaccine against Theileria parva. Int. J. Parasitol. 2016, 46, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, E.T.; Lott, M.J.; Eldridge, M.D.; Power, M.L. Evaluation of next generation sequencing for the analysis of Eimeria communities in wildlife. J. Microbiol. Methods 2016, 124, 1–9. [Google Scholar] [CrossRef]
- Hinsu, A.T.; Thakkar, J.R.; Koringa, P.G.; Vrba, V.; Jakhesara, S.J.; Psifidi, A.; Guitian, J.; Tomley, F.M.; Rank, D.N.; Raman, M.; et al. Illumina next generation sequencing for the analysis of Eimeria populations in commercial broilers and indigenous chickens. Front. Vet. Sci. 2018, 5, 176. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, R.; Mishra, A.K.; Raoult, D.; Fournier, P.E. Genomics and metagenomics in medical microbiology. J. Microbiol. Methods 2013, 95, 415–424. [Google Scholar] [CrossRef]
- Tyzzer, E. Coccidiosis in gallinaceous birds. Am. J. Hyg. 1929, 10, 269–383. [Google Scholar] [CrossRef]
- Beach, J.; Corl, J. Studies in the control of avian coccidiosis. Poultry Sci. 1925, 4, 83–93. [Google Scholar] [CrossRef]
- Gilbert, M.; Conchedda, G.; Van Boeckel, T.P.; Cinardi, G.; Linard, C.; Nicolas, G.; Thanapongtharm, W.; D’Aietti, L.; Wint, W.; Newman, S.H.; et al. Income disparities and the global distribution of intensively farmed chicken and pigs. PLoS ONE 2015, 10, e0133381. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Woods, W.G.; Richards, D.G.; Gasser, R.B. Investigating a persistent coccidiosis problem on a commercial broiler-breeder farm utilising PCR-coupled capillary electrophoresis. Parasitol. Res. 2007, 101, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, R.S.; Khan, S.M.; Franke-Fayard, B.; Janse, C.J.; Obbard, D.J.; Reece, S.E. Hybridization and pre-zygotic reproductive barriers in Plasmodium. Proc. Biol. Sci. 2015, 282, 20143027. [Google Scholar] [CrossRef] [PubMed]
| Parasite Species | Parasite Strain | SequencinG Platform | Assembly Size (Mb) | No. Contigs/Supercontigs | Reference |
|---|---|---|---|---|---|
| E. acervulina | Houghton | Illumina HiSeq 2000 | 45.8 | 3415 | [10] |
| E. brunetti | Houghton | Illumina HiSeq 2000 | 66.9 | 8575 | [10] |
| E. maxima | Houghton | Sanger capillary and Roche GS-FLX 454 | 46.0 | 22,259 | [39] |
| Weybridge | Illumina HiSeq 2000 | 42.5 | 3564 | [10] | |
| E. mitis | Houghton | Illumina HiSeq 2000 | 72.2 | 15,978 | [10] |
| E. necatrix | Houghton | Illumina HiSeq 2000 | 55.0 | 3707 | [10] |
| E. praecox | Houghton | Illumina HiSeq 2000 | 60.1 | 21,348 | [10] |
| E. tenella | Houghton | Sanger capillary and Illumina GAIIx | 51.9 | 4664 | [10] |
| Nippon-2 | Illumina GAIIx | na | na | [10,41] | |
| Wisconsin | Illumina GAIIx | na | na | [10,41] | |
| E. falciformis | Bayer Haberkorn 1970 | Illumina GAIIx | 43.7 | 753 | [38] |
| E. nieschulzi | Landers | Illumina HiSeq 2000 | 63.0 | 33,467 | [3] |
| C. cayetanensis | CHN_HEN01 | Roche GS-FLX 454, Illumina GAIIx and Illumina HiSeq 2500 | 46.8 | 4811 | [19] |
| NF1_C8 | Illumina MiSeq | 44.4 | 738 | [42] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blake, D.P.; Worthing, K.; Jenkins, M.C. Exploring Eimeria Genomes to Understand Population Biology: Recent Progress and Future Opportunities. Genes 2020, 11, 1103. https://doi.org/10.3390/genes11091103
Blake DP, Worthing K, Jenkins MC. Exploring Eimeria Genomes to Understand Population Biology: Recent Progress and Future Opportunities. Genes. 2020; 11(9):1103. https://doi.org/10.3390/genes11091103
Chicago/Turabian StyleBlake, Damer P., Kate Worthing, and Mark C. Jenkins. 2020. "Exploring Eimeria Genomes to Understand Population Biology: Recent Progress and Future Opportunities" Genes 11, no. 9: 1103. https://doi.org/10.3390/genes11091103
APA StyleBlake, D. P., Worthing, K., & Jenkins, M. C. (2020). Exploring Eimeria Genomes to Understand Population Biology: Recent Progress and Future Opportunities. Genes, 11(9), 1103. https://doi.org/10.3390/genes11091103





