Actions of L-thyroxine (T4) and Tetraiodothyroacetic Acid (Tetrac) on Gene Expression in Thyroid Cancer Cells
Abstract
1. Introduction
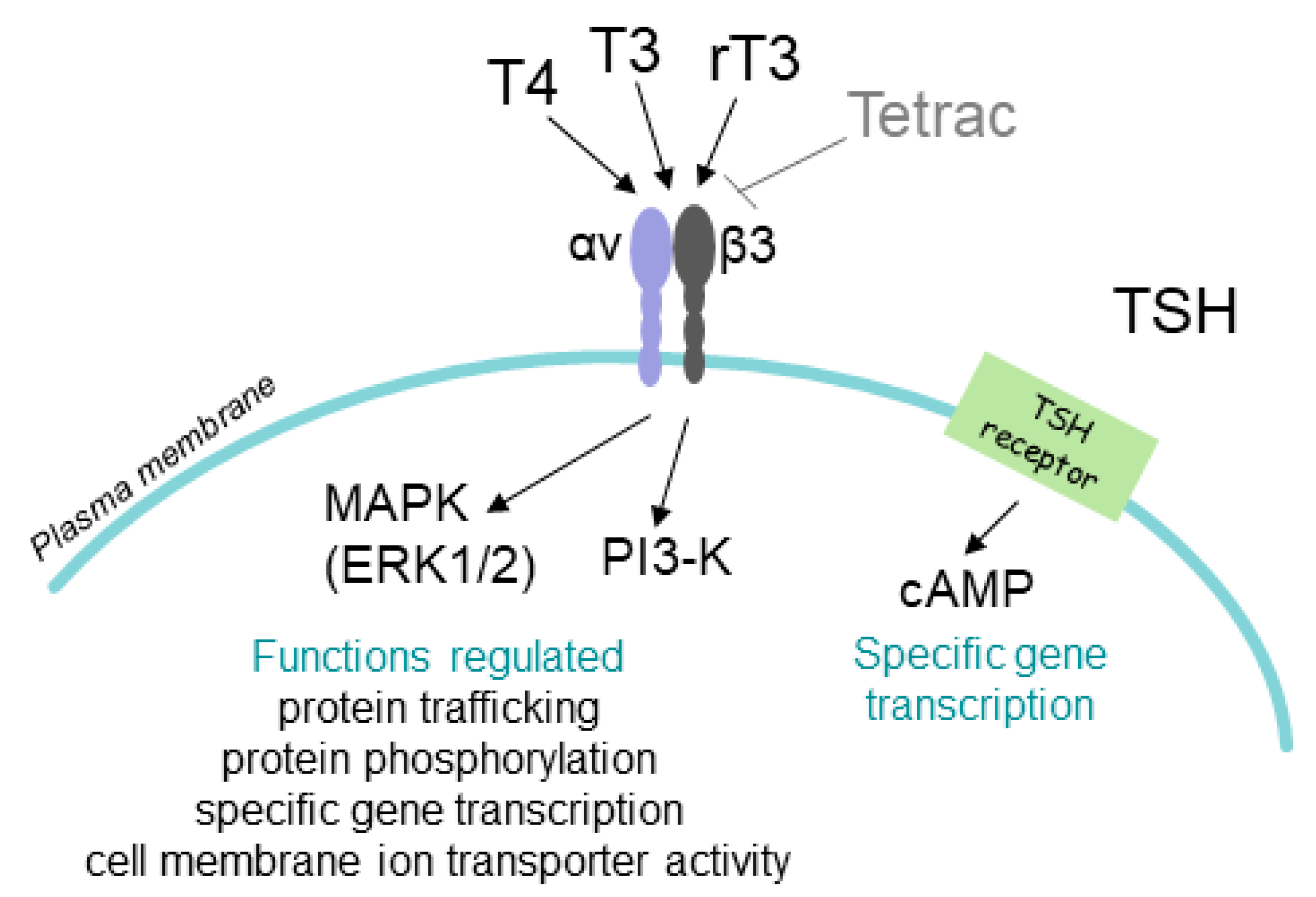
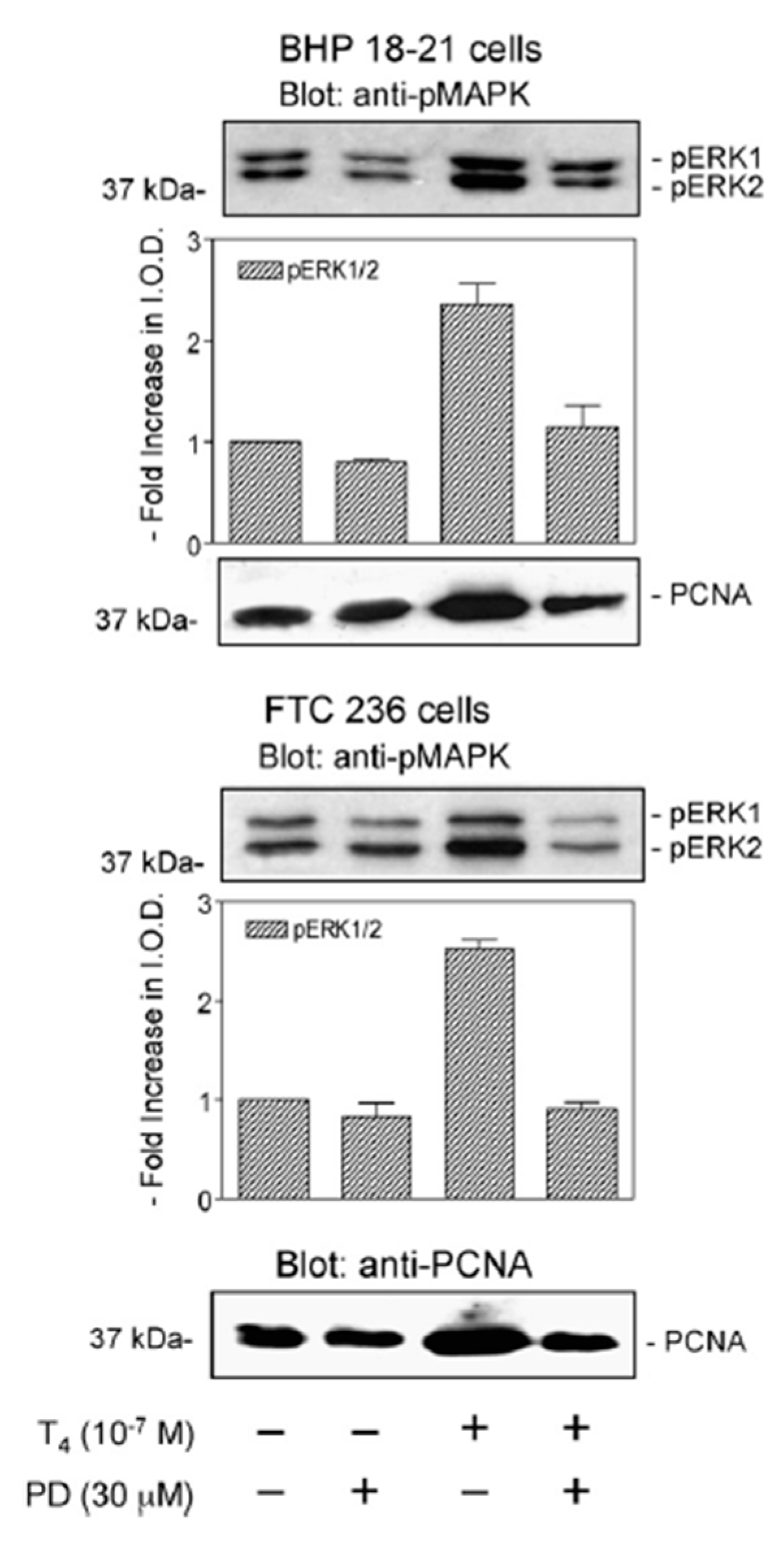
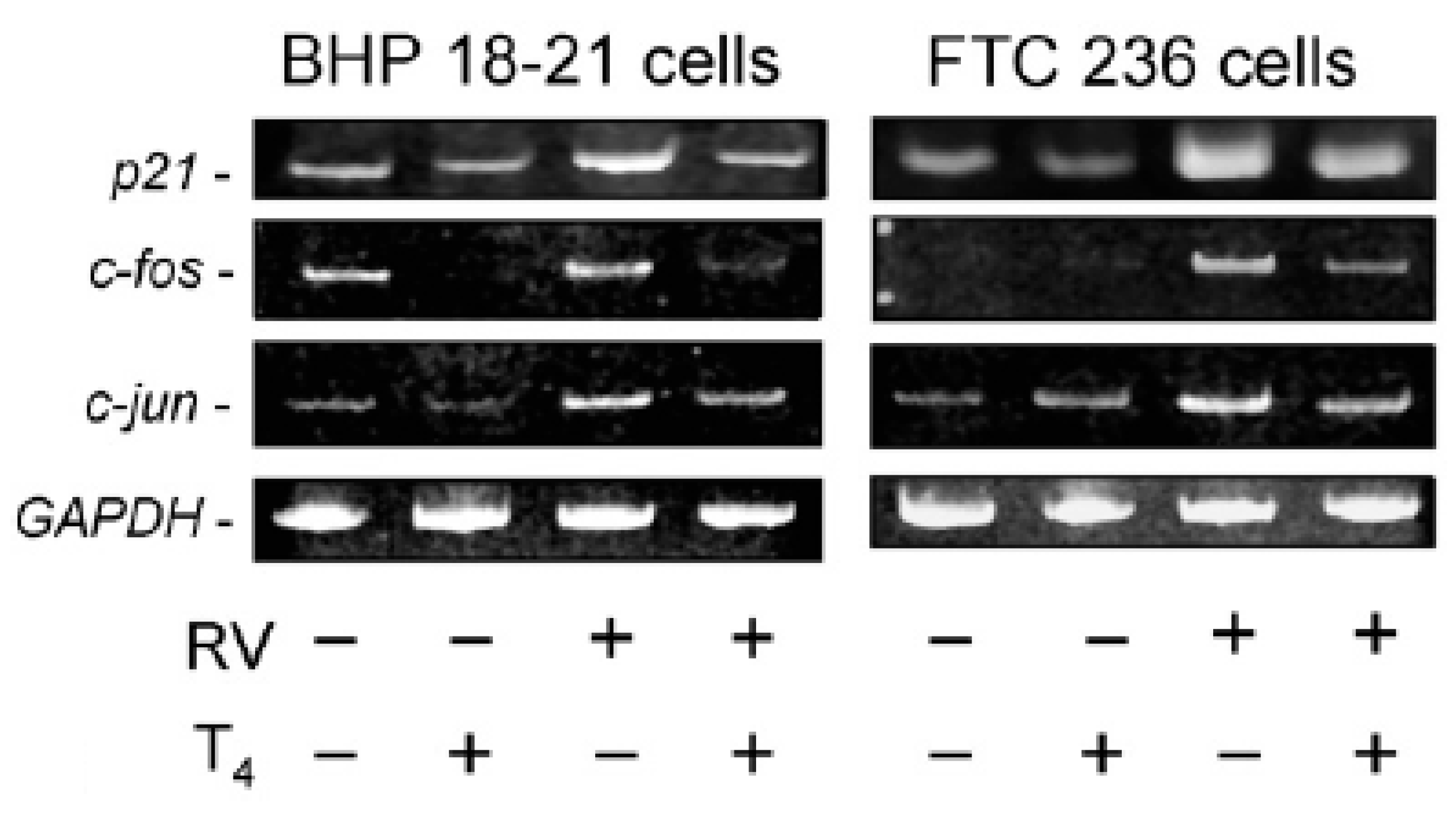
2. T4 Actions at the Integrin αvβ3 in Papillary and Follicular Thyroid Carcinoma Cells
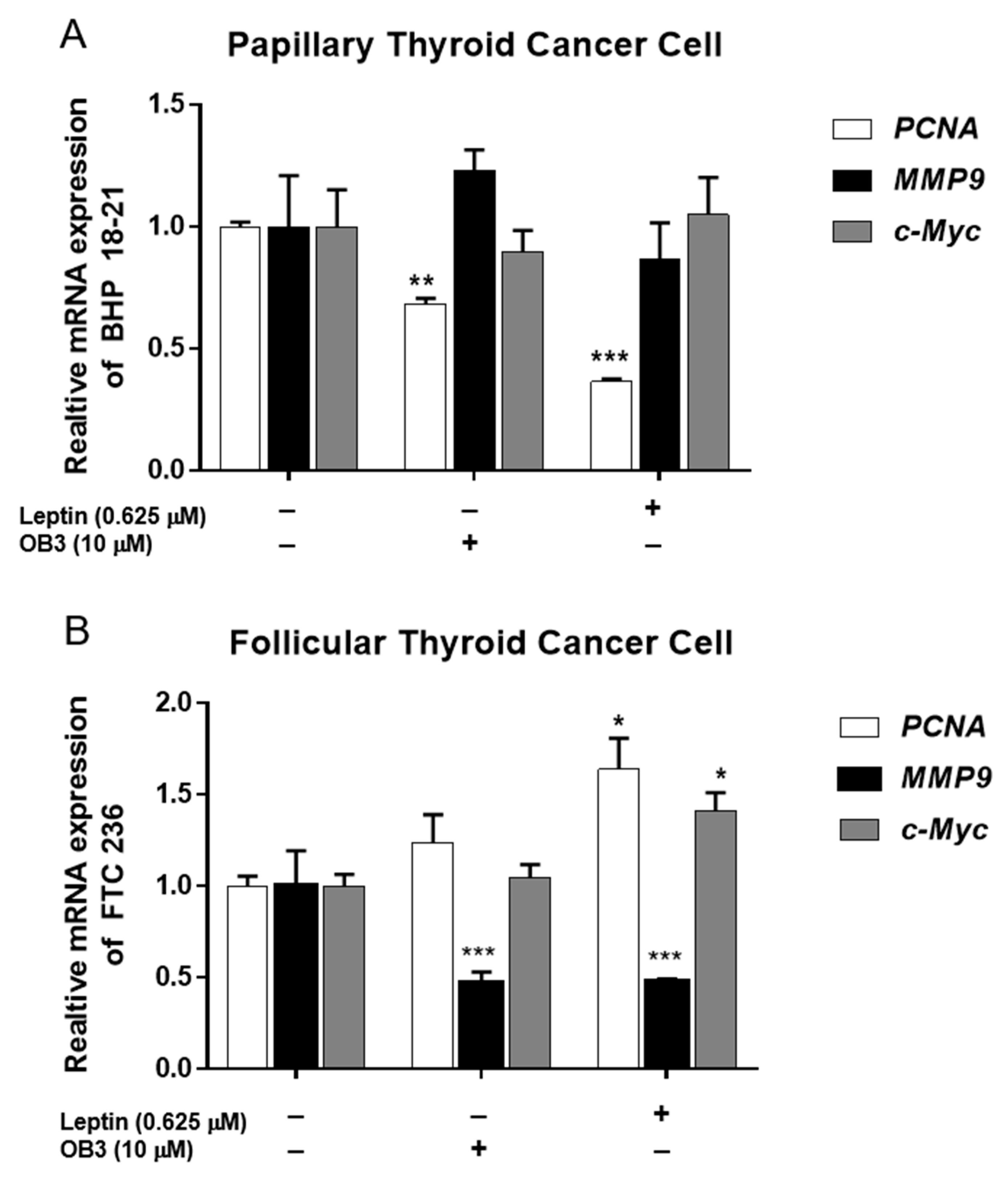
3. Gene Expression in Thyroid Cancer Cells Exposed to Leptin
4. Actions of Thyroid Hormone Analogues on Cells of Medullary Thyroid Carcinoma (MTC)
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.Y.; Witt, R.L.; Steward, D.L. Molecular testing for thyroid nodules: Review and current state. Cancer 2018, 124, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Hercbergs, A.; Luidens, M.K.; Lin, H.Y. Recurrence of differentiated thyroid carcinoma during full tsh suppression: Is the tumor now thyroid hormone dependent? Horm. Cancer 2015, 6, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Celano, M.; Maggisano, V.; Lepore, S.M.; Sponziello, M.; Pecce, V.; Verrienti, A.; Durante, C.; Maranghi, M.; Lucia, P.; Bulotta, S.; et al. Expression of leptin receptor and effects of leptin on papillary thyroid carcinoma cells. Int. J. Endocrinol. 2019, 2019, 5031696. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Chin, Y.T.; Hsieh, M.T.; Lai, H.Y.; Ke, C.C.; Crawford, D.R.; Lee, O.K.; Fu, E.; Mousa, S.A.; Grasso, P.; et al. Novel leptin OB3 peptide-induced signaling and progression in thyroid cancers: Comparison with leptin. Oncotarget 2016, 7, 27641–27654. [Google Scholar] [CrossRef][Green Version]
- Cheng, S.Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef]
- Mousa, S.A.; Glinsky, G.V.; Lin, H.Y.; Ashur-Fabian, O.; Hercbergs, A.; Keating, K.A.; Davis, P.J. Contributions of thyroid hormone to cancer metastasis. Biomedicines 2018, 6, 89. [Google Scholar] [CrossRef]
- Leonard, J.L.; Farwell, A.P. Thyroid hormone-regulated actin polymerization in brain. Thyroid 1997, 7, 147–151. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tang, H.Y.; Leinung, M.; Mousa, S.A.; Hercbergs, A.; Davis, P.J. Action of reverse T3 on cancer cells. Endocr. Res. 2019, 44, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Mousa, S.A.; Cody, V.; Tang, H.Y.; Lin, H.Y. Small molecule hormone or hormone-like ligands of integrin αVβ3: Implications for cancer cell behavior. Horm. Cancer 2013, 4, 335–342. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Schmidbauer, B.; Menhart, K.; Hellwig, D.; Grosse, J. Differentiated thyroid cancer-treatment: State of the art. Int. J. Mol. Sci. 2017, 18, 1292. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tang, H.Y.; Shih, A.; Keating, T.; Cao, G.; Davis, P.J.; Davis, F.B. Thyroid hormone is a MAPK-dependent growth factor for thyroid cancer cells and is anti-apoptotic. Steroids 2007, 72, 180–187. [Google Scholar] [CrossRef]
- Bergh, J.J.; Lin, H.Y.; Lansing, L.; Mohamed, S.N.; Davis, F.B.; Mousa, S.; Davis, P.J. Integrin αVβ3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 2005, 146, 2864–2871. [Google Scholar] [CrossRef]
- Davis, P.J.; Shih, A.; Lin, H.Y.; Martino, L.J.; Davis, F.B. Thyroxine promotes association of mitogen-activated protein kinase and nuclear thyroid hormone receptor (TR) and causes serine phosphorylation of TR. J. Biol. Chem. 2000, 275, 38032–38039. [Google Scholar] [CrossRef]
- Chen, Y.R.; Chen, Y.S.; Chin, Y.T.; Li, Z.L.; Shih, Y.J.; Yang, Y.S.H.; ChangOu, C.A.; Su, P.Y.; Wang, S.H.; Wu, Y.H.; et al. Thyroid hormone-induced expression of inflammatory cytokines interfere with resveratrol-induced anti-proliferation of oral cancer cells. Food Chem. Toxicol. 2019, 132, 110693. [Google Scholar] [CrossRef]
- Hercbergs, A.; Mousa, S.A.; Leinung, M.; Lin, H.Y.; Davis, P.J. Thyroid hormone in the clinic and breast cancer. Horm. Cancer 2018, 9, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Chin, Y.T.; Shih, Y.J.; Nana, A.W.; Chen, Y.R.; Wu, H.C.; Yang, Y.S.H.; Lin, H.Y.; Davis, P.J. Thyroid hormone promotes β-catenin activation and cell proliferation in colorectal cancer. Horm. Cancer 2018, 9, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Shinderman-Maman, E.; Cohen, K.; Weingarten, C.; Nabriski, D.; Twito, O.; Baraf, L.; Hercbergs, A.; Davis, P.J.; Werner, H.; Ellis, M.; et al. The thyroid hormone-avb3 integrin axis in ovarian cancer: Regulation of gene transcription and MAPK-dependent proliferation. Oncogene 2016, 35, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Ellis, M.; Khoury, S.; Davis, P.J.; Hercbergs, A.; Ashur-Fabian, O. Thyroid hormone is a MAPK-dependent growth factor for human myeloma cells acting via αvβ3 integrin. Mol. Cancer Res. 2011, 9, 1385–1394. [Google Scholar] [CrossRef]
- Davis, F.B.; Tang, H.Y.; Shih, A.; Keating, T.; Lansing, L.; Hercbergs, A.; Fenstermaker, R.A.; Mousa, A.; Mousa, S.A.; Davis, P.J.; et al. Acting via a cell surface receptor, thyroid hormone is a growth factor for glioma cells. Cancer Res. 2006, 66, 7270–7275. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chin, Y.T.; Nana, A.W.; Shih, Y.J.; Lai, H.Y.; Tang, H.Y.; Leinung, M.; Mousa, S.A.; Davis, P.J. Actions of L-thyroxine and nano-diamino-tetrac (Nanotetrac) on PD-L1 in cancer cells. Steroids 2016, 114, 59–67. [Google Scholar] [CrossRef]
- Shih, A.; Davis, F.B.; Lin, H.Y.; Davis, P.J. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK- and p53-dependent mechanism. J. Clin. Endocrinol. Metab. 2002, 87, 1223–1232. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S., Jr. Leptin function and regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef]
- Yoshida, T.; Monkawa, T.; Hayashi, M.; Saruta, T. Regulation of expression of leptin mRNA and secretion of leptin by thyroid hormone in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 1997, 232, 822–826. [Google Scholar] [CrossRef]
- Hedayati, M.; Yaghmaei, P.; Pooyamanesh, Z.; Zarif Yeganeh, M.; Hoghooghi Rad, L. Leptin: A correlated peptide to papillary thyroid carcinoma? J. Thyroid Res. 2011, 2011, 832163. [Google Scholar] [CrossRef]
- de Oliveira, E.; Teixeira Silva Fagundes, A.; Teixeira Bonomo, I.; Curty, F.H.; Fonseca Passos, M.C.; de Moura, E.G.; Lisboa, P.C. Acute and chronic leptin effect upon in vivo and in vitro rat thyroid iodide uptake. Life Sci. 2007, 81, 1241–1246. [Google Scholar] [CrossRef]
- Cheng, S.P.; Yin, P.H.; Chang, Y.C.; Lee, C.H.; Huang, S.Y.; Chi, C.W. Differential roles of leptin in regulating cell migration in thyroid cancer cells. Oncol. Rep. 2010, 23, 1721–1727. [Google Scholar] [PubMed]
- Peng, C.; Sun, Z.; Li, O.; Guo, C.; Yi, W.; Tan, Z.; Jiang, B. Leptin stimulates the epithelialmesenchymal transition and proangiogenic capability of cholangiocarcinoma cells through the miR122/PKM2 axis. Int. J. Oncol. 2019, 55, 298–308. [Google Scholar] [PubMed]
- Weingarten, C.; Jenudi, Y.; Tshuva, R.Y.; Moskovich, D.; Alfandari, A.; Hercbergs, A.; Davis, P.J.; Ellis, M.; Ashur-Fabian, O. The interplay between epithelial-mesenchymal transition (EMT) and the thyroid hormones-αvβ3 axis in ovarian cancer. Horm. Cancer 2018, 9, 22–32. [Google Scholar] [CrossRef]
- Raue, F.; Frank-Raue, K. Multiple endocrine neoplasia type 2: 2007 update. Horm. Res. 2007, 68, 101–104. [Google Scholar] [CrossRef]
- Yalcin, M.; Dyskin, E.; Lansing, L.; Bharali, D.J.; Mousa, S.S.; Bridoux, A.; Hercbergs, A.H.; Lin, H.Y.; Davis, F.B.; Glinsky, G.V.; et al. Tetraiodothyroacetic acid (tetrac) and nanoparticulate tetrac arrest growth of medullary carcinoma of the thyroid. J. Clin. Endocrinol. Metab. 2010, 95, 1972–1980. [Google Scholar] [CrossRef]
- Davis, P.J.; Glinsky, G.V.; Lin, H.Y.; Leith, J.T.; Hercbergs, A.; Tang, H.Y.; Ashur-Fabian, O.; Incerpi, S.; Mousa, S.A. Cancer cell gene expression modulated from plasma membrane integrin αvβ3 by thyroid hormone and nanoparticulate tetrac. Front. Endocrinol. (Lausanne) 2014, 5, 240. [Google Scholar]
- Meng, R.; Tang, H.Y.; Westfall, J.; London, D.; Cao, J.H.; Mousa, S.A.; Luidens, M.; Hercbergs, A.; Davis, F.B.; Davis, P.J.; et al. Crosstalk between integrin αvβ3 and estrogen receptor-α is involved in thyroid hormone-induced proliferation in human lung carcinoma cells. PLoS ONE 2011, 6, e27547. [Google Scholar] [CrossRef]
- Latteyer, S.; Christoph, S.; Theurer, S.; Hones, G.S.; Schmid, K.W.; Fuhrer, D.; Moeller, L.C. Thyroxine promotes lung cancer growth in an orthotopic mouse model. Endocr. Relat. Cancer 2019, 26, 565–574. [Google Scholar] [CrossRef]
- Cayrol, F.; Sterle, H.A.; Díaz Flaqué, M.C.; Barreiro Arcos, M.L.; Cremaschi, G.A. Non-genomic actions of thyroid hormones regulate the growth and angiogenesis of T cell lymphomas. Front. Endocrinol. 2019, 10, 63. [Google Scholar] [CrossRef]
- Leith, J.T.; Mousa, S.A.; Hercbergs, A.; Lin, H.Y.; Davis, P.J. Radioresistance of cancer cells, integrin αvβ3 and thyroid hormone. Oncotarget 2018, 9, 37069–37075. [Google Scholar] [CrossRef] [PubMed]
- Leith, J.T.; Hercbergs, A.; Kenney, S.; Mousa, S.A.; Davis, P.J. Activation of tumor cell integrin αvβ3 by radiation and reversal of activation by chemically modified tetraiodothyroacetic acid (tetrac). Endocr. Res. 2018, 43, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Hercbergs, A.; Johnson, R.E.; Ashur-Fabian, O.; Garfield, D.H.; Davis, P.J. Medically induced euthyroid hypothyroxinemia may extend survival in compassionate need cancer patients: An observational study. Oncologist 2015, 20, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Sibilio, A.; Ambrosio, R.; Bonelli, C.; De Stefano, M.A.; Torre, V.; Dentice, M.; Salvatore, D. Deiodination in cancer growth: The role of type III deiodinase. Minerva Endocrinol. 2012, 37, 315–327. [Google Scholar]
- Romitti, M.; Wajner, S.M.; Ceolin, L.; Ferreira, C.V.; Ribeiro, R.V.; Rohenkohl, H.C.; Weber Sde, S.; Lopez, P.L.; Fuziwara, C.S.; Kimura, E.T.; et al. MAPK and SHH pathways modulate type 3 deiodinase expression in papillary thyroid carcinoma. Endocr. Relat. Cancer 2016, 23, 135–146. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, P.J.; Lin, H.-Y.; Hercbergs, A.; Mousa, S.A. Actions of L-thyroxine (T4) and Tetraiodothyroacetic Acid (Tetrac) on Gene Expression in Thyroid Cancer Cells. Genes 2020, 11, 755. https://doi.org/10.3390/genes11070755
Davis PJ, Lin H-Y, Hercbergs A, Mousa SA. Actions of L-thyroxine (T4) and Tetraiodothyroacetic Acid (Tetrac) on Gene Expression in Thyroid Cancer Cells. Genes. 2020; 11(7):755. https://doi.org/10.3390/genes11070755
Chicago/Turabian StyleDavis, Paul J., Hung-Yun Lin, Aleck Hercbergs, and Shaker A. Mousa. 2020. "Actions of L-thyroxine (T4) and Tetraiodothyroacetic Acid (Tetrac) on Gene Expression in Thyroid Cancer Cells" Genes 11, no. 7: 755. https://doi.org/10.3390/genes11070755
APA StyleDavis, P. J., Lin, H.-Y., Hercbergs, A., & Mousa, S. A. (2020). Actions of L-thyroxine (T4) and Tetraiodothyroacetic Acid (Tetrac) on Gene Expression in Thyroid Cancer Cells. Genes, 11(7), 755. https://doi.org/10.3390/genes11070755






