Genome-Wide Association Study of Muscle Glycogen in Jingxing Yellow Chicken
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Resource Population
2.3. Phenotypic Measurement
2.4. Quality Control and Imputation
2.5. Whole-Genome Resequencing
2.6. Population Structure and Association Analysis
2.7. Gene Identification and Annotation
3. Results
3.1. Phenotypic Data and Structural Analysis
3.2. Genome-Wide Association Studies (GWAS) for Single Nucleotide Polymorphism (SNP) and Additive Effect
3.3. GWAS for Insertions and Deletions (INDEL) and Additive Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Walvekar, M.V.; Bhopale, L.P.; Sarvalkar, P.P. Sialoadenectomy effect on blood glucose level and skeletal muscle glycogen from male and female mice. J. Exp. Zool. India 2011, 14, 145–148. [Google Scholar]
- Febbraio, M.A.; Keenan, J.; Angus, D.J.; Campbell, S.E.; Garnham, A.P. Preexercise carbohydrate ingestion, glucose kinetics, and muscle glycogen use: Effect of the glycemic index. J. Appl. Physiol. 2000, 89, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Gariépy, C.; Godbout, D.; Fernandez, X.; Talmant, A.; Houde, A. The effect of RN gene on yields and quality of extended cooked cured hams. Meat Sci. 1999, 52, 57–64. [Google Scholar] [CrossRef]
- Hamilton, D.N.; Miller, K.D.; Ellis, M.; McKeith, F.K.; Wilson, E.R. Relationships between longissimus glycolytic potential and swine growth performance, carcass traits, and pork quality. J. Anim. Sci. 2003, 81, 2206–2212. [Google Scholar] [CrossRef]
- Rosenvold, K.; Petersen, J.S.; Lwerke, H.N.; Jensen, S.; Therkildsen, M.; Karlsson, A.H.; Møller, H.S.; Andersen, H.J. Muscle glycogen stores and meat quality as affected by strategic finishing feeding of slaughter pigs. J. Anim. Sci. 2001, 79, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Bee, G.; Biolley, C.; Guex, G.; Herzog, W.; Lonergan, S.M.; Huff-Lonergan, E. Effects of available dietary carbohydrate and preslaughter treatment on glycolytic potential, protein degradation, and quality traits of pig muscles. J. Anim. Sci. 2006, 84, 191–203. [Google Scholar] [CrossRef]
- Le Bihan-Duval, É.; Millet, N.; Remignon, H. Broiler meat quality: Effect of selection for increased carcass quality and estimates of genetic parameters. Poult. Sci. 1999, 78, 822–826. [Google Scholar] [CrossRef]
- Le Bihan-Duval, É.; Berri, C.; Baeza, E.; Millet, N.; Beaumont, C. Estimation of the genetic parameters of meat characteristics and of their genetic correlations with growth and body composition in an experimental broiler line. Poult. Sci. 2001, 80, 839–843. [Google Scholar] [CrossRef]
- Sudre, K.; Cassar-Malek, I.; Listrat, A.; Ueda, Y.; Leroux, C.; Jurie, C.; Auffray, C.; Renand, G.; Martin, P.; Hocquette, J.F. Biochemical and transcriptomic analyses of two bovine skeletal muscles in Charolais bulls divergently selected for muscle growth. Meat Sci. 2005, 70, 267–277. [Google Scholar] [CrossRef]
- Le Bihan-Duval, É.; Debut, M.; Berri, C.; Sellier, N.; Santé-Lhoutellier, V.; Jégo, Y.; Beaumont, C. Chicken meat quality: Genetic variability and relationship with growth and muscle characteristics. BMC Genet. 2008, 9, 53. [Google Scholar] [CrossRef]
- Larzul, C.; Roy, P.L.; Gogué, J.M.; Talmant, A.; Jacquet, B.; Lefaucheur, L.; Ecolan, P.; Sellier, P.; Monin, G. Selection for reduced muscle glycolytic potential in Large White pigs. II. Correlated responses in meat quality and muscle compositional traits. Genet. Sel. Evol. 1999, 31, 61–76. [Google Scholar] [CrossRef]
- Stratton, M. Genome resequencing and genetic variation. Nat. Biotechnol. 2008, 26, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.C. Next-generation sequencing transforms today’s biology. Nat. Methods 2008, 5, 16. [Google Scholar] [CrossRef]
- Hardy, J.; Singleton, A. Genome-wide association studies and human disease. New Engl. J. Med. 2009, 360, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.A. Statistical false positive or true disease pathway? Nat. Genet. 2006, 38, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-W.; Yang, J.; Zhou, L.; Ren, J.; Liu, X.; Zhang, H.; Yang, B.; Zhang, Z.; Ma, H.; Xie, X.; et al. A splice mutation in the PHKG1 gene causes high glycogen content and low meat quality in pig skeletal muscle. PLoS Genet. 2014, 10, e1004710. [Google Scholar] [CrossRef]
- Browning, B.; Zhou, Y.; Browning, S. A One-penny imputed genome from next-generation reference panels. Am. J. Hum. Genet. 2018, 103, 338–348. [Google Scholar] [CrossRef]
- Browning, S.; Browning, B. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 2007, 81, 1084–1097. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience 2015, 4, 7. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Xiang, Z.; Matthew, S. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012, 44, 821–824. [Google Scholar]
- McLaren, W.; Pritchard, B.; Rios, D.; Chen, Y.; Flicek, P.; Cunningham, F. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics 2010, 26, 2069–2070. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, R.J.; Kähäri, A.; Haider, S.; Zamora, J.; Proctor, G.; Spudich, G.; Almeida-King, J.; Staines, D.; Derwent, P.; Kerhornou, A.; et al. Ensembl BioMarts: A hub for data retrieval across taxonomic space. Database 2011, 2011, bar030. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, A. BioMart: Driving a paradigm change in biological data management. Database 2011, 2011, bar049. [Google Scholar] [CrossRef]
- Pearson, T.A.; Manolio, T.A. How to interpret a genome-wide association study. JAMA 2008, 299, 1335–1344. [Google Scholar] [CrossRef]
- Essén-Gustavsson, B.; Jensen-Waern, M.; Jonasson, R.; Andersson, L. Effect of exercise on proglycogen and macroglycogen content in skeletal muscles of pigs with the Rendement Napole mutation. Am. J. Vet. Res. 2005, 66, 1197–1201. [Google Scholar] [CrossRef]
- Weber, J.L.; David, D.; Heil, J.; Fan, Y.; Zhao, C.; Marth, G. Human diallelic insertion/deletion polymorphisms. Am. J. Hum. Genet. 2002, 71, 854–862. [Google Scholar] [CrossRef]
- Jander, G.; Norris, S.R.; Rounsley, S.D.; Bush, D.F.; Levin, I.M.; Last, R.L. Arabidopsis map-based cloning in the post-genome era. Plant Physiol. 2002, 129, 440–450. [Google Scholar] [CrossRef]
- Christudass, C.S.; Veltri, R.W.; Lelin, A.; Chai, E.; De Marzo, A.; Partin, A.W.; Marzo, A.D. Abstract 3640: Overexpression of five novel biomarkers in human prostate tissues and cell lines. Clin. Trials 2012, 72, 3640. [Google Scholar]
- Pellerin, L.; Magistretti, P.J. NEUROSCIENCE: Let there be (NADH) light. Science 2004, 305, 50–52. [Google Scholar] [CrossRef]
- Caesar, K.; Hashemi, P.; Douhou, A.; Bonvento, G.; Boutelle, M.G.; Walls, A.B.; Lauritzen, M. Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J. Physiol. 2008, 586, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, H.; Zhong, F.; Zhang, W.; Lee, K.; He, J.C. Expression of glutamate receptor subtype 3 is epigenetically regulated in podocytes under diabetic conditions. Kidney Dis. (Basel) 2019, 5, 34–42. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, T.M.; Pontsler, A.V.; Silva, A.R.; Hilaire, A.S.; Xu, Y.; Hinshaw, J.C.; Zimmerman, G.A.; Hama, K.; Aoki, J.; Arai, H.; et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARγ agonist. Proc. Natl. Acad. Sci. USA 2002, 100, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Chouvarine, P.; Legchenko, E.; Hoffmann, N.; Geldner, J.; Borchert, P.; Jonigk, D.; Mozes, M.M.; Hansmann, G. PPARγ Links BMP2 and TGFβ1 pathways in vascular smooth muscle cells, regulating cell proliferation and glucose metabolism. Cell Metab. 2017, 25, 1118–1134. [Google Scholar] [CrossRef]
- Festuccia, W.T.; Blanchard, P.-G.; Deshaies, Y. Control of brown adipose tissue glucose and lipid metabolism by PPARγ. Front. Endocrinol. 2011, 2, 84. [Google Scholar] [CrossRef]
- Schmidt, C.; Otto, A.; Luke, G.; Valášek, P.; Otto, W.R.; Patel, K. Expression and regulation of Nkd-1, an intracellular component of Wnt signalling pathway in the chick embryo. Brain Struct. Funct. 2006, 211, 525–534. [Google Scholar] [CrossRef]
- Woodgett, J.R. Regulation and functions of the glycogen synthase kinase-3 subfamily. Semin. Cancer Biol. 1994, 5, 269–275. [Google Scholar]
- Ali, A.; Hoeflich, K.P.; Woodgett, J.R. Glycogen synthase kinase-3: Properties, functions, and regulation. Chem. Rev. 2001, 101, 2527–2540. [Google Scholar] [CrossRef]
- Rayasam, G.V.; Tulasi, V.K.; Sodhi, R.; Davis, J.A.; Ray, A. Glycogen synthase kinase 3: More than a namesake. Br. J. Pharmacol. 2009, 156, 885–898. [Google Scholar] [CrossRef]
- Hamilton, D.N.; Ellis, M.; Hemann, M.D.; McKeith, F.K.; Miller, K.D.; Purser, K.W. The impact of longissimus glycolytic potential and short-term feeding of magnesium sulfate heptahydrate prior to slaughter on carcass characteristics and pork quality. J. Anim. Sci. 2002, 80, 1586–1592. [Google Scholar] [CrossRef]
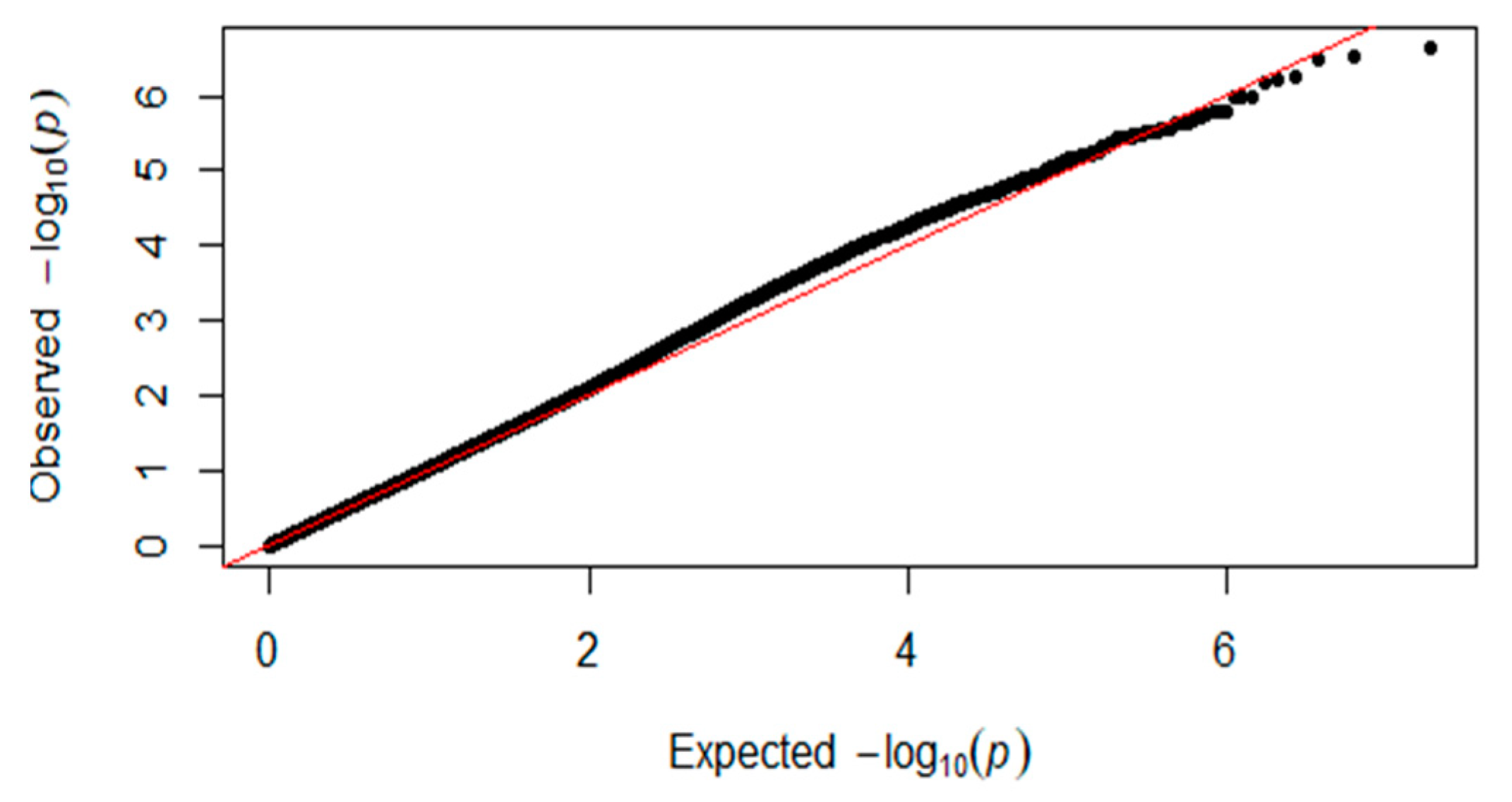
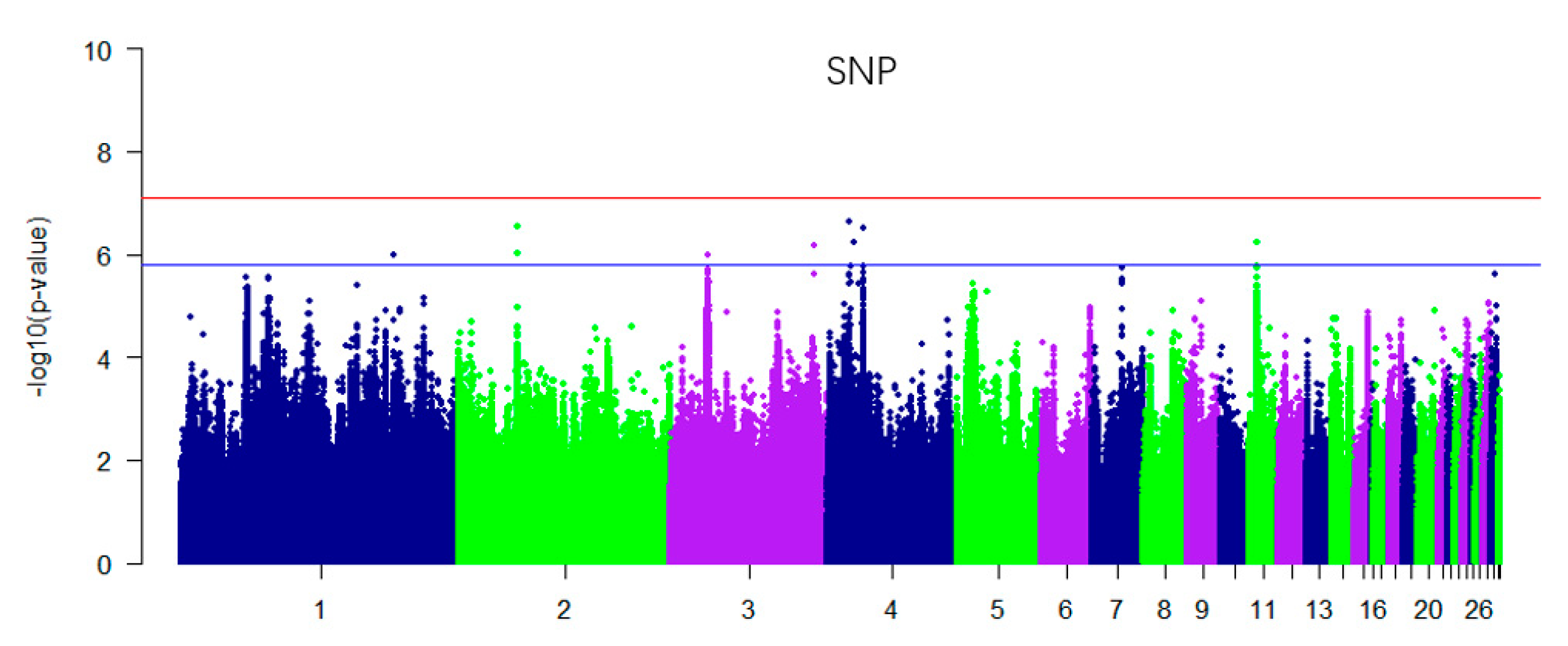

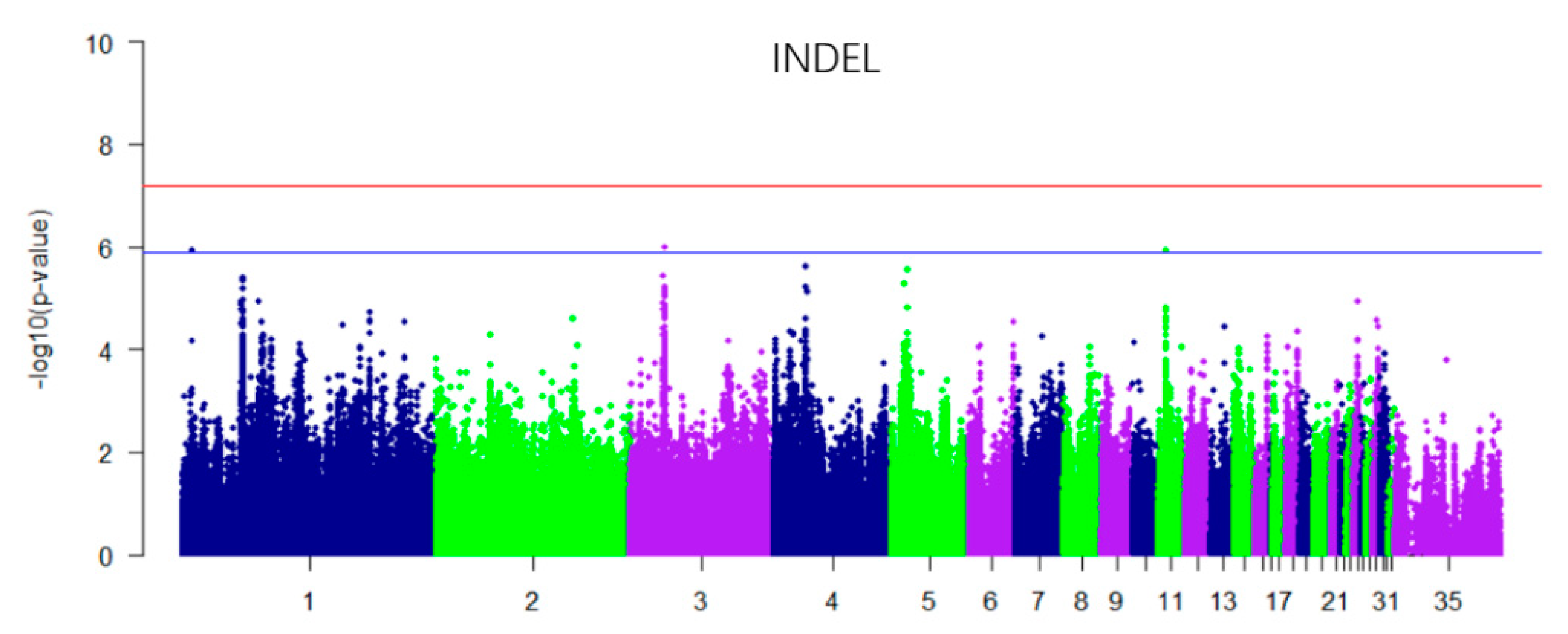
| Chromosome | SNP ID | Position (BP) | p_Wald | Nearest Gene | Location (Kb) |
|---|---|---|---|---|---|
| 1 | rs314624646 | 150449184 | 1.05 × 10−06 | / | / |
| 2 | rs313265900 | 42074647 | 2.89 × 10−07 | CPNE4 | Intronic |
| 2 | NEW | 42078810 | 9.95 × 10−07 | CPNE4 | Intronic |
| 3 | rs13720604 | 26983490 | 1.04 × 10−06 | MCFD2 | D12.4 |
| TTC7A | U26.6 | ||||
| SOCS5 | D72.7 | ||||
| 3 | rs740265511 | 101450476 | 6.58 × 10−07 | OSR1 | D18.5 |
| 4 | rs733544036 | 15746019 | 2.29 × 10−07 | GRIA3 | U62.1 |
| 4 | rs741487544 | 18209706 | 5.66 × 10−07 | IDS | U53.5 |
| 4 | rs315075611 | 25507719 | 3.14 × 10−07 | MFAP3L | D30.8 |
| CLCN3 | U32.8 | ||||
| HPF1 | D22.1 | ||||
| 11 | rs734443657 | 6224928 | 6.02 × 10−07 | CYLD | D50.2 |
| SNX20 | U73.2 |
| Chr | Position (BP) | ref | alt | freq_ref|ref | freq_ref|alt/alt|ref | freq_alt|alt | pheno_ref|ref | pheno_ref|alt | pheno_alt|alt | Additive Effect |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 150449184 | C | T | 0.1308 | 0.3059 | 0.5633 | 3.1108 | 2.4512 | 2.1559 | –0.4775 |
| 2 | 42074647 | C | T | 0.8376 | 0.1414 | 0.0211 | 2.2755 | 2.5604 | 4.8972 | 1.3108 |
| 2 | 42078810 | A | C | 0.8586 | 0.1224 | 0.0190 | 2.2726 | 2.7663 | 4.2772 | 1.0023 |
| 3 | 26983490 | G | A | 0.4515 | 0.4051 | 0.1435 | 2.6424 | 2.2138 | 1.9615 | –0.3405 |
| 3 | 101450476 | C | T | 0.5380 | 0.2025 | 0.2595 | 2.6404 | 2.1277 | 2.0027 | –0.3188 |
| 4 | 15746019 | G | T | 0.8658 | 0.1216 | 0.0126 | 2.2689 | 2.8665 | 4.5671 | 1.1491 |
| 4 | 18209706 | A | G | 0.8692 | 0.1097 | 0.0211 | 2.2713 | 2.8023 | 4.2408 | 0.9847 |
| 4 | 25507719 | A | G | 0.8847 | 0.1048 | 0.0105 | 2.2757 | 2.9680 | 4.3935 | 1.0589 |
| 11 | 6224928 | G | A | 0.6603 | 0.2869 | 0.0527 | 2.1946 | 2.6683 | 2.9648 | 0.3851 |
| Chr | Position (BP) | ref | alt | freq_ref|ref | freq_ref| alt/alt |ref | freq_ alt|alt | pheno_ref|ref | pheno_ref| alt | pheno_ alt|alt | Additive Effect |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7169549 | G | GACAA | 0.8421 | 0.1308 | 0.0219 | 2.2696 | 2.8068 | 3.9756 | 0.8530 |
| 3 | 27425548 | T | TA | 0.3861 | 0.3991 | 0.2148 | 2.1143 | 2.3646 | 2.8366 | 0.3612 |
| 11 | 6376492 | AG | A | 0.5416 | 0.3539 | 0.1045 | 2.1395 | 2.5656 | 2.9383 | 0.3994 |
| Chromosome | Position (BP) | p_Wald | Nearest Gene | Location (Kb) |
|---|---|---|---|---|
| 1 | 7169549 | 1.23 × 10−06 | / | / |
| 3 | 27425548 | 1.03 × 10−06 | FOSL2 | U29.7Kb |
| 11 | 6376492 | 1.18 × 10−06 | NKD1 | Intronic |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, L.; Wang, J.; Cui, H.; Chu, H.; Bi, H.; Zhao, G.; Wen, J. Genome-Wide Association Study of Muscle Glycogen in Jingxing Yellow Chicken. Genes 2020, 11, 497. https://doi.org/10.3390/genes11050497
Liu X, Liu L, Wang J, Cui H, Chu H, Bi H, Zhao G, Wen J. Genome-Wide Association Study of Muscle Glycogen in Jingxing Yellow Chicken. Genes. 2020; 11(5):497. https://doi.org/10.3390/genes11050497
Chicago/Turabian StyleLiu, Xiaojing, Lu Liu, Jie Wang, Huanxian Cui, Huanhuan Chu, Huijuan Bi, Guiping Zhao, and Jie Wen. 2020. "Genome-Wide Association Study of Muscle Glycogen in Jingxing Yellow Chicken" Genes 11, no. 5: 497. https://doi.org/10.3390/genes11050497
APA StyleLiu, X., Liu, L., Wang, J., Cui, H., Chu, H., Bi, H., Zhao, G., & Wen, J. (2020). Genome-Wide Association Study of Muscle Glycogen in Jingxing Yellow Chicken. Genes, 11(5), 497. https://doi.org/10.3390/genes11050497




