MiR-184 Combined with STC2 Promotes Endometrial Epithelial Cell Apoptosis in Dairy Goats via RAS/RAF/MEK/ERK Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Cell Culture
2.2. Cell Treatment and Total RNA Extraction
2.3. Luciferase Activity Assay
2.4. RT-qPCR
2.5. Protein Extraction and Western Blot (WB) Analysis
2.6. Vector Construction
2.7. Cell Proliferation Analysis
2.8. Cell Apoptosis Assay
2.9. Statistical Analysis
3. Results
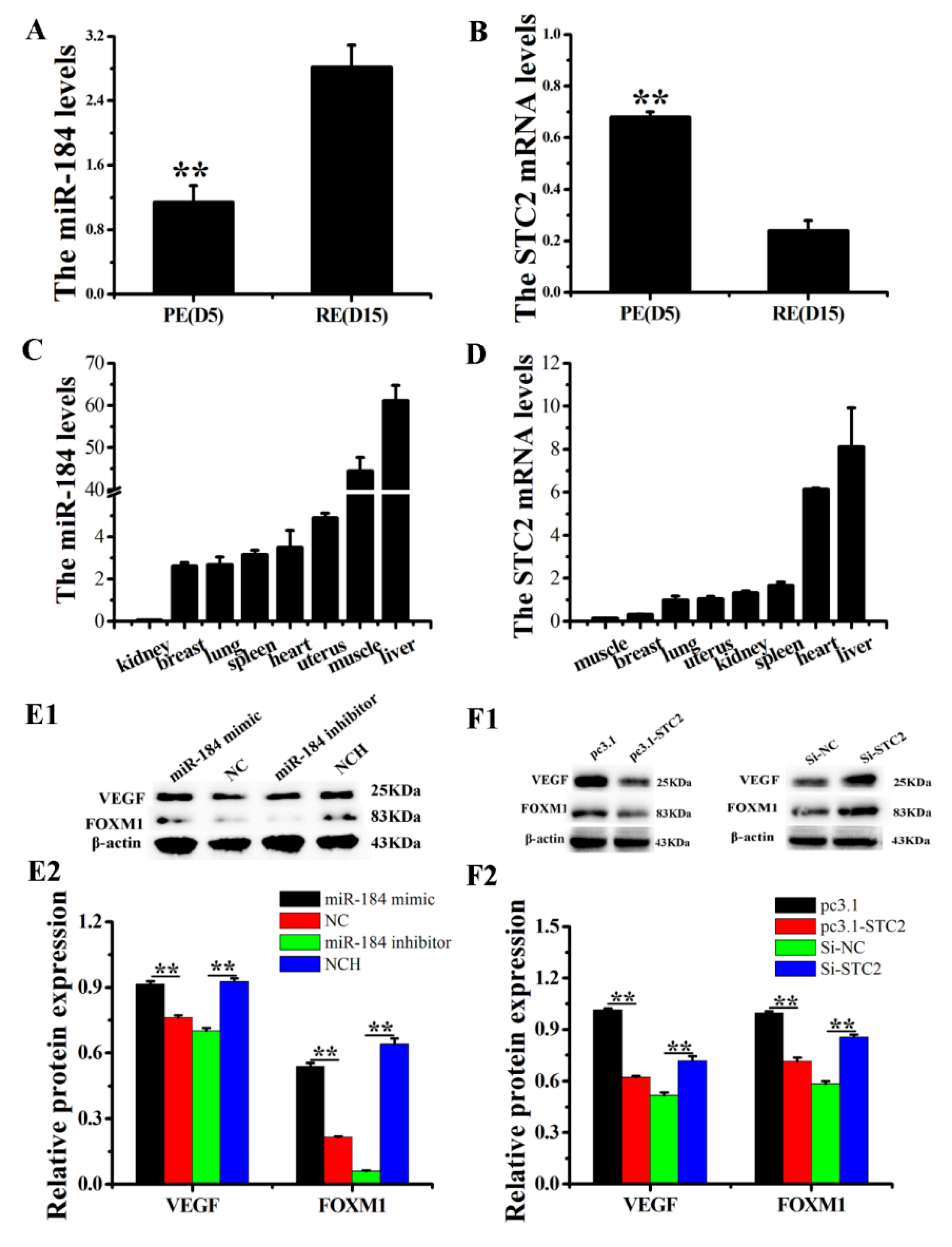
3.1. Differential Expression of miR-184 and STC2 in PE and RE Endometrial Tissues
3.2. miR-184 and STC2 Regulate the Expression of Some RE Markers in Dairy Goats
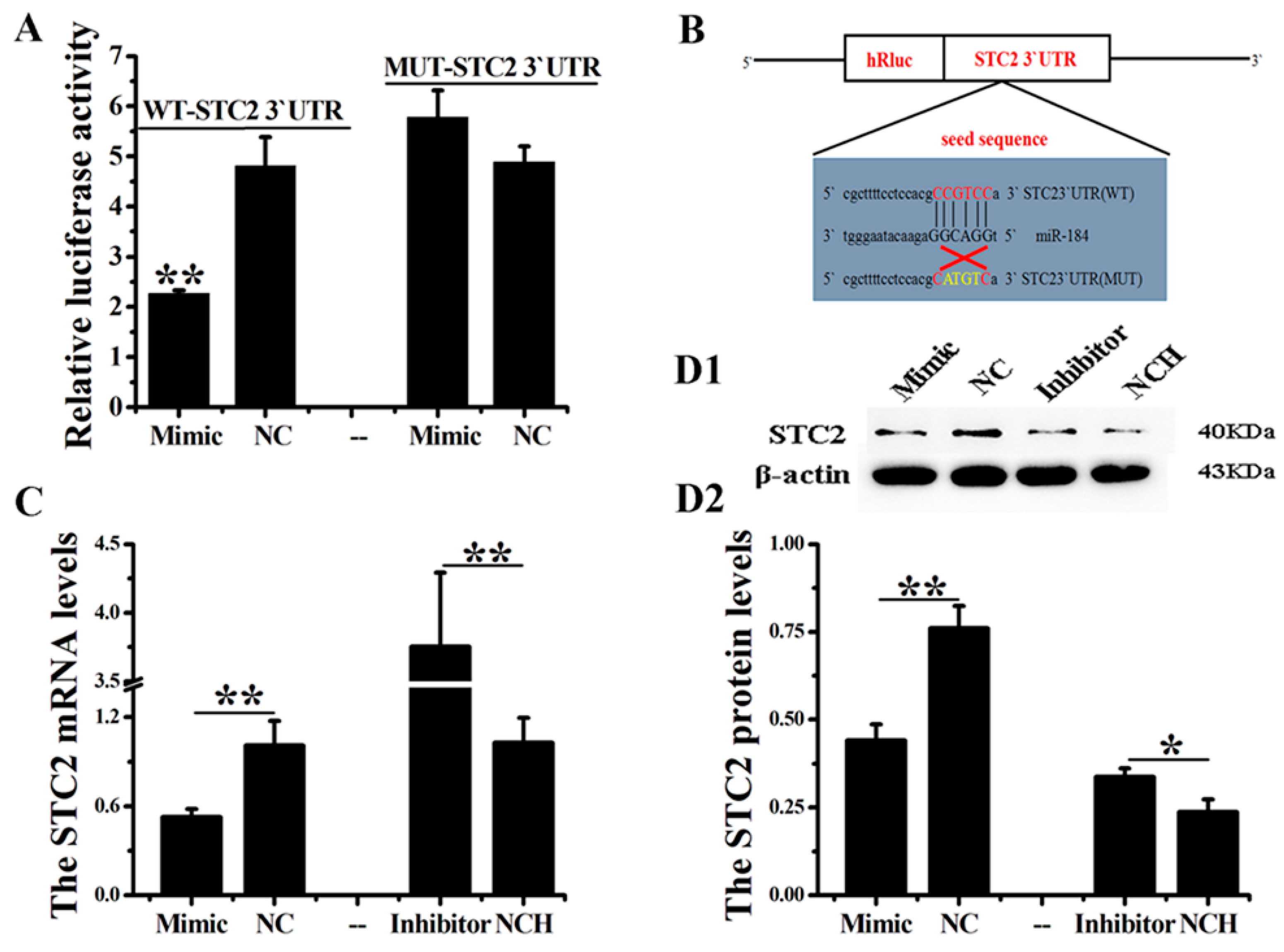
3.3. STC2 Is the Target of miR-184
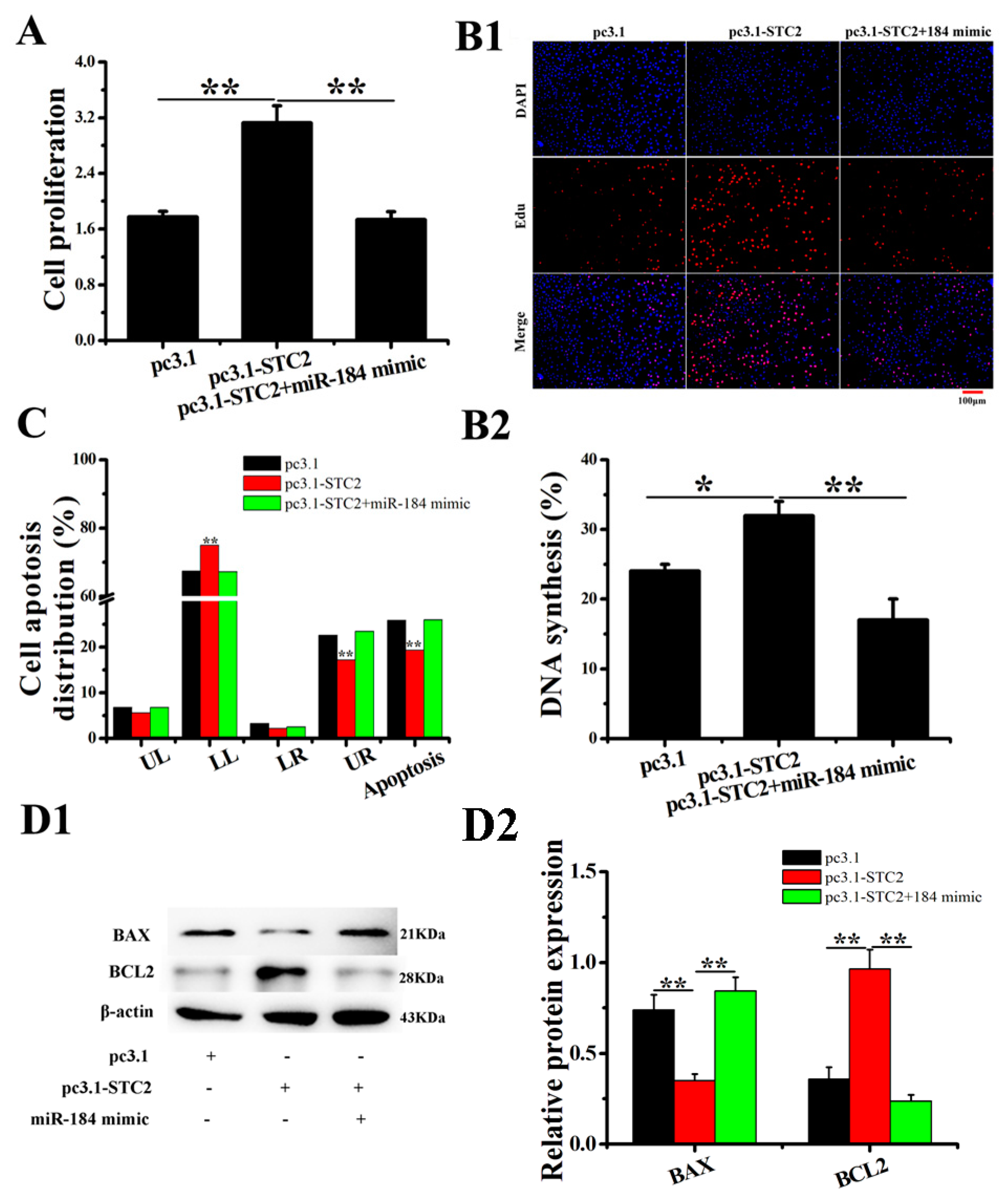
3.4. miR-184 Inhibits the Proliferation and Promotes EECs Apoptosis
3.5. The Effect of STC2 on EECs
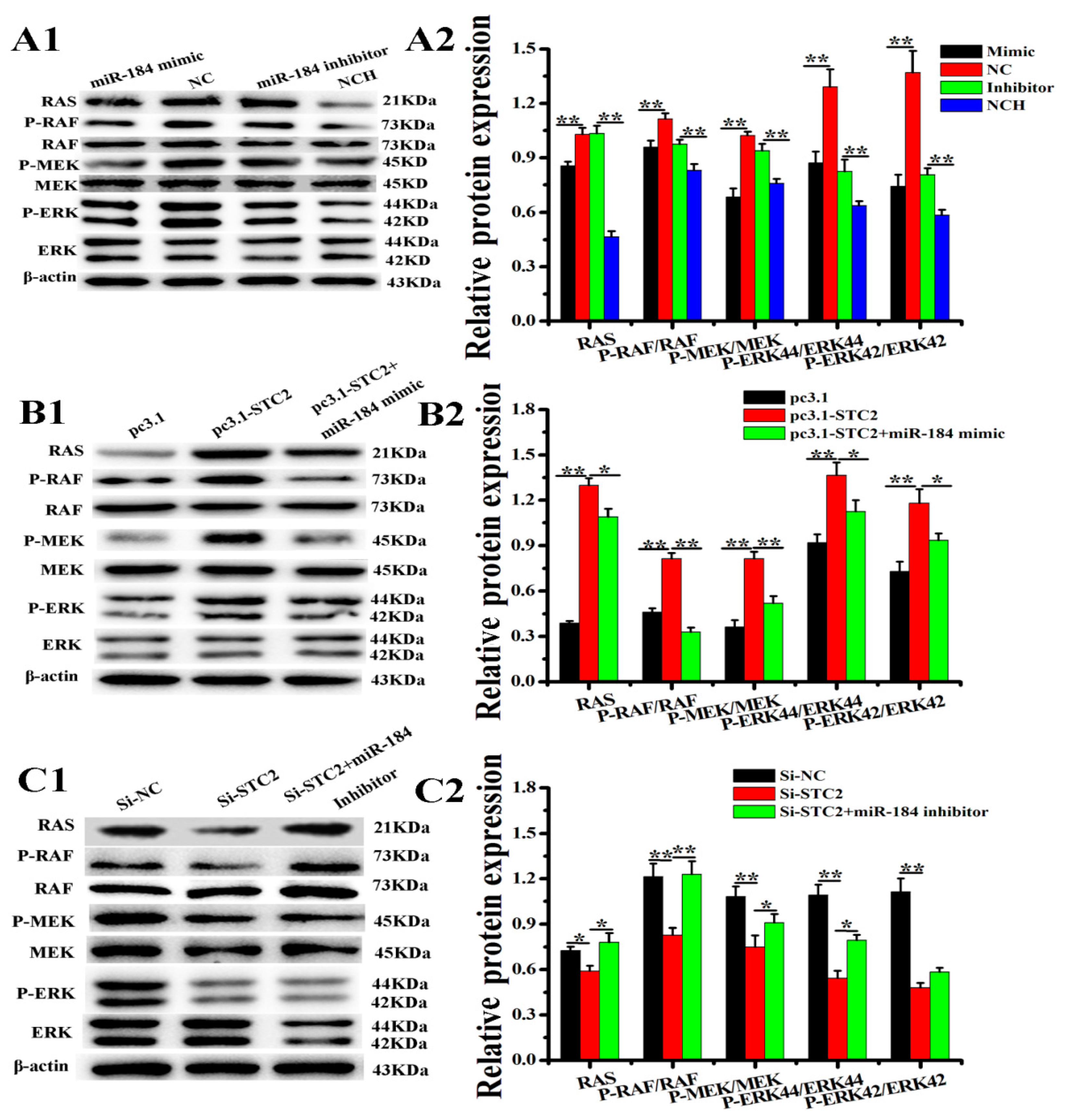
3.6. miR-184 Regulates the RAS/RAF/MEK/ERK Pathway Through the STC2 in EECs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, S.J.; Ren, G.; Liu, J.L.; Zhao, Z.A.; Yu, Y.S.; Su, R.W.; Ma, X.H.; Ni, H.; Lei, W.; Yang, Z.M. MicroRNA expression and regulation in mouse uterus during embryo implantation. J. Biol. Chem. 2008, 283, 23473–23484. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.M.; Tsai, J.H.; Moley, K.H. Obesity and PCOS. The effect of metabolic derangements on endometrial receptivity at the time of implantation. Reprod. Sci. 2015, 22, 6–14. [Google Scholar] [CrossRef]
- Achache, H.; Revel, A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum. Reprod. Update 2006, 12, 731–746. [Google Scholar] [CrossRef]
- Diedrich, K.; Fauser, B.C.; Devroey, P.; Griesinger, G. Evian Annual Reproduction Workshop G: The role of the endometrium and embryo in human implantation. Hum. Reprod. Update 2007, 13, 365–377. [Google Scholar] [CrossRef]
- Galan, A.; O’Connor, J.E.; Valbuena, D.; Herrer, R.; Remohi, J.; Pampfer, S.; Pellicer, A.; Simon, C. The human blastocyst regulates endometrial epithelial apoptosis in embryonic adhesion. Biol. Reprod. 2000, 63, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Bidarimath, M.; Khalaj, K.; Wessels, J.M.; Tayade, C. MicroRNAs, immune cells and pregnancy. Cell Mol. Immunol. 2014, 11, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, S.; Wang, Z. Role of microRNAs in embryo implantation. Reprod. Biol. Endocrinol. 2017, 15, 90. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Liu, J.; Ma, X.; Zhou, Z.; Song, Y.; Cao, B. miR-26a promoted endometrial epithelium cells (EECs) proliferation and induced stromal cells (ESCs) apoptosis via the PTEN-PI3K/AKT pathway in dairy goats. J. Cell Physiol. 2018, 233, 4688–4706. [Google Scholar] [CrossRef]
- An, X.; Liu, X.; Zhang, L.; Liu, J.; Zhao, X.; Chen, K.; Ma, H.; Li, G.; Cao, B.; Song, Y. MiR-449a regulates caprine endometrial stromal cell apoptosis and endometrial receptivity. Sci. Rep. 2017, 7, 12248. [Google Scholar] [CrossRef]
- Huang, W.; Huang, F.; Lei, Z.; Luo, H. LncRNA SNHG11 Promotes, Proliferation, Migration, Apoptosis, and Autophagy by Regulating hsa-miR-184/AGO2 in HCC. OncoTargets Ther. 2020, 13, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Dong, L. Inhibitory effect of miR-184 on the potential of proliferation and invasion in human glioma and breast cancer cells in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 9376–9382. [Google Scholar] [PubMed]
- Li, Q.; Zhou, X.; Fang, Z.; Pan, Z. Effect of STC2 gene silencing on colorectal cancer cells. Mol. Med. Rep. 2019, 20, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xu, X.; He, S.; Zhang, J.; Wang, X.; Wu, P.; Liu, J.; Jiang, H.; Zheng, M.; Li, W.; et al. STC2 modulates ERK1/2 signaling to suppress adipogenic differentiation of human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2020, 524, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, T.Y.; Su, S.C.; Yu, J.S.; Zhang, H.L.; Tan, G.Q.; Liu, J.W.; Wang, B.L. STC2 as a novel mediator for Mus81-dependent proliferation and survival in hepatocellular carcinoma. Cancer Lett. 2017, 388, 177–186. [Google Scholar] [CrossRef]
- Minchenko, D.O.; Kharkova, A.P.; Halkin, O.V.; Karbovskyi, L.L.; Minchenko, O.H. Effect of hypoxia on the expression of genes encoding insulin-like growth factors and some related proteins in U87 glioma cells without IRE1 function. Endocr. Regul. 2016, 50, 43–54. [Google Scholar] [CrossRef]
- Al-Khafaji, H.; Noer, P.R.; Alkharobi, H.; Alhodhodi, A.; Meade, J.; El-Gendy, R.; Oxvig, C.; Beattie, J. A characteristic signature of insulin-like growth factor (IGF) axis expression during osteogenic differentiation of human dental pulp cells (hDPCs): Potential co-ordinated regulation of IGF action. Growth Horm. IGF Res. 2018, 42, 14–21. [Google Scholar] [CrossRef]
- Yang, S.W.; Ji, Q.H.; Chang, B.; Wang, Y.; Zhu, Y.X.; Li, D.S.; Huang, C.P.; Wang, Y.L.; Sun, G.H.; Zhang, L.; et al. STC2 promotes head and neck squamous cell carcinoma metastasis through modulating the PI3K/AKT/Snail signaling. Oncotarget 2017, 8, 5976–5991. [Google Scholar] [CrossRef]
- Song, Y.; An, X.; Zhang, L.; Fu, M.; Peng, J.; Han, P.; Hou, J.; Zhou, Z.; Cao, B. Identification and profiling of microRNAs in goat endometrium during embryo implantation. PLoS ONE 2015, 10, e0122202. [Google Scholar] [CrossRef]
- Igwebuike, U.M. A review of uterine structural modifications that influence conceptus implantation and development in sheep and goats. Anim. Reprod. Sci. 2009, 112, 1–7. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Liu, J.; Zhou, Z.; Song, Y.; Cao, B.; An, X. miR-182 aids in receptive endometrium development in dairy goats by down-regulating, P.TN expression. PLoS ONE 2017, 12, e0179783. [Google Scholar]
- Liu, X.; Zhang, L.; Liu, Y.; Cui, J.; Che, S.; An, X.; Song, Y.; Cao, B. Circ-8073 regulates CEP55 by sponging miR-449a to promote caprine endometrial epithelial cells proliferation via the PI3K/AKT/mTOR pathway. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1130–1147. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, K.S.; Omar, I.S.; Kwong, S.C.; Mohamed, Z.; Woo, Y.L.; Adenan, N.A.M.; Chung, I. Cancer-associated fibroblasts promote endometrial cancer growth via activation of interleukin-6/STAT-3/c-Myc pathway. Am. J. Cancer Res. 2016, 6, 200–213. [Google Scholar] [PubMed]
- Li, H.; Yang, J.; Wei, X.; Song, C.; Dong, D.; Huang, Y.; Lan, X.; Plath, M.; Lei, C.; Ma, Y.; et al. CircFUT10 reduces proliferation and facilitates differentiation of myoblasts by sponging miR-133a. J. Cell Physiol. 2018, 233, 4643–4651. [Google Scholar] [CrossRef]
- Luo, Z.; Rong, Z.; Zhang, J.; Zhu, Z.; Yu, Z.; Li, T.; Fu, Z.; Qiu, Z.; Huang, C. Circular RNA circCCDC9 acts as a miR-6792-3p sponge to suppress the progression of gastric cancer through regulating CAV1 expression. Mol. Cancer 2020, 19, 86. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, J.; Zhang, M.; Seleh-Zo, E.; Wang, J.; Cao, B.; An, X. Circ-016910 sponges miR-574-5p to regulate cell physiology and milk synthesis via MAPK and PI3K/AKT-mTOR pathways in GMECs. J. Cell Physiol. 2020, 235, 4198–4216. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Che, S.; Cui, J.; Ma, X.; An, X.; Cao, B.; Song, Y. Endometrial Epithelial Cell Apoptosis Is Inhibited by a ciR8073-miR181a-Neurotensis Pathway during Embryo Implantation. Mol. Ther. Nucleic Acids 2019, 14, 262–273. [Google Scholar]
- Zhang, Y.; Zhou, J.; Li, M.Q.; Xu, J.; Zhang, J.P.; Jin, L.P. MicroRNA-184 promotes apoptosis of trophoblast cells via targeting WIG1 and induces early spontaneous abortion. Cell Death Dis. 2019, 10, 223. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.R.; Han, P.; Zhou, Z.Q.; Cao, B.Y.; Song, Y.X. The Study of Endometrium at Gestational Days 5 and 15 in Dairy Goats (Capra hircus). Czech J. Anim. Sci. 2017, 62, 357–368. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Yang, L.; Cui, J.; Che, S.; Liu, Y.; Han, J.; An, X.; Cao, B.; Song, Y. miR-34a/c induce caprine endometrial epithelial cell apoptosis by regulating circ-8073/CEP55 via the RAS/RAF/MEK/ERK and PI3K/AKT/mTOR pathways. J. Cell Physiol. 2020. [Google Scholar] [CrossRef]
- Zhen, Y.; Liu, Z.; Yang, H.; Yu, X.; Wu, Q.; Hua, S.; Long, X.; Jiang, Q.; Song, Y.; Cheng, C.; et al. Tumor suppressor PDCD4 modulates miR-184-mediated direct suppression of C-MYC and BCL2 blocking cell growth and survival in nasopharyngeal carcinoma. Cell Death Dis. 2013, 4, e872. [Google Scholar] [CrossRef]
- Soriano, M.E.; Scorrano, L. The Interplay between BCL-2 Family Proteins and Mitochondrial Morphology in the Regulation of Apoptosis. In Bcl-2 Protein Family: Essential Regulators of Cell Death; Springer: Berlin/Heidelberg, Germany, 2010; pp. 97–114. [Google Scholar]
- Oltvai, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 Heterodimerizes in-Vivo with a Conserved Homolog, Bax, That Accelerates Programmed Cell-Death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Zhang, C. MicroRNomics: A newly emerging approach for disease biology. Physiol. Genom. 2008, 33, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Jian, Y.; Fu, H.; Li, B. MiR-532 downregulation of the Wnt/β-catenin signaling via targeting Bcl-9 and induced human intervertebral disc nucleus pulposus cells apoptosis. J. Pharmacol. Sci. 2018, 138, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zeng, X.; He, Y.; Wang, X.X.; Liang, Z.W.; Liu, J.J.; Zhang, P.; Zhu, H.X.; Xu, N. Z, Liang, S.F. STC2 promotes the epithelial-mesenchymal transition of colorectal cancer cells through AKT-ERK signaling pathways. Oncotarget 2016, 7, 71400–71416. [Google Scholar] [CrossRef]
- Law, A.Y.; Wong, C.K. Stanniocalcin-2 is a HIF-1 target gene that promotes cell proliferation in hypoxia. Exp. Cell Res. 2010, 316, 466–476. [Google Scholar] [CrossRef]
- Paul, S.; Jain, P.; Tuli, A.; Gupta, U.; Jain, M. Serum VEGF as a marker of endometrial receptivity in infertile women. FASEB J. 2013, 27, 750. [Google Scholar]
- Miravet-Valenciano, J.A.; Rincon-Bertolin, A.; Vilella, F.; Simon, C. Understanding and improving endometrial receptivity. Curr. Opin. Obstet. Gyn. 2015, 27, 187–192. [Google Scholar] [CrossRef]
- Xie, Y.; Cui, D.; Kong, Y. FoxM1 influences embryo implantation and is regulated by 17 β-estradiol and progesterone in mouse uteri and endometrium cells. Int. J. Clin. Exp. Pathol. 2014, 7, 6585–6595. [Google Scholar]
- Butler, D.E.; Marlein, C.; Walker, H.F.; Frame, F.M.; Mann, V.M.; Simms, M.S.; Davies, B.R.; Collins, A.T.; Maitland, N.J. Inhibition of the PI3K/AKT/mTOR pathway activates autophagy and compensatory Ras/Raf/MEK/ERK signalling in prostate cancer. Oncotarget 2017, 8, 56698–56713. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Mei, Y.; Geng, W.; Yang, J.; Hong, Q.; Feng, Z.; Cai, G.; Zhu, H.; Shi, S.; et al. miR-34a regulates mesangial cell proliferation via the PDGFR-β/Ras-MAPK signaling pathway. Cell Mol. Life Sci. 2014, 71, 4027–4042. [Google Scholar] [CrossRef]


| Gene | GenBank Accession No. | Primer Sequence (5′–3′) |
|---|---|---|
| STC2 (qPCR) | XM_005694539.3 | F: CGGAAGTGTCCAGCCATCAAGG |
| R:GCAGCAGTCACACACAGTCA | ||
| STC2 (Check2) | / | F: CGCTCGAGTCGGGAGACCGGCCGAGG |
| R: ATGCGGCCGCTCGCTGCCCAGGGAGCCT | ||
| STC2 (pcDNA3.1) | / | F: CAGGGTACCATGTGTGCCGAGCGGCTG |
| R:CGCTCGAGTCACCTCCGGATATCGGAATACTCAGACTGTTC | ||
| β-actin | XM_018039831.1 | F: GATCTGGCACCACACCTTCT |
| R: GGGTCATCTTCTCACGGTTG | ||
| U6 | / | F: CTCGCTTCGGCAGCACA |
| R: AACGCTTCACGAATTTGCGT | ||
| miR-184-Loop | / | gtcgtatccagtgcagggtccgaggtattcgcactggatacgacACCCTTAT |
| miR-184 | / | gcgcgcTGGACGGAGAACTG |
| Reverse Primer | / | GTGCAGGGTCCGAGGT |
| Name | Manufacturer | Product Number |
|---|---|---|
| STC2 | Gene Tex, America | GTX82231 |
| β-actin | Beyotime, Shanghai, China | AA128 |
| BAX | Beyotime, Shanghai, China | AB026 |
| BCL2 | Beyotime, Shanghai, China | AB112 |
| VEGF | Boster, Wnhan, China | BA0407 |
| FOXM1 | SAB, United States | #32671 |
| RAS | Gene Tex, America | GTX132480 |
| RAF | BBI, Shanghai, China | D220484 |
| P-RAF (Ser642) | BBI, Shanghai, China | D151385 |
| MEK1 | Abways, Shanghai, China | CY5168 |
| P-MEK1 (Ser298) | Abways, Shanghai, China | CY5277 |
| ERK | Cell Signaling, America | #9101 |
| P-ERK (Thr202/Tyr204) | Cell Signaling, America | #9102 |
| HRP-labeled goat anti-rabbit IgG (H + L) | Beyotime, Shanghai, China | A0208 |
| HRP-labeled goat anti-mouse IgG (H + L) | Beyotime, Shanghai, China | A0216 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Liu, X.; Yang, L.; Che, S.; Guo, H.; Han, J.; Zhu, Z.; Cao, B.; An, X.; Zhang, L.; et al. MiR-184 Combined with STC2 Promotes Endometrial Epithelial Cell Apoptosis in Dairy Goats via RAS/RAF/MEK/ERK Pathway. Genes 2020, 11, 1052. https://doi.org/10.3390/genes11091052
Cui J, Liu X, Yang L, Che S, Guo H, Han J, Zhu Z, Cao B, An X, Zhang L, et al. MiR-184 Combined with STC2 Promotes Endometrial Epithelial Cell Apoptosis in Dairy Goats via RAS/RAF/MEK/ERK Pathway. Genes. 2020; 11(9):1052. https://doi.org/10.3390/genes11091052
Chicago/Turabian StyleCui, Jiuzeng, Xiaorui Liu, Lichun Yang, Sicheng Che, Hongran Guo, Jincheng Han, Zhongshi Zhu, Binyun Cao, Xiaopeng An, Lei Zhang, and et al. 2020. "MiR-184 Combined with STC2 Promotes Endometrial Epithelial Cell Apoptosis in Dairy Goats via RAS/RAF/MEK/ERK Pathway" Genes 11, no. 9: 1052. https://doi.org/10.3390/genes11091052
APA StyleCui, J., Liu, X., Yang, L., Che, S., Guo, H., Han, J., Zhu, Z., Cao, B., An, X., Zhang, L., & Song, Y. (2020). MiR-184 Combined with STC2 Promotes Endometrial Epithelial Cell Apoptosis in Dairy Goats via RAS/RAF/MEK/ERK Pathway. Genes, 11(9), 1052. https://doi.org/10.3390/genes11091052




