Two Novel Pathogenic Variants Confirm RMND1 Causative Role in Perrault Syndrome with Renal Involvement
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Nephrological and Neurological Examinations
2.3. Targeted HL Gene Panel, Data Analysis and Interpretation
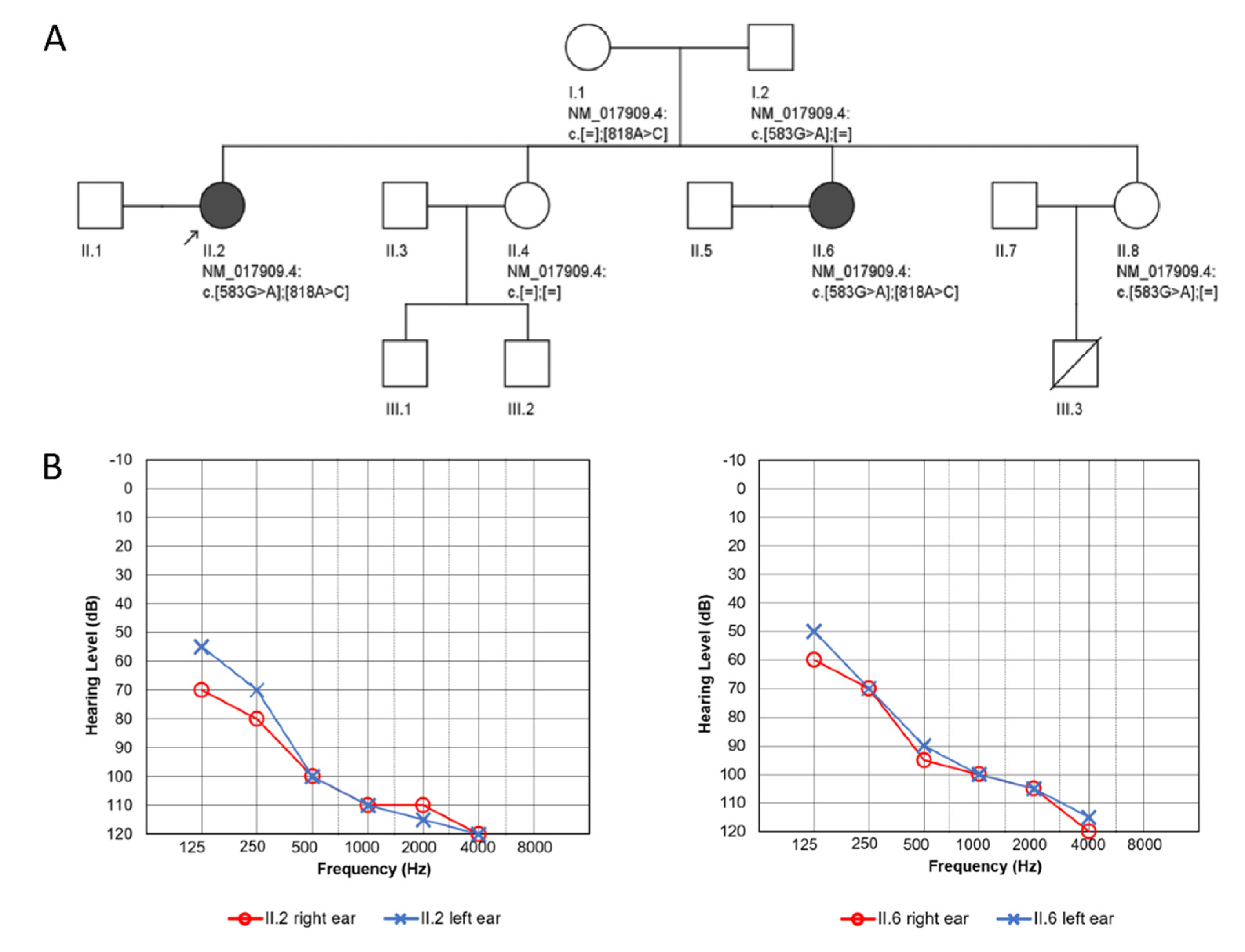
3. Results
3.1. Clinical Presentation
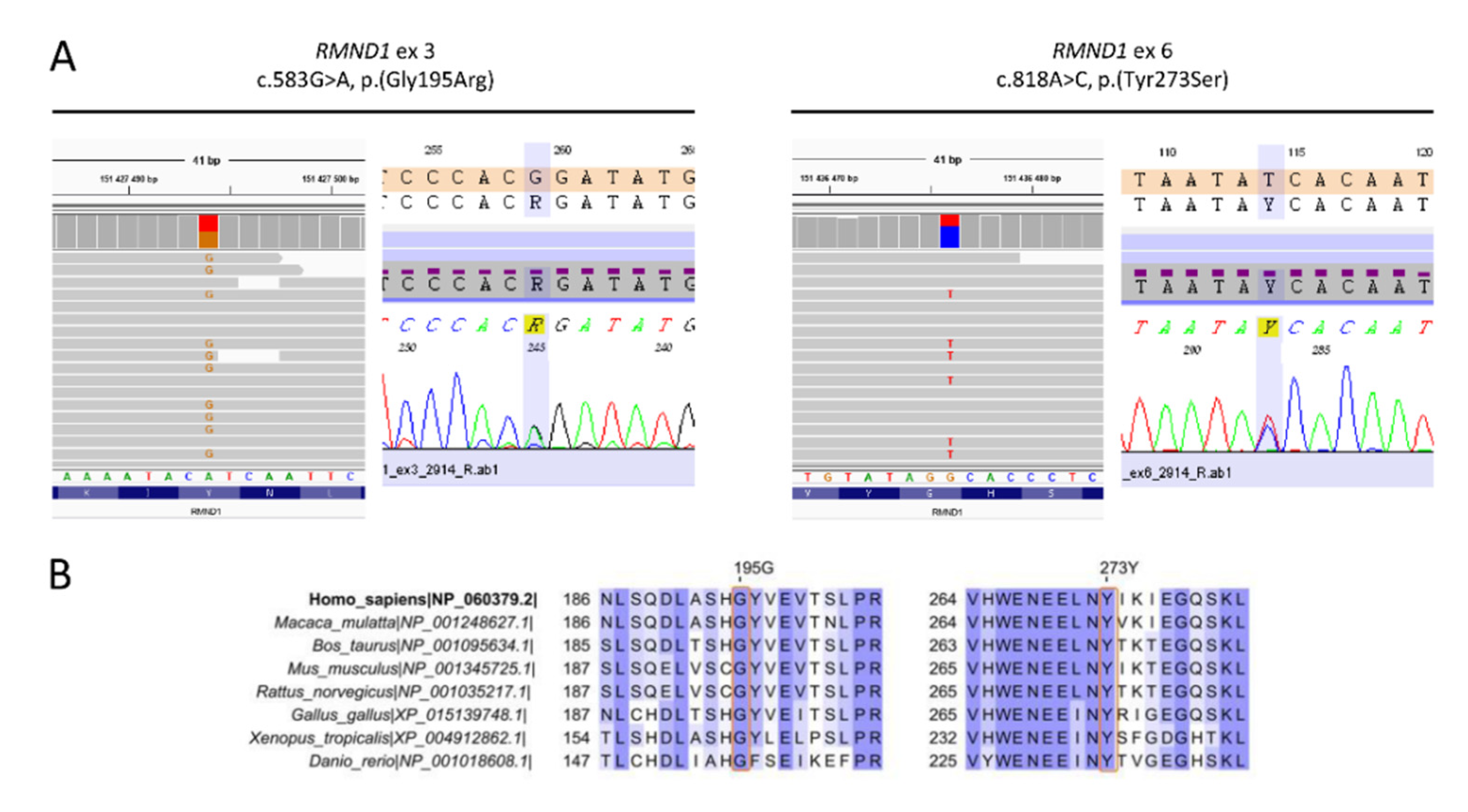
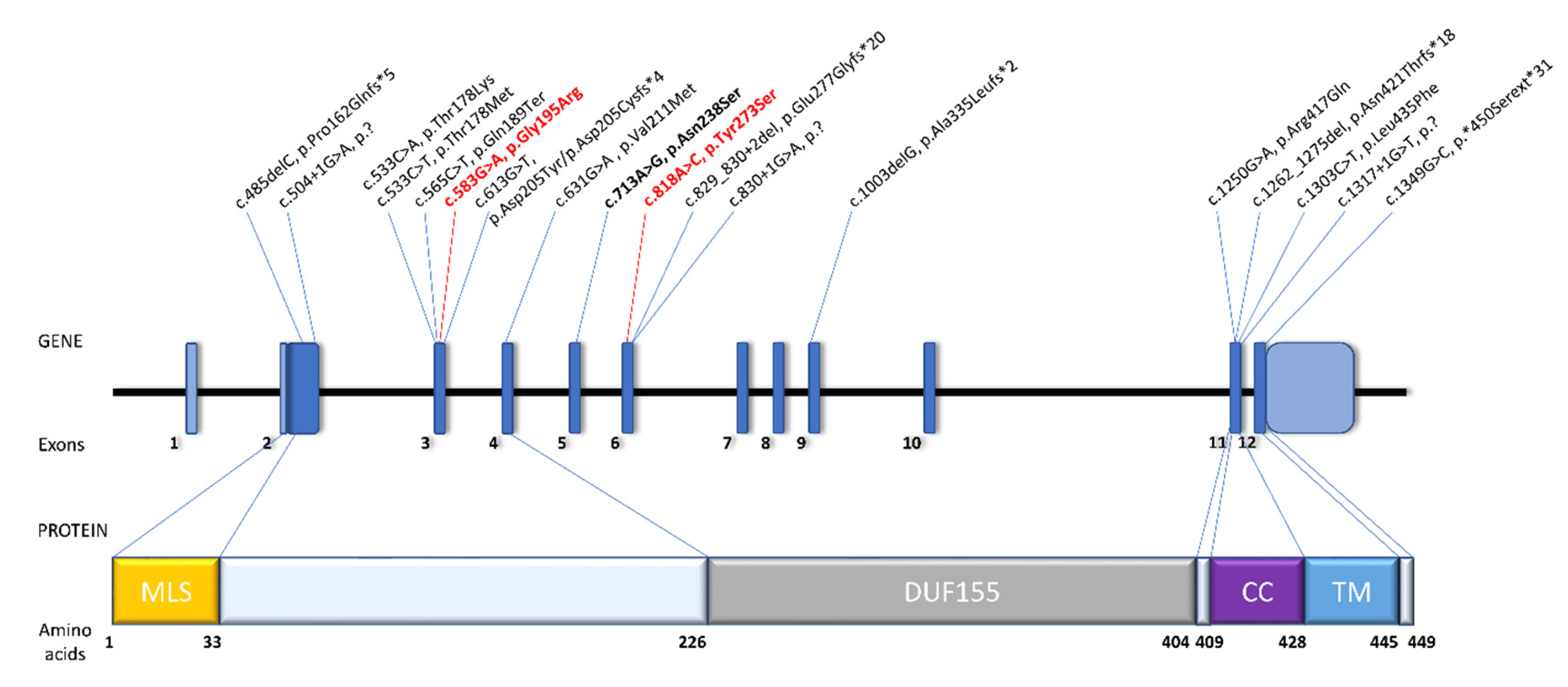
3.2. Identification of Pathogenic Variants
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garcia-Diaz, B.; Barros, M.H.; Sanna-Cherchi, S.; Emmanuele, V.; Akman, H.O.; Ferreiro-Barros, C.C.; Horvath, R.; Tadesse, S.; El Gharaby, N.; DiMauro, S.; et al. Infantile encephaloneuromyopathy and defective mitochondrial translation are due to a homozygous RMND1 mutation. Am. J. Hum. Genet. 2012, 91, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Janer, A.; Antonicka, H.; Lalonde, E.; Nishimura, T.; Sasarman, F.; Brown, G.K.; Brown, R.M.; Majewski, J.; Shoubridge, E.A. An RMND1 Mutation causes encephalopathy associated with multiple oxidative phosphorylation complex deficiencies and a mitochondrial translation defect. Am. J. Hum. Genet. 2012, 91, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Janer, A.; van Karnebeek, C.D.; Sasarman, F.; Antonicka, H.; Al Ghamdi, M.; Shyr, C.; Dunbar, M.; Stockler-Ispiroglu, S.; Ross, C.J.; Vallance, H.; et al. RMND1 deficiency associated with neonatal lactic acidosis, infantile onset renal failure, deafness, and multiorgan involvement. Eur. J. Hum. Genet. 2015, 23, 1301–1307. [Google Scholar] [CrossRef]
- Ng, Y.S.; Alston, C.L.; Diodato, D.; Morris, A.A.; Ulrick, N.; Kmoch, S.; Houstek, J.; Martinelli, D.; Haghighi, A.; Atiq, M.; et al. The clinical, biochemical and genetic features associated with RMND1-related mitochondrial disease. J. Med. Genet. 2016, 53, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Shayota, B.J.; Le, N.T.; Bekheirnia, N.; Rosenfeld, J.A.; Goldstein, A.C.; Moritz, M.; Bartholomew, D.W.; Pastore, M.T.; Xia, F.; Eng, C.; et al. Characterization of the renal phenotype in RMND1-related mitochondrial disease. Mol. Genet. Genomic Med. 2019, 7, e973. [Google Scholar] [CrossRef]
- Demain, L.A.M.; Antunes, D.; O’Sullivan, J.; Bhaskhar, S.S.; O’Keefe, R.T.; Newman, W.G. A known pathogenic variant in the essential mitochondrial translation gene RMND1 causes a Perrault-like syndrome with renal defects. Clin. Genet. 2018, 94, 276–277. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am. J. Kidney Dis. 2010, 55, 622–627. [Google Scholar] [CrossRef]
- Schmitz-Hubsch, T.; du Montcel, S.T.; Baliko, L.; Berciano, J.; Boesch, S.; Depondt, C.; Giunti, P.; Globas, C.; Infante, J.; Kang, J.S.; et al. Scale for the assessment and rating of ataxia: Development of a new clinical scale. Neurology 2006, 66, 1717–1720. [Google Scholar] [CrossRef]
- Schmitz-Hubsch, T.; Coudert, M.; Bauer, P.; Giunti, P.; Globas, C.; Baliko, L.; Filla, A.; Mariotti, C.; Rakowicz, M.; Charles, P.; et al. Spinocerebellar ataxia types 1, 2, 3, and 6: Disease severity and nonataxia symptoms. Neurology 2008, 71, 982–989. [Google Scholar] [CrossRef]
- Lohse, M.; Bolger, A.M.; Nagel, A.; Fernie, A.R.; Lunn, J.E.; Stitt, M.; Usadel, B. RobiNA: A user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Res. 2012, 40, W622–W627. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Faust, G.G.; Hall, I.M. SAMBLASTER: Fast duplicate marking and structural variant read extraction. Bioinformatics 2014, 30, 2503–2505. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Garrison, E.; Marth, G. Haplotype-based variant detection from short-read sequencing. arXiv Prepr. 2012, arXiv:1207.3907. [Google Scholar]
- Poplin, R.; Chang, P.C.; Alexander, D.; Schwartz, S.; Colthurst, T.; Ku, A.; Newburger, D.; Dijamco, J.; Nguyen, N.; Afshar, P.T.; et al. A universal SNP and small-indel variant caller using deep neural networks. Nat. Biotechnol. 2018, 36, 983–987. [Google Scholar] [CrossRef]
- Cibulskis, K.; Lawrence, M.S.; Carter, S.L.; Sivachenko, A.; Jaffe, D.; Sougnez, C.; Gabriel, S.; Meyerson, M.; Lander, E.S.; Getz, G. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 2013, 31, 213–219. [Google Scholar] [CrossRef]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef]
- Liu, X.; Wu, C.; Li, C.; Boerwinkle, E. dbNSFP v3.0: A One-Stop Database of Functional Predictions and Annotations for Human Nonsynonymous and Splice-Site SNVs. Hum. Mutat. 2016, 37, 235–241. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. bioRxiv 2020, 10, 531210. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Riley, G.R.; Jang, W.; Rubinstein, W.S.; Church, D.M.; Maglott, D.R. ClinVar: Public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014, 42, D980–D985. [Google Scholar] [CrossRef]
- Stenson, P.D.; Ball, E.V.; Mort, M.; Phillips, A.D.; Shiel, J.A.; Thomas, N.S.; Abeysinghe, S.; Krawczak, M.; Cooper, D.N. Human Gene Mutation Database (HGMD): 2003 update. Hum. Mutat. 2003, 21, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.; Jordan, D.M.; Sunyaev, S.R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013, 76, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009, 4, 1073–1081. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods 2014, 11, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.; Fay, J.C. Identification of deleterious mutations within three human genomes. Genome Res. 2009, 19, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.; Witten, D.M.; Jain, P.; O’Roak, B.J.; Cooper, G.M.; Shendure, J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014, 46, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Davydov, E.V.; Goode, D.L.; Sirota, M.; Cooper, G.M.; Sidow, A.; Batzoglou, S. Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLoS Comput. Biol. 2010, 6, e1001025. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, J.S.; Agarwala, R. COBALT: Constraint-based alignment tool for multiple protein sequences. Bioinformatics 2007, 23, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Oza, A.M.; DiStefano, M.T.; Hemphill, S.E.; Cushman, B.J.; Grant, A.R.; Siegert, R.K.; Shen, J.; Chapin, A.; Boczek, N.J.; Schimmenti, L.A.; et al. Expert specification of the ACMG/AMP variant interpretation guidelines for genetic hearing loss. Hum. Mutat. 2018, 39, 1593–1613. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.M.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 150. [Google Scholar] [CrossRef]
- Tucker, E.J.; Rius, R.; Jaillard, S.; Bell, K.; Lamont, P.J.; Travessa, A.; Dupont, J.; Sampaio, L.; Dulon, J.; Vuillaumier-Barrot, S.; et al. Genomic sequencing highlights the diverse molecular causes of Perrault syndrome: A peroxisomal disorder (PEX6), metabolic disorders (CLPP, GGPS1), and mtDNA maintenance/translation disorders (LARS2, TFAM). Hum. Genet. 2020. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Shimbo, T.; Horio, M.; Ando, M.; Yasuda, Y.; Komatsu, Y.; Masuda, K.; Matsuo, S.; Maruyama, S. Longitudinal Study of the Decline in Renal Function in Healthy Subjects. PLoS ONE 2015, 10, e0129036. [Google Scholar] [CrossRef] [PubMed]
- Broenen, E.; Ranchin, B.; Besmond, C.; Freychet, C.; Fouilhoux, A.; Perouse de Montclos, T.; Ville, D.; Bacchetta, J. RMND1 mutations in two siblings: Severe renal hypoplasia but different levels of extrarenal abnormality severity: The ethics of decision making. Arch. Pediatr. 2019, 26, 377–380. [Google Scholar] [CrossRef]
- Ravn, K.; Neland, M.; Wibrand, F.; Duno, M.; Ostergaard, E. Hearing impairment and renal failure associated with RMND1 mutations. Am. J. Med. Genet. Part A 2016, 170, 142–147. [Google Scholar] [CrossRef]
- Parikh, S.; Karaa, A.; Goldstein, A.; Ng, Y.S.; Gorman, G.; Feigenbaum, A.; Christodoulou, J.; Haas, R.; Tarnopolsky, M.; Cohen, B.K.; et al. Solid organ transplantation in primary mitochondrial disease: Proceed with caution. Mol. Genet. Metab. 2016, 118, 178–184. [Google Scholar] [CrossRef]
- Theunissen, T.E.; Szklarczyk, R.; Gerards, M.; Hellebrekers, D.M.; Mulder-Den Hartog, E.N.; Vanoevelen, J.; Kamps, R.; de Koning, B.; Rutledge, S.L.; Schmitt-Mechelke, T.; et al. Specific MRI Abnormalities Reveal Severe Perrault Syndrome due to CLPP Defects. Front. Neurol. 2016, 7, 203. [Google Scholar] [CrossRef]
- Lerat, J.; Jonard, L.; Loundon, N.; Christin-Maitre, S.; Lacombe, D.; Goizet, C.; Rouzier, C.; Van Maldergem, L.; Gherbi, S.; Garabedian, E.N.; et al. An Application of NGS for Molecular Investigations in Perrault Syndrome: Study of 14 Families and Review of the Literature. Hum. Mutat. 2016, 37, 1354–1362. [Google Scholar] [CrossRef]
- Dursun, F.; Mohamoud, H.S.; Karim, N.; Naeem, M.; Jelani, M.; Kirmizibekmez, H. A Novel Missense Mutation in the CLPP Gene Causing Perrault Syndrome Type 3 in a Turkish Family. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 472–477. [Google Scholar] [CrossRef]
- Demain, L.A.; Urquhart, J.E.; O’Sullivan, J.; Williams, S.G.; Bhaskar, S.S.; Jenkinson, E.M.; Lourenco, C.M.; Heiberg, A.; Pearce, S.H.; Shalev, S.A.; et al. Expanding the Genotypic Spectrum of Perrault syndrome. Clin. Genet. 2017, 91, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Jelani, M.; Alrayes, N.; Mohamoud, H.S.; Almramhi, M.M.; Anshasi, W.; Ahmed, N.A.; Wang, J.; Nasir, J.; Al-Aama, J.Y. Exome analysis identified a novel missense mutation in the CLPP gene in a consanguineous Saudi family expanding the clinical spectrum of Perrault Syndrome type-3. J. Neurol. Sci. 2015, 353, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, E.M.; Rehman, A.U.; Walsh, T.; Clayton-Smith, J.; Lee, K.; Morell, R.J.; Drummond, M.C.; Khan, S.N.; Naeem, M.A.; Rauf, B.; et al. Perrault syndrome is caused by recessive mutations in CLPP, encoding a mitochondrial ATP-dependent chambered protease. Am. J. Hum. Genet. 2013, 92, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Chatzispyrou, I.A.; Alders, M.; Guerrero-Castillo, S.; Zapata Perez, R.; Haagmans, M.A.; Mouchiroud, L.; Koster, J.; Ofman, R.; Baas, F.; Waterham, H.R.; et al. A homozygous missense mutation in ERAL1, encoding a mitochondrial rRNA chaperone, causes Perrault syndrome. Hum. Mol. Genet. 2017, 26, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Foley, A.R.; Zou, Y.; Dunford, J.E.; Rooney, J.; Chandra, G.; Xiong, H.; Straub, V.; Voit, T.; Romero, N.; Donkervoort, S.; et al. GGPS1 Mutations Cause Muscular Dystrophy/Hearing Loss/Ovarian Insufficiency Syndrome. Ann. Neurol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Karstensen, H.G.; Rendtorff, N.D.; Hindbaek, L.S.; Colombo, R.; Stein, A.; Birkebaek, N.H.; Hartmann-Petersen, R.; Lindorff-Larsen, K.; Hojland, A.T.; Petersen, M.B.; et al. Novel HARS2 missense variants identified in individuals with sensorineural hearing impairment and Perrault syndrome. Eur. J. Med. Genet. 2020, 63, 103733. [Google Scholar] [CrossRef]
- Pierce, S.B.; Chisholm, K.M.; Lynch, E.D.; Lee, M.K.; Walsh, T.; Opitz, J.M.; Li, W.; Klevit, R.E.; King, M.C. Mutations in mitochondrial histidyl tRNA synthetase HARS2 cause ovarian dysgenesis and sensorineural hearing loss of Perrault syndrome. Proc. Natl. Acad. Sci. USA 2011, 108, 6543–6548. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.J.; Kim, J.; Chae, H.; Kim, M.; Kim, Y. Genotype and phenotype heterogeneity in perrault syndrome. J. Pediatr. Adolesc. Gynecol. 2013, 26, e25–e27. [Google Scholar] [CrossRef]
- Pierce, S.B.; Walsh, T.; Chisholm, K.M.; Lee, M.K.; Thornton, A.M.; Fiumara, A.; Opitz, J.M.; Levy-Lahad, E.; Klevit, R.E.; King, M.C. Mutations in the DBP-deficiency protein HSD17B4 cause ovarian dysgenesis, hearing loss, and ataxia of Perrault Syndrome. Am. J. Hum. Genet. 2010, 87, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Lieber, D.S.; Hershman, S.G.; Slate, N.G.; Calvo, S.E.; Sims, K.B.; Schmahmann, J.D.; Mootha, V.K. Next generation sequencing with copy number variant detection expands the phenotypic spectrum of HSD17B4-deficiency. BMC Med. Genet. 2014, 15, 30. [Google Scholar] [CrossRef]
- Chen, K.; Yang, K.; Luo, S.S.; Chen, C.; Wang, Y.; Wang, Y.X.; Li, D.K.; Yang, Y.J.; Tang, Y.L.; Liu, F.T.; et al. A homozygous missense variant in HSD17B4 identified in a consanguineous Chinese Han family with type II Perrault syndrome. BMC Med. Genet. 2017, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.B.; Gersak, K.; Michaelson-Cohen, R.; Walsh, T.; Lee, M.K.; Malach, D.; Klevit, R.E.; King, M.C.; Levy-Lahad, E. Mutations in LARS2, encoding mitochondrial leucyl-tRNA synthetase, lead to premature ovarian failure and hearing loss in Perrault syndrome. Am. J. Hum. Genet. 2013, 92, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Solda, G.; Caccia, S.; Robusto, M.; Chiereghin, C.; Castorina, P.; Ambrosetti, U.; Duga, S.; Asselta, R. First independent replication of the involvement of LARS2 in Perrault syndrome by whole-exome sequencing of an Italian family. J. Hum. Genet. 2016, 61, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Kosaki, R.; Horikawa, R.; Fujii, E.; Kosaki, K. Biallelic mutations in LARS2 can cause Perrault syndrome type 2 with neurologic symptoms. Am. J. Med. Genet. A 2018, 176, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Carminho-Rodrigues, M.T.; Klee, P.; Laurent, S.; Guipponi, M.; Abramowicz, M.; Cao-van, H.; Guinand, N.; Paoloni-Giacobino, A. LARS2-Perrault syndrome: A new case report and literature review. BMC Med. Genet. 2020, 21, 109. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaroudi, D.; Enabi, S.; AlThagafi, M.S. Perrault syndrome with amenorrhea, infertility, Tarlov cyst, and degenerative disc. Gynecol. Endocrinol. 2019, 35, 1037–1039. [Google Scholar] [CrossRef]
- Riley, L.G.; Rudinger-Thirion, J.; Frugier, M.; Wilson, M.; Luig, M.; Alahakoon, T.I.; Nixon, C.Y.; Kirk, E.P.; Roscioli, T.; Lunke, S.; et al. The expanding LARS2 phenotypic spectrum: HLASA, Perrault syndrome with leukodystrophy, and mitochondrial myopathy. Hum. Mutat. 2020, 41, 1425–1434. [Google Scholar] [CrossRef]
- Pan, Z.; Xu, H.; Tian, Y.; Liu, D.; Liu, H.; Li, R.; Dou, Q.; Zuo, B.; Zhai, R.; Tang, W.; et al. Perrault syndrome: Clinical report and retrospective analysis. Mol. Genet. Genomic Med. 2020, e1445. [Google Scholar] [CrossRef]
- van der Knaap, M.S.; Bugiani, M.; Mendes, M.I.; Riley, L.G.; Smith, D.E.C.; Rudinger-Thirion, J.; Frugier, M.; Breur, M.; Crawford, J.; van Gaalen, J.; et al. Biallelic variants in LARS2 and KARS cause deafness and (ovario)leukodystrophy. Neurology 2019, 92, e1225–e1237. [Google Scholar] [CrossRef]
- Cherot, E.; Keren, B.; Dubourg, C.; Carre, W.; Fradin, M.; Lavillaureix, A.; Afenjar, A.; Burglen, L.; Whalen, S.; Charles, P.; et al. Using medical exome sequencing to identify the causes of neurodevelopmental disorders: Experience of 2 clinical units and 216 patients. Clin. Genet. 2018, 93, 567–576. [Google Scholar] [CrossRef]
- Gotta, F.; Lamp, M.; Geroldi, A.; Trevisan, L.; Origone, P.; Fugazza, G.; Fabbri, S.; Nesti, C.; Rubegni, A.; Morani, F.; et al. A novel mutation of Twinkle in Perrault syndrome: A not rare diagnosis? Ann. Hum. Genet. 2020, 84, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Oldak, M.; Ozieblo, D.; Pollak, A.; Stepniak, I.; Lazniewski, M.; Lechowicz, U.; Kochanek, K.; Furmanek, M.; Tacikowska, G.; Plewczynski, D.; et al. Novel neuro-audiological findings and further evidence for TWNK involvement in Perrault syndrome. J. Transl. Med. 2017, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Ruiz, M.; Garcia-Martinez, A.; Corral-Juan, M.; Perez-Alvarez, A.I.; Plasencia, A.M.; Villamar, M.; Moreno-Pelayo, M.A.; Matilla-Duenas, A.; Menendez-Gonzalez, M.; Del Castillo, I. Perrault syndrome with neurological features in a compound heterozygote for two TWNK mutations: Overlap of TWNK-related recessive disorders. J. Transl. Med. 2019, 17, 290. [Google Scholar] [CrossRef] [PubMed]
- Fekete, B.; Pentelenyi, K.; Rudas, G.; Gal, A.; Grosz, Z.; Illes, A.; Idris, J.; Csukly, G.; Domonkos, A.; Molnar, M.J. Broadening the phenotype of the TWNK gene associated Perrault syndrome. BMC Med. Genet. 2019, 20, 198. [Google Scholar] [CrossRef]
- Morino, H.; Pierce, S.B.; Matsuda, Y.; Walsh, T.; Ohsawa, R.; Newby, M.; Hiraki-Kamon, K.; Kuramochi, M.; Lee, M.K.; Klevit, R.E.; et al. Mutations in Twinkle primase-helicase cause Perrault syndrome with neurologic features. Neurology 2014, 83, 2054–2061. [Google Scholar] [CrossRef]
| RBC (T/L) | Hb (g/dL) | Creatinine (mg/dL) | eGFR CKD EPI (mL/min) | Acid Base Venous Balance | Blood Lipids (mg/dL) | Calcium (mg/dL) | Phosphates (mg/dL) | PTH (pg/mL) | UACR (mg/g) | |
|---|---|---|---|---|---|---|---|---|---|---|
| proband | 4.49 (4.2–6.3) | 13.3 (12–16) | 1.53 (0.6–1.3) | 41 (>90) | pH 7.31 (7.35–7.45) HCO3− 20.7 (22–26 mmol/L) BE −1.7 (−2 to +2mmol/l) Anion gap 12 (12 ± 4 mEq/L) Cl− 105 (98–106 mmol/L) Lactic acid 1.2 (0.5–1.6 mmol/L) K+ 5.4 (3.4–4.5 mEq/L) Na+ 137 (136–146 mEq/L) | T chol 159 (<190) HDL 71 (35–65) LDL 72 (<115) TG 76 (<150) | 9.2 (8.5–10.1) | 3.6 (2.5–4.9) | 72.6 (12–68.3) | 2.9 (<30) |
| proband’s sister | 4.08 (4.2–6.3) | 12.4 (12–16) | 1.38 (0.6–1.3) | 49 (>90) | pH 7.35 (7.35–7.45) HCO3− 22 (22–26 mmol/L) BE −0.6 (−2 to +2mmol/L) Anion gap 8.9 (12 ± 4 mEq/L) Cl− 105 (98–106 mmol/L) Lactic acid 1.7 (0.5–1.6 mmol/L) K+ 5.2 (3.4–4.5 mEq/L) Na+ 138 (136–146 mEq/L) | T chol 213 (<190) HDL 68 (35–65)LDL 145 (<115) TG 57 (<150) | 9.4 (8.5–10.1) | 3.6 (2.5–4.9) | 148.8 (12–68.3) | 6.5 (<30) |
| Variant cDNA Level | Variant Protein Level | Reference SNP ID | Population Frequencies | Pathogenicity Predictions | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| gnomAD | 1000 Genomes | ESP 6500 | SIFT | PolyPhen-2 | Mutation Taster | LRT | CADD | ACMG Classification * | |||
| c.583G>A | p.(Gly195Arg) | rs776083030 | 0.00002388 (6/251308) | 0 | 0 | D (0.011) | PD (0.997) | D (1) | D (0) | D (29.7) | LP (PM2, PP1_M, PP3, PP4) |
| c.818A>C | p.(Tyr273Ser) | rs766739125 | 0.00000399 (1/250612) | 0 | 0 | D (0) | PD (1) | D (0.99) | N (0.001742) | D (26.4) | LP (PM2, PP1_M, PP3, PP4) |
| Gene (Locus) | Protein | Subcellular Localization | Function | Additional Clinical Features * | Inheritance Mode | Ref. |
|---|---|---|---|---|---|---|
| CLPP (19p13.3) | caseinolytic mitochondrial matrix peptidase proteolytic subunit | mitochondrial | mitochondrial protein degradation (component of a proteolytic complex) |
| AR | [33,38,39,40,41,42,43] |
| ERAL1 (17q11.2) | Era like 12S mitochondrial rRNA chaperone 1 | mitochondrial | mitochondrial protein translation (assembly of mitochondrial ribosomal subunit) |
| AR | [44] |
| GGPS1 (1q42.3) | geranylgeranyl diphosphate synthase 1 | cytoplasmic | acts on peroxisomal products, part of mevalonate pathway |
| AR | [33,45] |
| HARS2 (5q31.3) | histidyl-tRNA synthetase 2 | mitochondrial | mitochondrial protein translation (synthesis of histidyl-transfer RNA) |
| AR | [39,46,47] |
| HSD17B4 (17q21.2) | hydroxysteroid 17-β dehydrogenase 4 | peroxisomal | β-oxidation pathway for fatty acids in peroxisomes |
| AR | [39,41,48,49,50,51] |
| LARS2 (3p21.31) | leucyl-tRNA synthetase | mitochondrial | mitochondrial protein translation (synthesis of leucyl-transfer RNA) |
| AR | [33,39,41,52,53,54,55,56,57,58,59,60] |
| PEX6 (6p21.1) | peroxisomal biogenesis factor 6 | peroxisomal | peroxisomal protein import (ATPase activity) |
| AR | [33] |
| RMND1 (6q25.1) | required for meiotic nuclear division 1 homolog | mitochondrial | mitochondrial protein translation |
| AR | [6] Present study |
| TFAM (10q21.1) | transcription factor A, mitochondrial | mitochondrial | key mitochondrial transcription factor |
| AR | [33] |
| TWNK (10q24.31) | twinkle mtDNA helicase | mitochondrial | mitochondrial DNA replication and transcription (unwinds double-stranded DNA) |
| AR | [39,41,61,62,63,64,65] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oziębło, D.; Pazik, J.; Stępniak, I.; Skarżyński, H.; Ołdak, M. Two Novel Pathogenic Variants Confirm RMND1 Causative Role in Perrault Syndrome with Renal Involvement. Genes 2020, 11, 1060. https://doi.org/10.3390/genes11091060
Oziębło D, Pazik J, Stępniak I, Skarżyński H, Ołdak M. Two Novel Pathogenic Variants Confirm RMND1 Causative Role in Perrault Syndrome with Renal Involvement. Genes. 2020; 11(9):1060. https://doi.org/10.3390/genes11091060
Chicago/Turabian StyleOziębło, Dominika, Joanna Pazik, Iwona Stępniak, Henryk Skarżyński, and Monika Ołdak. 2020. "Two Novel Pathogenic Variants Confirm RMND1 Causative Role in Perrault Syndrome with Renal Involvement" Genes 11, no. 9: 1060. https://doi.org/10.3390/genes11091060
APA StyleOziębło, D., Pazik, J., Stępniak, I., Skarżyński, H., & Ołdak, M. (2020). Two Novel Pathogenic Variants Confirm RMND1 Causative Role in Perrault Syndrome with Renal Involvement. Genes, 11(9), 1060. https://doi.org/10.3390/genes11091060







