The Changes of Expression and Methylation of Genes Involved in Oxidative Stress in Course of Chronic Mild Stress and Antidepressant Therapy with Agomelatine
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chronic Mild Stress Procedure
2.3. Specimen Collection of Peripheral Blood Mononuclear Cells and Homogenates of Animal Brain Tissue
2.4. RNA Isolation, cDNA Synthesis, and Real-Time Analysis of the Expression of Genes Encoding Antioxidant Enzymes
2.5. DNA Isolation, Methylation, and HRM Analysis
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Correlation between Differences in Sucrose Intake of Rats Subjected to Stress and Agomelatine Treatment
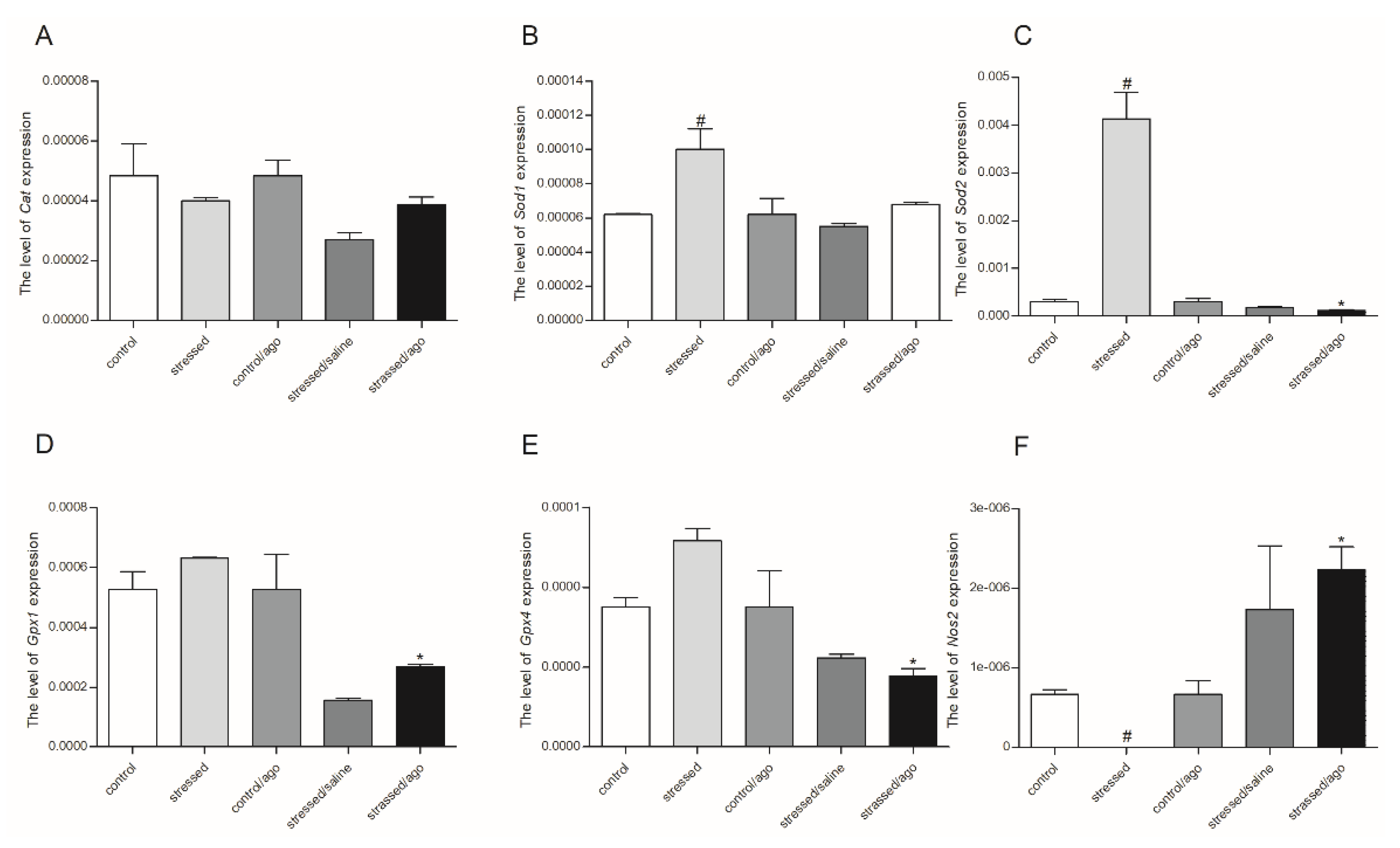
3.2. mRNA Expression Level of the Studied Genes
3.2.1. Gene Expression in PBMCs and Brain Structures
3.2.2. Comparison of Agomelatine Impact on Gene Expressions in PMBCs and Brain Structure
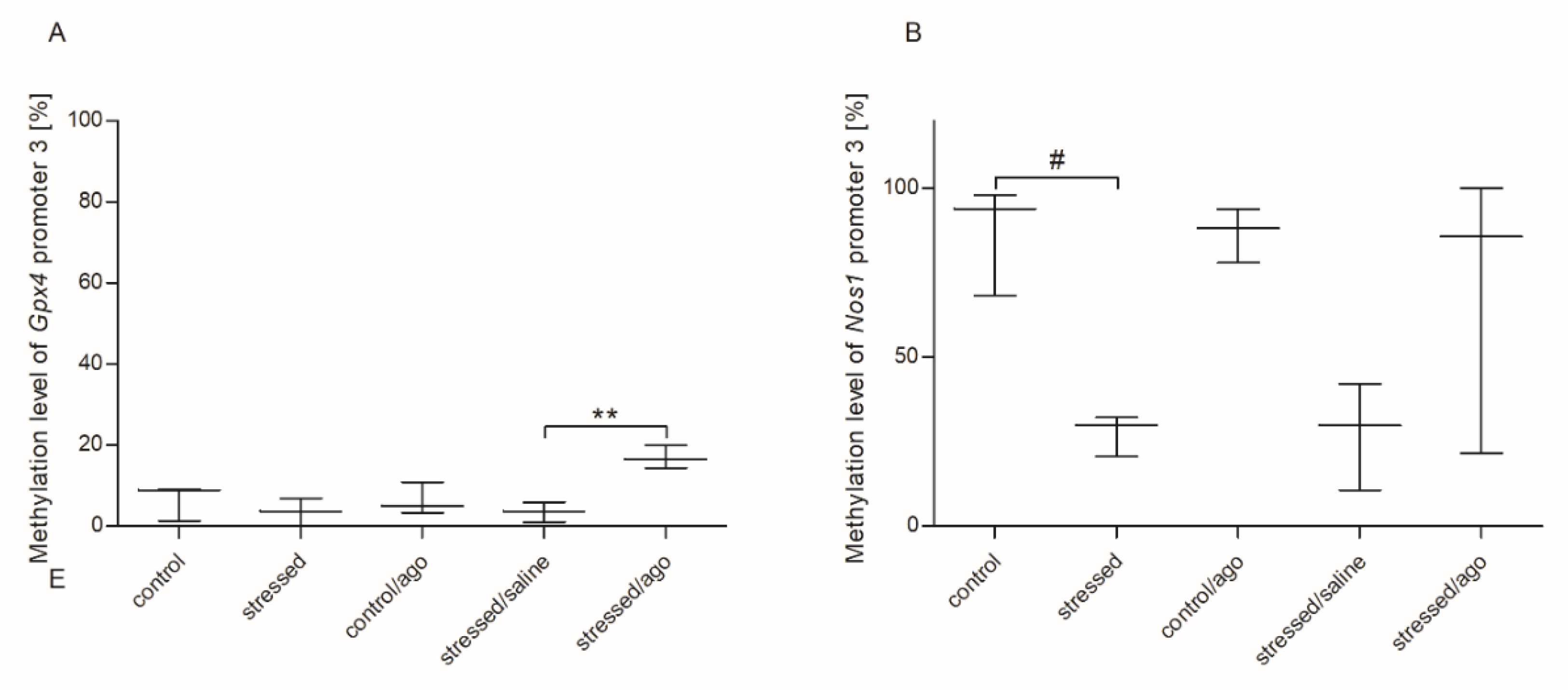
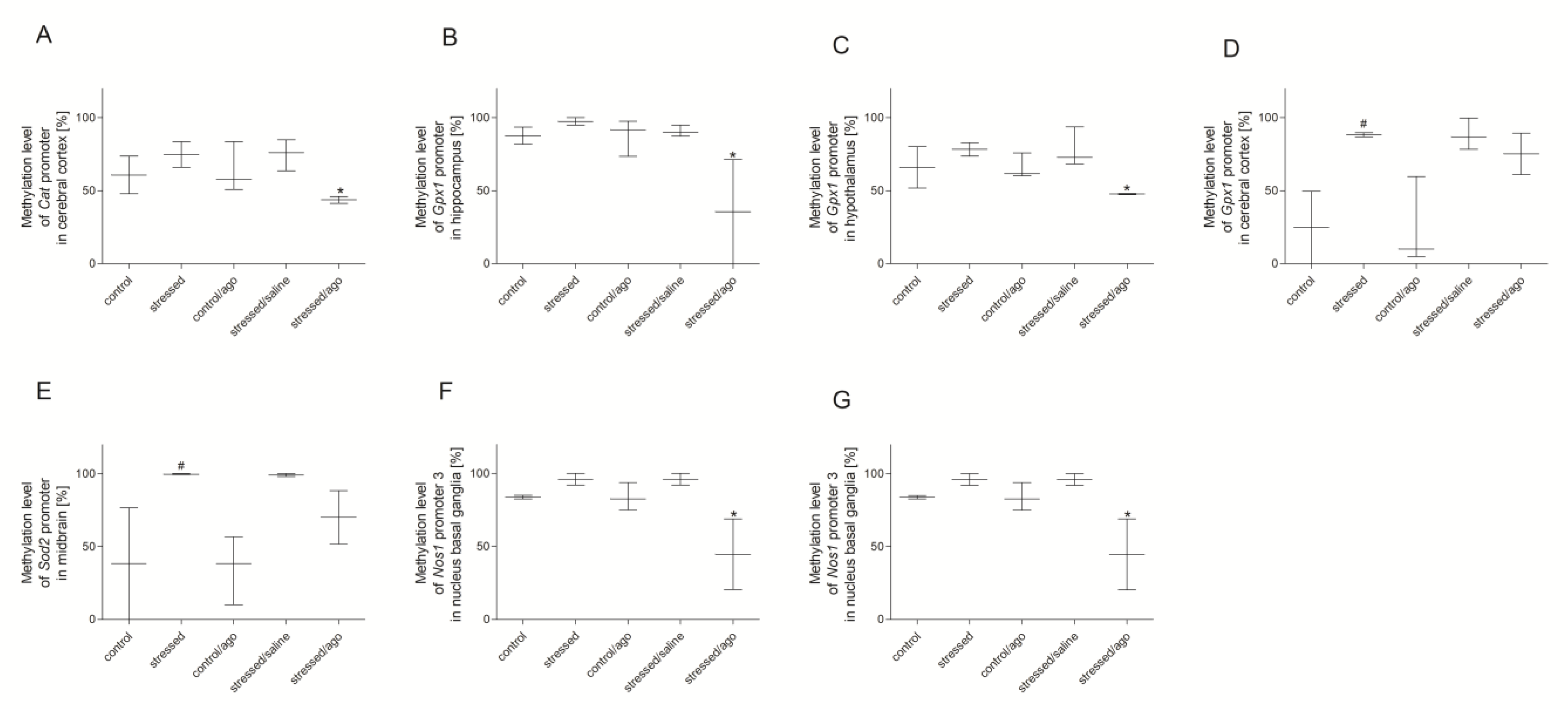
3.3. Methylation Changes in the Studied Gene Promotors
3.3.1. Methylation Status of Gene Promoters in Peripheral Blood Mononuclear Cells and Brain Structures
3.3.2. Comparison of Agomelatine Impact on the Methylation Status of Gene Promoters in PBMCs and Brain Structure
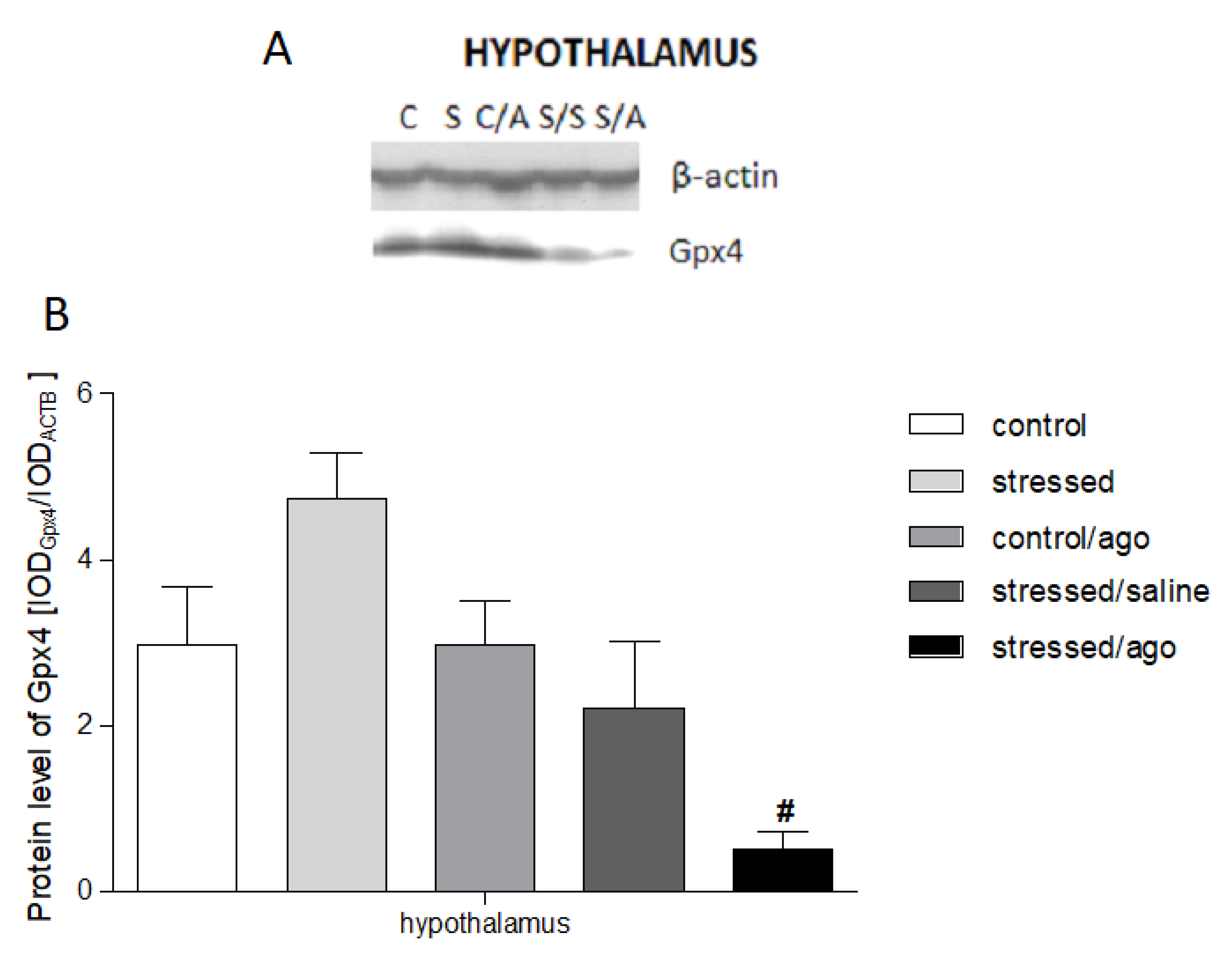
3.4. Gene Expression at Protein Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Availability Statement
References
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 1 January 2020).
- Greden, J.F. The burden of recurrent depression: Causes, consequences, and future prospects. J. Clin. Psychiatry 2001, 62, 5–9. [Google Scholar] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Eren, I.; Nazıroğlu, M.; Demirdaş, A. Protective effects of lamotrigine, aripiprazole and escitalopram on depression-induced oxidative stress in rat brain. Neurochem. Res. 2007, 32, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Eren, I.; Naziroğlu, M.; Demirdaş, A.; Celik, O.; Uğuz, A.C.; Altunbaşak, A.; Ozmen, I.; Uz, E. Venlafaxine modulates depressioninduced oxidative stress in brain and medulla of rat. Neurochem. Res. 2007, 32, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Sharma, B.M.; Sharma, B. Benefits of agomelatine in behavioral, neurochemical and blood brain barrier alterations in prenatal valproic acid induced autism spectrum disorder. Neurochem. Int. 2015, 91, 34–45. [Google Scholar] [CrossRef]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A review on the oxidative andnitrosative stress (O&NS) pathways in major depression and their possiblecontribution to the (neuro) degenerative processes in that illness. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar]
- Kodydková, J.; Vavrova, L.; Zeman, M.; Jirák, R.; Macášek, J.; Stankova, B.; Tvrzicka, E.; Žák, A.; Rychlikova, J. Antioxidative enzymes and increased oxidative stress in depressive women. Clin. Biochem. 2009, 42, 1368–1374. [Google Scholar] [CrossRef]
- Maes, M.; De Vos, N.; Pioli, R.; Demedts, P.; Wauters, A.; Neels, H.; Christophe, A. Lower serum vitamin E concentrations in major depression: Another marker of lowered antioxidant defenses in that illness. J Affect. Disord. 2000, 58, 240–246. [Google Scholar] [CrossRef]
- Maes, M.; Mihaylova, I.; Kubera, M.; Uytterhoeven, M.; Vrydags, N.; Bosmans, E. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol. Lett. 2009, 30, 470–476. [Google Scholar]
- Samuelsson, M.; Gerdin, G.; Ollinger, K.; Vrethem, M. Taurine and glutathione levels in plasma before and after ECT treatment. Psychiatry Res. 2012, 198, 53–57. [Google Scholar] [CrossRef]
- Suh, S.W.; Won, S.J.; Hamby, A.M.; Yoo, B.H.; Fan, Y.; Sheline, C.T.; Tamano, H.; Takeda, A.; Liu, J. Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. J. Cereb. Blood Flow Metab. 2009, 29, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhong, S.; Liao, X.; Chen, J.; He, T.; Lai, S.; Jia, Y. A meta-analysis of oxidative stress markers in depression. PLoS ONE 2015, 10, e0138904. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Mihaylova, I.; Kubera, M.; Uytterhoeven, M.; Vrydags, N.; Bosmans, E. Lower whole blood glutathione peroxidase (GPX) activity in depression, but not in myalgic encephalomyelitis / chronic fatigue syndrome: Another pathway that may be associated with coronary artery disease and neuroprogression in depression. Neuro Endocrinol. Lett. 2011, 32, 133–140. [Google Scholar] [PubMed]
- Rybka, J.; Kedziora-Kornatowska, K.; Banas-Lezanska, P.; Majsterek, I.; Carvalho, L.A.; Cattaneo, A.; Anacker, C.; Kędziora, J. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic. Biol. Med. 2013, 63, 187–194. [Google Scholar] [CrossRef]
- Herken, H.; Gurel, A.; Selek, S.; Armutcu, F.; Ozen, M.E.; Bulut, M.; Kap, O.; Yumru, M.; Savas, H.A.; Akyol, O. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: Impact of antidepressant treatment. Arch. Med. Res. 2007, 38, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Stefanescu, C.; Ciobica, A. The relevance of oxidative stress status in first episode and recurrent depression. J. Affect. Disord. 2012, 143, 34–38. [Google Scholar] [CrossRef]
- Herken, H.; Akyol, O.; Yilmaz, H.R.; Tutkun, H.; Savas, H.A.; Ozen, M.E.; Kalenderoglu, A.; Gulec, M. Nitric oxide, adenosine deaminase, xanthine oxidase and superoxide dismutase in patients with panic disorder: Alterations by antidepressant treatment. Hum. Psychopharmacol. Clin. Exp. 2006, 21, 53–59. [Google Scholar] [CrossRef]
- Michel, T.M.; Camara, S.; Tatschner, T.; Frangou, S.; Sheldrick, A.J.; Riederer, P.; Grünblatt, E. Increased xanthine oxidase in the thalamus and putamen in depression. World J. Biol. Psychiatry 2010, 11, 314–320. [Google Scholar] [CrossRef]
- Bakunina, N.; Pariante, C.M.; Zunszain, P.A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015, 144, 365–373. [Google Scholar] [CrossRef]
- Lange, C.; Irle, E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol. Med. 2004, 34, 1059–1064. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Srinivasan, V.; Cardinali, D.P.; Monti, M.J. Could agomelatine be the ideal antidepressant? Expert Rev. Neurother. 2006, 6, 1595–1608. [Google Scholar] [CrossRef]
- Joffe, R.T.; Levitt, A.J.; Sokolov, S.T. Augmentation strategies: Focus on anxiolytics. J. Clin. Psychiatry 1996, 57, 25–31. [Google Scholar] [PubMed]
- Michel, T.M.; Pülschen, D.; Thome, J. The role of oxidative stress in depressive disorders. Curr. Pharm. Des. 2012, 18, 5890–5899. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Bejarano, I.; Paredes, S.D.; Barriga, C.; Rodríguez, A.B.; Pariente, J.A. Protective effect of melatonin against human leukocyte apoptosis induced by intracellular calcium overload: Relation with its antioxidant actions. J. Pineal Res. 2011, 51, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.R. Melatonin and Circadian Rhythmicity in Vertebrates. In Neurobiology of Sleep and Circadian Rhythms; Turek, F.W., Zee, P., Eds.; Dekker: New York, NY, USA, 1999; pp. 197–262. [Google Scholar]
- Yous, S.; Andrieux, J.; Howell, H.E.; Morgan, P.J.; Renard, P.; Pfeiffer, B.; Lesieur, D.; Guardiola-Lemaître, B. Novel naphthalenic ligands with high affinity for the melatonin receptor. J. Med. Chem. 1992, 35, 1484–1486. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J.; Gobert, A.; Lejeune, F.; Dekeyne, A.; Newman-Tancredi, A.; Pasteau, V.; Rivet, J.M.; Cussac, D. The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J. Pharmacol. Exp. Ther. 2003, 306, 954–964. [Google Scholar] [CrossRef] [PubMed]
- de Bodinat, C.; Guardiola-Lemaitre, B.; Mocaer, E.; Renard, P.; Munoz, C.; Millan, M.J. Agomelatine, the first melatonergic antidepressant: Discovery, characterization and development. Nat. Rev. Drug Discov. 2010, 9, 628–642. [Google Scholar] [CrossRef]
- Krauchi, K.; Cajochen, C.; Mori, D.; Graw, P.; Wirz-Justice, A. Early evening melatonin and S-20098 advance circadian phase and nocturnal regulation of core body temperature. Am. J. Physiol. 1997, 272, R1178–R1188. [Google Scholar] [CrossRef]
- Grassi-Zucconi, G.; Semprevivo, M.; Mocaer, E.; Kristensson, K.; Bentivoglio, M. Melatonin and its new agonist S-20098 restore synchronized sleep fragmented by experimental trypanosome infection in the rat. Brain Res. Bull. 1996, 39, 63–68. [Google Scholar] [CrossRef]
- Redman, J.R.; Guardiola-Lemaitre, B.; Brown, M.; Delagrange, P.; Armstrong, S.M. Dose dependent effects of S-20098, a melatonin agonist, on direction of re-entrainment of rat circadian activity rhythms. Psychopharmacology 1995, 118, 385–390. [Google Scholar] [CrossRef]
- Van Reeth, O.; Weibel, L.; Olivares, E.; Maccari, S.; Mocaer, E.; Turek, F.W. Melatonin or a melatonin agonist corrects age-related changes in circadian response to environmental stimulus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1582–R1591. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; Van Onderbergen, A.; L’Hermite-Baleriaux, M.; Van Cauter, E.; Copinschi, G. Phase-shifts of 24-h rhythms of hormonal release and body temperature following early evening administration of the melatonin agonist agomelatine in healthy older men. Clin. Endocrinol. 2005, 63, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, B. Pharmacological benefits of agomelatine and vanillin in experimental model of Huntington's disease. Pharmacol. Biochem. Behav. 2014, 122, 122–135. [Google Scholar] [CrossRef]
- Inanir, S.; Copoglu, U.S.; Kokacya, H.; Dokuyucu, R.; Erbas, O.; Inanır, A. Agomelatine protection in an LPS-induced psychosis-relevant behavior model. Med. Sci. Monit. 2015, 21, 3834–3839. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karakus, E.; Halici, Z.; Albayrak, A.; Polat, B.; Bayir, Y.; Kiki, I.; Cadirci, E.; Topcu, A.; Aksak, S. Agomelatine: An antidepressant with new potent hepatoprotective effects on paracetamol-induced liver damage in rats. Hum. Exp. Toxicol. 2013, 32, 846–857. [Google Scholar] [CrossRef]
- Wigner, P.; Synowiec, E.; Czarny, P.; Bijak, M.; Jóźwiak, P.; Szemraj, J.; Gruca, P.; Papp, M.; Śliwiński, T. Effects of venlafaxine on the expression level and methylation status of genes involved in oxidative stress in rats exposed to a chronic mild stress. J. Cell Mol. Med. 2020, 24. [Google Scholar] [CrossRef]
- Papp, M. Models of affective illness: Chronic mild stress in the rat. Curr. Protoc. Pharmacol. 2012, 57. [Google Scholar] [CrossRef]
- Papp, M.; Gruca, P.; Boyer, P.-A.; Mocaër, E. Effect of agomelatine in the chronic mild stress model of depression in the rat. Neuropsychopharmacol 2003, 28, 694–703. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–8110. [Google Scholar] [CrossRef]
- Wojdacz, T.K.; Dobrovic, A. Methylation-sensitive high resolution melting (MS-HRM): A new approach for sensitive and high-throughput assessment of methylation. Nucleic Acids Res. 2007, 35, e41. [Google Scholar] [CrossRef]
- Wojdacz, T.K.; Dobrovic, A.; Hansen, L.L. Methylation-sensitive high-resolution melting. Nat. Protoc. 2008, 3, 1903–1908. [Google Scholar] [CrossRef]
- Wojdacz, T.K.; Borgbo, T.; Hansen, L.L. Primer design versus PCR bias in methylation independent PCR amplifications. Epigenetics 2009, 4, 231–234. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Gamaro, G.D.; Manoli, L.P.; Torres, I.L.; Silveira, R.; Dalmaz, C. Effects of chronic variate stress on feeding behavior and on monoamine levels in different rat brain structures. Neurochem. Int. 2003, 42, 107–114. [Google Scholar] [CrossRef]
- Bekris, S.; Antoniou, K.; Daskas, S.; Papadopoulou-Daifoti, Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav. Brain Res. 2005, 161, 45–59. [Google Scholar] [CrossRef]
- Demyttenaere, K. Agomelatine: A narrative review. Eur. Neuro Psychopharmacol. 2011, 21, S703–S709. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Shigenaga, M.K.; Yeo, H.C.; Mori, A.; Ames, B.N. Immobilization stress causes oxidative damage to lipid, protein, and DNA in the brain of rats. FASEB J. 1996, 10, 1532–1538. [Google Scholar] [CrossRef]
- Fontella, F.U.; Siqueira, I.R.; Vasconcellos, A.P.; Tabajara, A.S.; Netto, C.A.; Dalmaz, C. Repeated restraint stress induces oxidative damage in rat hippocampus. Neurochem. Res. 2005, 30, 105–111. [Google Scholar] [CrossRef]
- Lucca, G.; Comim, C.M.; Valvassori, S.S.; Réus, G.Z.; Vuolo, F.; Petronilho, F.; Dal-Pizzol, F.; Gavioli, E.C.; Quevedo, J. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem. Int. 2009, 54, 358–362. [Google Scholar] [CrossRef]
- Bilici, M.; Efe, H.; Köroğlu, M.; Uydu, H.A.; Bekaroğlu, M.; Değer, O. Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments. J. Affect. Disord. 2001, 64, 43–51. [Google Scholar] [CrossRef]
- Demirdaş, A.; Nazıroğlu, M.; Ünal, G.Ö. Agomelatine reduces brain, kidney and liver oxidative stress but increases plasma cytokine production in the rats with chronic mild stress-induced depression. Metab. Brain Dis. 2016, 31, 1445–1453. [Google Scholar] [CrossRef]
- Akpinar, A.; Uğuz, A.C.; Nazıroğlu, M. Agomelatine and duloxetine synergistically modulates apoptotic pathway by inhibiting oxidative stress triggered intracellular calcium entry in neuronal PC12 cells: Role of TRPM2 and voltage-gated calcium channels. J. Membr. Biol. 2014, 247, 451–459. [Google Scholar] [CrossRef]
- Aguiar, C.C.T.; Almeida, A.B.; Araújo, P.V.P.; Vasconcelos, G.S.; Chaves, E.M.C.; Vale, O.C.D.; Macedo, D.S.; Leal, L.K.A.M.; Viana, G.S.D.B.; Vasconcelos, S. Effects of agomelatine on oxidative stress in the brain of mice after chemically induced seizures. Cell Mol. Neurobiol. 2013, 33, 825–835. [Google Scholar] [CrossRef]
- Kharwar, R.K.; Haldar, C. Daily variation in antioxidant enzymes and lipid peroxidation in lungs of a tropical bird Perdicula asiatica: Role of melatonin and nuclear receptor RORα. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 162, 296–302. [Google Scholar] [CrossRef]
- Tsai, M.C.; Huang, T.L. Increased activities of both superoxide dismutase and catalase were indicators of acute depressive episodes in patients with major depressive disorder. Psychiatry Res. 2016, 235, 38–42. [Google Scholar] [CrossRef]
- De Mello, A.H.; Souza, L.D.R.; Cereja, A.C.M.; Schraiber, R.D.B.; Florentino, D.; Martins, M.M.; Rezin, G.T.; Petronilho, F.; Quevedo, J. Effect of subchronic administration of agomelatine on brain energy metabolism and oxidative stress parameters in rats. Psychiatry Clin. Neurosci. 2016, 70, 159–166. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.X.; Chen, X.; Qin, X.Y.; Kardami, E.; Cheng, Y. Antidepressant-like effects of low- and high-molecular weight FGF-2 on chronic unpredictable mild stress mice. Front. Mol. Neurosci. 2018, 11, 377. [Google Scholar] [CrossRef]
- Selek, S.; Savas, H.A.; Gergerlioglu, H.S.; Bulbul, F.; Uz, E.; Yumru, M. The course of nitric oxide and superoxide dismutase during treatment of bipolar depressive episode. J. Affect. Disord. 2008, 107, 89–94. [Google Scholar] [CrossRef]
- Suzuki, E.; Yagi, G.; Nakaki, T.; Kanba, S.; Asai, M. Elevated plasma nitrate levels in depressive states. J. Affect. Disord. 2001, 63, 221–224. [Google Scholar] [CrossRef]
- Scapagnini, G.; Davinelli, S.; Drago, F.; De Lorenzo, A.; Oriani, G. Antioxidants as antidepressants: Fact or fiction? CNS Drugs 2012, 26, 477–490. [Google Scholar] [CrossRef]
- Pozo, D.; Reiter, R.J.; Calvo, J.R.; Guerrero, J.M. Inhibition of cerebellar nitric oxide synthase and cyclic GMP production by melatonin via complex formation with calmodulin. J. Cell Biochem. 1997, 65, 430–442. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; López, L.C.; Hitos, A.B.; León, J. Melatonin and nitric oxide. Endocrine 2005, 27, 159–168. [Google Scholar] [CrossRef]
- Storr, M.; Koppitz, P.; Sibaev, A.; Saur, D.; Kurjak, M.; Franck, H.; Schusdziarra, V.; Allescher, H.-D. Melatonin reduces non-adrenergic, non-cholinergic relaxant neurotransmission by inhibition of nitric oxide synthase activity in the gastrointestinal tract of rodents in vitro. J. Pineal Res. 2002, 33, 101–108. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K. Synaptic dysfunction in depression: Potential therapeutic targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef]
- Wigner, P.; Czarny, P.; Galecki, P.; Su, K.P.; Sliwinski, T. The molecular aspects of oxidative & nitrosative stress and the tryptophan catabolites pathway (TRYCATs) as potential causes of depression. Psychiatry Res. 2018, 262, 566–574. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wigner, P.; Synowiec, E.; Jóźwiak, P.; Czarny, P.; Bijak, M.; Barszczewska, G.; Białek, K.; Szemraj, J.; Gruca, P.; Papp, M.; et al. The Changes of Expression and Methylation of Genes Involved in Oxidative Stress in Course of Chronic Mild Stress and Antidepressant Therapy with Agomelatine. Genes 2020, 11, 644. https://doi.org/10.3390/genes11060644
Wigner P, Synowiec E, Jóźwiak P, Czarny P, Bijak M, Barszczewska G, Białek K, Szemraj J, Gruca P, Papp M, et al. The Changes of Expression and Methylation of Genes Involved in Oxidative Stress in Course of Chronic Mild Stress and Antidepressant Therapy with Agomelatine. Genes. 2020; 11(6):644. https://doi.org/10.3390/genes11060644
Chicago/Turabian StyleWigner, Paulina, Ewelina Synowiec, Paweł Jóźwiak, Piotr Czarny, Michał Bijak, Gabriela Barszczewska, Katarzyna Białek, Janusz Szemraj, Piotr Gruca, Mariusz Papp, and et al. 2020. "The Changes of Expression and Methylation of Genes Involved in Oxidative Stress in Course of Chronic Mild Stress and Antidepressant Therapy with Agomelatine" Genes 11, no. 6: 644. https://doi.org/10.3390/genes11060644
APA StyleWigner, P., Synowiec, E., Jóźwiak, P., Czarny, P., Bijak, M., Barszczewska, G., Białek, K., Szemraj, J., Gruca, P., Papp, M., & Śliwiński, T. (2020). The Changes of Expression and Methylation of Genes Involved in Oxidative Stress in Course of Chronic Mild Stress and Antidepressant Therapy with Agomelatine. Genes, 11(6), 644. https://doi.org/10.3390/genes11060644




