Association between Family Histories of Thyroid Cancer and Thyroid Cancer Incidence: A Cross-Sectional Study Using the Korean Genome and Epidemiology Study Data
Abstract
1. Introduction
2. Materials and Methods
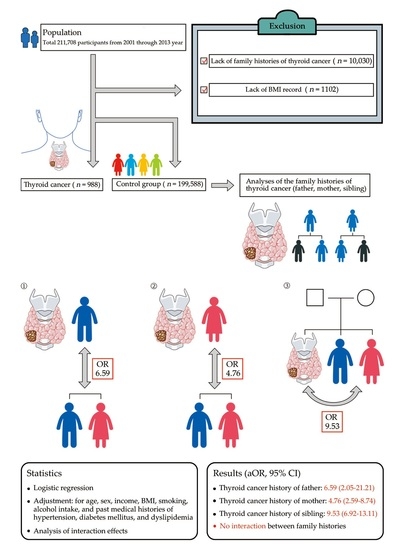
2.1. Study Population and Data Collection
2.2. Participant Selection
2.3. Survey
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vigneri, R.; Malandrino, P.; Vigneri, P. The changing epidemiology of thyroid cancer: Why is incidence increasing? Curr. Opin. Oncol. 2015, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.G.; Sikora, A.G.; Tosteson, T.D.; Davies, L. The increasing incidence of thyroid cancer: The influence of access to care. Thyroid 2013, 23, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferri, E.L. Management of a solitary thyroid nodule. N. Engl. J. Med. 1993, 328, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Wartofsky, L. Increasing world incidence of thyroid cancer: Increased detection or higher radiation exposure? Hormones 2010, 9, 103–108. [Google Scholar] [CrossRef]
- Ahn, H.S.; Kim, H.J.; Welch, H.G. Korea’s thyroid-cancer “epidemic”—Screening and overdiagnosis. N. Engl. J. Med. 2014, 371, 1765–1767. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Kust, D.; Stanicic, J.; Matesa, N. Bethesda thyroid categories and family history of thyroid disease. Clin. Endocrinol. 2018, 88, 468–472. [Google Scholar] [CrossRef]
- Schmidbauer, B.; Menhart, K.; Hellwig, D.; Grosse, J. Differentiated Thyroid Cancer-Treatment: State of the Art. Int. J. Mol. Sci. 2017, 18, 1292. [Google Scholar] [CrossRef]
- Park, S.; Oh, C.M.; Cho, H.; Lee, J.Y.; Jung, K.W.; Jun, J.K.; Won, Y.J.; Kong, H.J.; Choi, K.S.; Lee, Y.J.; et al. Association between screening and the thyroid cancer "epidemic" in South Korea: Evidence from a nationwide study. BMJ 2016, 355, i5745. [Google Scholar] [CrossRef]
- Xing, M.; Haugen, B.R.; Schlumberger, M. Progress in molecular-based management of differentiated thyroid cancer. Lancet 2013, 381, 1058–1069. [Google Scholar] [CrossRef]
- Spano, J.P.; Vano, Y.; Vignot, S.; De La Motte Rouge, T.; Hassani, L.; Mouawad, R.; Menegaux, F.; Khayat, D.; Leenhardt, L. GEMOX regimen in the treatment of metastatic differentiated refractory thyroid carcinoma. Med. Oncol. 2012, 29, 1421–1428. [Google Scholar] [CrossRef]
- Crouzeix, G.; Michels, J.J.; Sevin, E.; Aide, N.; Vaur, D.; Bardet, S.; French, T.N. Unusual short-term complete response to two regimens of cytotoxic chemotherapy in a patient with poorly differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2012, 97, 3046–3050. [Google Scholar] [CrossRef]
- Xu, L.; Li, G.; Wei, Q.; El-Naggar, A.K.; Sturgis, E.M. Family history of cancer and risk of sporadic differentiated thyroid carcinoma. Cancer 2012, 118, 1228–1235. [Google Scholar] [CrossRef]
- Albi, E.; Cataldi, S.; Lazzarini, A.; Codini, M.; Beccari, T.; Ambesi-Impiombato, F.S.; Curcio, F. Radiation and Thyroid Cancer. Int. J. Mol. Sci. 2017, 18, 911. [Google Scholar] [CrossRef]
- Pacini, F.; Castagna, M.G.; Brilli, L.; Pentheroudakis, G.; Group, E.G.W. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. 7), vii110–vii119. [Google Scholar] [CrossRef]
- Cong, X. Air pollution from industrial waste gas emissions is associated with cancer incidences in Shanghai, China. Environ. Sci. Pollut. Res. Int. 2018, 25, 13067–13078. [Google Scholar] [CrossRef]
- Fiore, M.; Oliveri Conti, G.; Caltabiano, R.; Buffone, A.; Zuccarello, P.; Cormaci, L.; Cannizzaro, M.A.; Ferrante, M. Role of Emerging Environmental Risk Factors in Thyroid Cancer: A Brief Review. Int. J. Environ. Res. Public Health 2019, 16, 1185. [Google Scholar] [CrossRef]
- Mack, W.J.; Preston-Martin, S.; Dal Maso, L.; Galanti, R.; Xiang, M.; Franceschi, S.; Hallquist, A.; Jin, F.; Kolonel, L.; La Vecchia, C.; et al. A pooled analysis of case-control studies of thyroid cancer: Cigarette smoking and consumption of alcohol, coffee, and tea. Cancer Causes Control. 2003, 14, 773–785. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Tseng, G.C.; Steward, D.; Diorio, D.; Nikiforov, Y.E. MicroRNA expression profiling of thyroid tumors: Biological significance and diagnostic utility. J. Clin. Endocrinol. Metab. 2008, 93, 1600–1608. [Google Scholar] [CrossRef]
- Abdullah, M.I.; Junit, S.M.; Ng, K.L.; Jayapalan, J.J.; Karikalan, B.; Hashim, O.H. Papillary Thyroid Cancer: Genetic Alterations and Molecular Biomarker Investigations. Int. J. Med. Sci. 2019, 16, 450–460. [Google Scholar] [CrossRef]
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; Schlumberger, M.; et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009, 19, 1167–1214. [Google Scholar] [CrossRef]
- Fallah, M.; Sundquist, K.; Hemminki, K. Risk of thyroid cancer in relatives of patients with medullary thyroid carcinoma by age at diagnosis. Endocr. Relat. Cancer 2013, 20, 717–724. [Google Scholar] [CrossRef]
- Goldgar, D.E.; Easton, D.F.; Cannon-Albright, L.A.; Skolnick, M.H. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J. Natl. Cancer Inst. 1994, 86, 1600–1608. [Google Scholar] [CrossRef]
- Amundadottir, L.T.; Thorvaldsson, S.; Gudbjartsson, D.F.; Sulem, P.; Kristjansson, K.; Arnason, S.; Gulcher, J.R.; Bjornsson, J.; Kong, A.; Thorsteinsdottir, U.; et al. Cancer as a complex phenotype: Pattern of cancer distribution within and beyond the nuclear family. PLoS Med. 2004, 1, e65. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, E.K.; Moon, H.J.; Yoon, J.H.; Kwak, J.Y. Risk of Thyroid Cancer in Euthyroid Asymptomatic Patients with Thyroid Nodules with an Emphasis on Family History of Thyroid Cancer. Korean J. Radiol. 2016, 17, 255–263. [Google Scholar] [CrossRef][Green Version]
- Myung, S.K.; Lee, C.W.; Lee, J.; Kim, J.; Kim, H.S. Risk Factors for Thyroid Cancer: A Hospital-Based Case-Control Study in Korean Adults. Cancer Res. Treat. 2017, 49, 70–78. [Google Scholar] [CrossRef]
- Byun, S.H.; Min, C.; Hong, S.J.; Choi, H.G.; Koh, D.H. Analysis of the Relation between Periodontitis and Chronic Gastritis/Peptic Ulcer: A Cross-Sectional Study Using KoGES HEXA Data. Int. J. Environ. Res. Public Health 2020, 17, 4387. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G.; KoGES Group. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, 1350. [Google Scholar] [CrossRef]
- Byun, S.H.; Min, C.; Kim, Y.B.; Kim, H.; Kang, S.H.; Park, B.J.; Wee, J.H.; Choi, H.G.; Hong, S.J. Analysis of Chronic Periodontitis in Tonsillectomy Patients: A Longitudinal Follow-Up Study Using a National Health Screening Cohort. Appl. Sci. 2020, 10, 3663. [Google Scholar] [CrossRef]
- Byun, S.H.; Min, C.; Park, I.S.; Kim, H.; Kim, S.K.; Park, B.J.; Choi, H.G.; Hong, S.J. Increased Risk of Chronic Periodontitis in Chronic Rhinosinusitis Patients: A Longitudinal Follow-Up Study Using a National Health-Screening Cohort. J. Clin. Med. 2020, 9, 1170. [Google Scholar] [CrossRef]
- Byun, S.H.; Lee, S.; Kang, S.H.; Choi, H.G.; Hong, S.J. Cross-Sectional Analysis of the Association between Periodontitis and Cardiovascular Disease Using the Korean Genome and Epidemiology Study Data. Int. J. Environ. Res. Public Health 2020, 17, 5237. [Google Scholar] [CrossRef]
- Tavarelli, M.; Russo, M.; Terranova, R.; Scollo, C.; Spadaro, A.; Sapuppo, G.; Malandrino, P.; Masucci, R.; Squatrito, S.; Pellegriti, G. Familial Non-Medullary Thyroid Cancer Represents an Independent Risk Factor for Increased Cancer Aggressiveness: A Retrospective Analysis of 74 Families. Front. Endocrinol. 2015, 6, 117. [Google Scholar] [CrossRef]
- Oakley, G.M.; Curtin, K.; Pimentel, R.; Buchmann, L.; Hunt, J. Establishing a familial basis for papillary thyroid carcinoma using the Utah Population Database. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1171–1174. [Google Scholar] [CrossRef]
- Ahn, H.S.; Kim, H.J.; Kim, K.H.; Lee, Y.S.; Han, S.J.; Kim, Y.; Ko, M.J.; Brito, J.P. Thyroid Cancer Screening in South Korea Increases Detection of Papillary Cancers with No Impact on Other Subtypes or Thyroid Cancer Mortality. Thyroid 2016, 26, 1535–1540. [Google Scholar] [CrossRef]
- Brito, J.P.; Kim, H.J.; Han, S.J.; Lee, Y.S.; Ahn, H.S. Geographic Distribution and Evolution of Thyroid Cancer Epidemic in South Korea. Thyroid 2016, 26, 864–865. [Google Scholar] [CrossRef]
- Oh, C.M.; Kong, H.J.; Kim, E.; Kim, H.; Jung, K.W.; Park, S.; Won, Y.J. National Epidemiologic Survey of Thyroid cancer (NEST) in Korea. Epidemiol. Health 2018, 40, e2018052. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Guo, X.G.; Ba, C.X.; Wang, W.; Yang, Y.Y.; Wang, J.; Cao, H.Y. Overweight, obesity and thyroid cancer risk: A meta-analysis of cohort studies. J. Int. Med. Res. 2012, 40, 2041–2050. [Google Scholar] [CrossRef]
- Han, J.M.; Kim, T.Y.; Jeon, M.J.; Yim, J.H.; Kim, W.G.; Song, D.E.; Hong, S.J.; Bae, S.J.; Kim, H.K.; Shin, M.H.; et al. Obesity is a risk factor for thyroid cancer in a large, ultrasonographically screened population. Eur. J. Endocrinol. 2013, 168, 879–886. [Google Scholar] [CrossRef]
- Cho, Y.A.; Kim, J. Thyroid cancer risk and smoking status: A meta-analysis. Cancer Causes Control. 2014, 25, 1187–1195. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Linet, M.S.; Beane Freeman, L.E.; Check, D.P.; Church, T.R.; Park, Y.; Purdue, M.P.; Schairer, C.; Berrington de Gonzalez, A. Cigarette smoking, alcohol intake, and thyroid cancer risk: A pooled analysis of five prospective studies in the United States. Cancer Causes Control. 2012, 23, 1615–1624. [Google Scholar] [CrossRef]
- Balhara, Y.P.; Deb, K.S. Impact of alcohol use on thyroid function. Indian J. Endocrinol. Metab. 2013, 17, 580–587. [Google Scholar] [CrossRef]
- Hong, S.H.; Myung, S.K.; Kim, H.S.; Korean Meta-Analysis Study, G. Alcohol Intake and Risk of Thyroid Cancer: A Meta-Analysis of Observational Studies. Cancer Res. Treat. 2017, 49, 534–547. [Google Scholar] [CrossRef]
- Hong, S.; Won, Y.J.; Park, Y.R.; Jung, K.W.; Kong, H.J.; Lee, E.S.; The Community of Population-Based Regional Cancer Registries. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2017. Cancer Res. Treat. 2020, 52, 335–350. [Google Scholar] [CrossRef]

| Characteristics | Total Participants | ||
|---|---|---|---|
| Thyroid Cancer (n, %) | Control (n, %) | p-Value | |
| Total number (n, %) | 988 (100.0) | 199,588 (100.0) | |
| Age (years, mean, SD) | 53.2 (7.6) | 53.9 (8.7) | 0.007 1 |
| Sex (n, %) | <0.001 1 | ||
| Male | 79 (8.0) | 69,614 (34.9) | |
| Female | 909 (92.0) | 129,974 (65.1) | |
| Income (n, %) | <0.001 1 | ||
| No information | 95 (9.6) | 40,590 (20.3) | |
| Low | 166 (16.8) | 41,060 (20.6) | |
| Middle | 292 (29.6) | 51,899 (26.0) | |
| High | 435 (44.0) | 66,039 (33.1) | |
| Hypertension (n, %) | 220 (22.3) | 40,930 (20.5) | 0.172 |
| Diabetes (n, %) | 50 (5.1) | 14,584 (7.3) | 0.007 1 |
| Dyslipidemia (n, %) | 117 (11.8) | 17,834 (8.9) | 0.001 1 |
| Obesity (BMI, kg/m2, mean, SD) | 23.8 (2.9) | 24.0 (3.0) | <0.001 1 |
| Tobacco index (pack-year, mean, SD) | 1.63 (6.5) | 6.62 (12.5) | <0.001 1 |
| alcohol consumption (g/day, mean, SD) | 2.0 (10.1) | 7.2 (22.1) | <0.001 1 |
| Family history of father (n, %) | 3 (0.3) | 86 (0.0) | 0.010 1 |
| Family history of mother (n, %) | 12 (1.2) | 307 (0.2) | <0.001 1 |
| Family history of siblings (n, %) | 44 (4.5) | 653 (0.3) | <0.001 1 |
| Family History | ORs of Thyroid Cancer for the Thyroid Cancer Family Histories | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude | p-Value | Model 1 | p-Value | Model 2 | p-Value | Model 3 | p-Value | |
| ORs of the thyroid cancer history of father | ||||||||
| Thyroid cancer | 7.07 (2.23–22.38) | 0.001 1 | 6.72 (2.10–21.50) | 0.001 1 | 6.75 (2.10–21.67) | 0.001 1 | 6.59 (2.05–21.21) | 0.002 1 |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| ORs of the thyroid cancer history of mother | ||||||||
| Thyroid cancer | 7.98 (4.47–14.26) | <0.001 1 | 6.33 (3.52–11.36) | <0.001 1 | 5.55 (3.02–10.19) | <0.001 1 | 4.76 (2.59–8.74) | <0.001 1 |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| ORs of the thyroid cancer history of siblings | ||||||||
| Thyroid cancer | 14.20 (10.40–19.40) | <0.001 1 | 10.16 (7.42–13.93) | <0.001 1 | 13.09 (9.53–17.99) | <0.001 1 | 9.53 (6.92–13.11) | <0.001 1 |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Family History | ORs of Thyroid Cancer for the Thyroid Cancer Histories of Families | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude | p-Value | Model 1 | p-Value | Model 2 | p-Value | Model 3 | p-Value | |
| Men (n = 69,693) | ||||||||
| ORs of the thyroid cancer history of father | ||||||||
| Thyroid cancer | 27.88 (3.76–206.55) | 0.001 1 | 28.21 (3.73–213.69) | 0.001 1 | 28.53 (3.85–211.44) | 0.001 1 | 29.09 (3.84–220.29) | 0.001 1 |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| ORs of the thyroid cancer history of mother | ||||||||
| Thyroid cancer | No convergence | 0.997 | No convergence | 0.997 | No convergence | 0.997 | No convergence | 0.997 |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| ORs of the thyroid cancer history of siblings | ||||||||
| Thyroid cancer | 15.43 (3.75–63.54) | <0.001 1 | 13.90 (3.34–57.94) | <0.001 1 | 16.02 (3.89–66.00) | <0.001 1 | 14.15 (3.39–58.95) | <0.001 1 |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Women (n = 130,883) | ||||||||
| ORs of the thyroid cancer history of father | ||||||||
| Thyroid cancer | 5.31 (1.29–21.79) | 0.021 1 | 4.84 (1.18–19.94) | 0.029 1 | 4.92 (1.18–20.61) | 0.029 1 | 4.71 (1.13–19.52) | 0.033 1 |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| ORs of the thyroid cancer history of mother | ||||||||
| Thyroid cancer | 7.89 (4.40–14.16) | <0.001 1 | 6.75 (3.75–12.14) | <0.001 1 | 5.70 (3.09–10.51) | <0.001 1 | 5.06 (2.75–9.31) | <0.001 1 |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| ORs of the thyroid cancer history of siblings | ||||||||
| Thyroid cancer | 11.70 (8.49–16.13) | <0.001 1 | 10.04 (7.27–13.87) | <0.001 1 | 10.81 (7.80–14.98) | <0.001 1 | 9.37 (6.75–13.00) | <0.001 1 |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Variable | ORs for Thyroid Cancer | |
|---|---|---|
| Model 4 | p-Value | |
| Father | No convergence | 1.00 |
| Mother | 12.84 (2.02–81.86) | 0.007 |
| Siblings | 26.87 (5.69–126.91) | <0.001 |
| Father × siblings | No convergence | 1.000 |
| Mother × siblings | 0.51 (0.12–2.17) | 0.362 |
| Histories | Onset of Thyroid Cancer | ||
|---|---|---|---|
| <50 Years Old | ≥50 Years Old | p-Value | |
| Thyroid cancer histories of father (n, %) | |||
| Yes | 3 (0.6) | 0 (0.0) | 0.253 |
| No | 520 (99.4) | 456 (100.0) | |
| Thyroid cancer histories of mother (n, %) | |||
| Yes | 11 (2.1) | 1 (0.2) | 0.007 1 |
| No | 512 (97.9) | 455 (99.8) | |
| Thyroid cancer histories of siblings (n, %) | |||
| Yes | 23 (4.4) | 21 (4.6) | 0.876 |
| No | 500 (95.6) | 435 (95.4) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, S.-H.; Min, C.; Choi, H.-G.; Hong, S.-J. Association between Family Histories of Thyroid Cancer and Thyroid Cancer Incidence: A Cross-Sectional Study Using the Korean Genome and Epidemiology Study Data. Genes 2020, 11, 1039. https://doi.org/10.3390/genes11091039
Byun S-H, Min C, Choi H-G, Hong S-J. Association between Family Histories of Thyroid Cancer and Thyroid Cancer Incidence: A Cross-Sectional Study Using the Korean Genome and Epidemiology Study Data. Genes. 2020; 11(9):1039. https://doi.org/10.3390/genes11091039
Chicago/Turabian StyleByun, Soo-Hwan, Chanyang Min, Hyo-Geun Choi, and Seok-Jin Hong. 2020. "Association between Family Histories of Thyroid Cancer and Thyroid Cancer Incidence: A Cross-Sectional Study Using the Korean Genome and Epidemiology Study Data" Genes 11, no. 9: 1039. https://doi.org/10.3390/genes11091039
APA StyleByun, S.-H., Min, C., Choi, H.-G., & Hong, S.-J. (2020). Association between Family Histories of Thyroid Cancer and Thyroid Cancer Incidence: A Cross-Sectional Study Using the Korean Genome and Epidemiology Study Data. Genes, 11(9), 1039. https://doi.org/10.3390/genes11091039