Exploring the Mechanisms of Multiple Insecticide Resistance in a Highly Plasmodium-Infected Malaria Vector Anopheles funestus Sensu Stricto from Sahel of Northern Nigeria
Abstract
1. Introduction
2. Materials and Methods
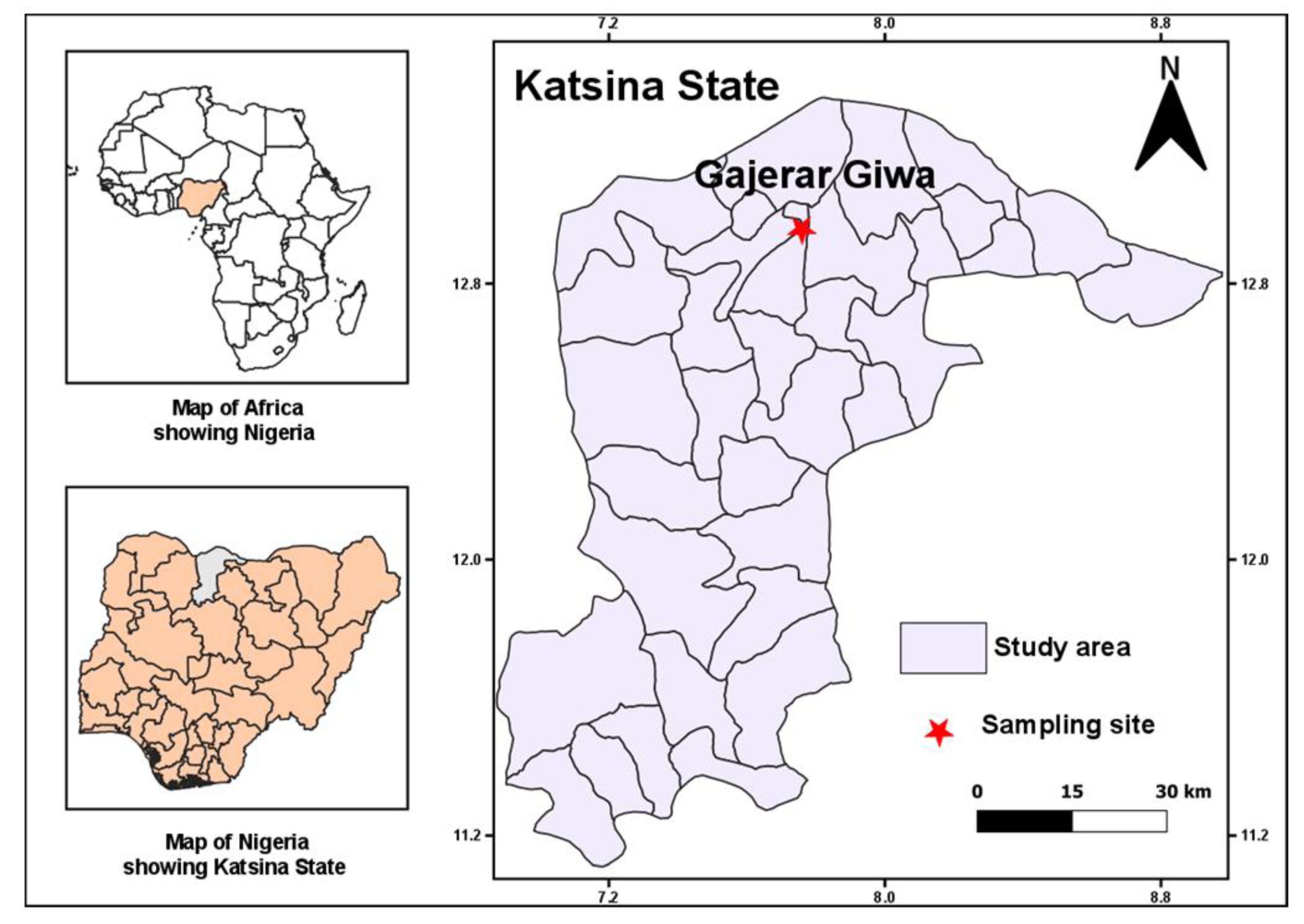
2.1. Study Site and Mosquito Sampling
2.2. Molecular Identification of Mosquito Species
2.3. Estimation of Entomological and Parasitological Parameters
2.3.1. Estimation of Indoor Resting Density, Human Blood Index and Biting Rate
2.3.2. Estimation of Sporozoite Rate
2.4. WHO Insecticides Susceptibility Tests
2.5. Estimation of Resistance Intensity with Time-Course Bioassay
2.6. Determination of LLIN Efficacy with Cone Bioassays
2.7. Synergist Bioassay with Piperonyl Butoxide (PBO) and Diethyl Maleate
2.8. Genotyping of L119F Glutathione S-Transferase (GSTe2) Mutation Associated with DDT/Permethrin Resistance
2.9. Polymorphism Analysis of Acetylcholinesterase-1 (ace-1) for G119S and N485I Target-Site Mutations
2.10. Polymorphism Analysis of Voltage-Gated Sodium Channel Gene Spanning the 1014 kdr Locus
2.11. Data Analysis
2.12. Ethical Approval
3. Results
3.1. Composition of the Mosquito Species Collected Indoor
3.2. Entomological and Parasitological Parameters of Transmission
3.2.1. Indoor Resting Density, Human Blood Index and Biting Rate
3.2.2. Estimation of Sporozoite Infection Rate
3.3. Insecticide Resistance Profile of An. funestus Population
3.4. Pyrethroid Resistance Intensity
3.5. Bed Net Efficacy Using Cone Bioassay
3.6. Investigating the Role of Metabolic Resistance Using Synergist Bioassays
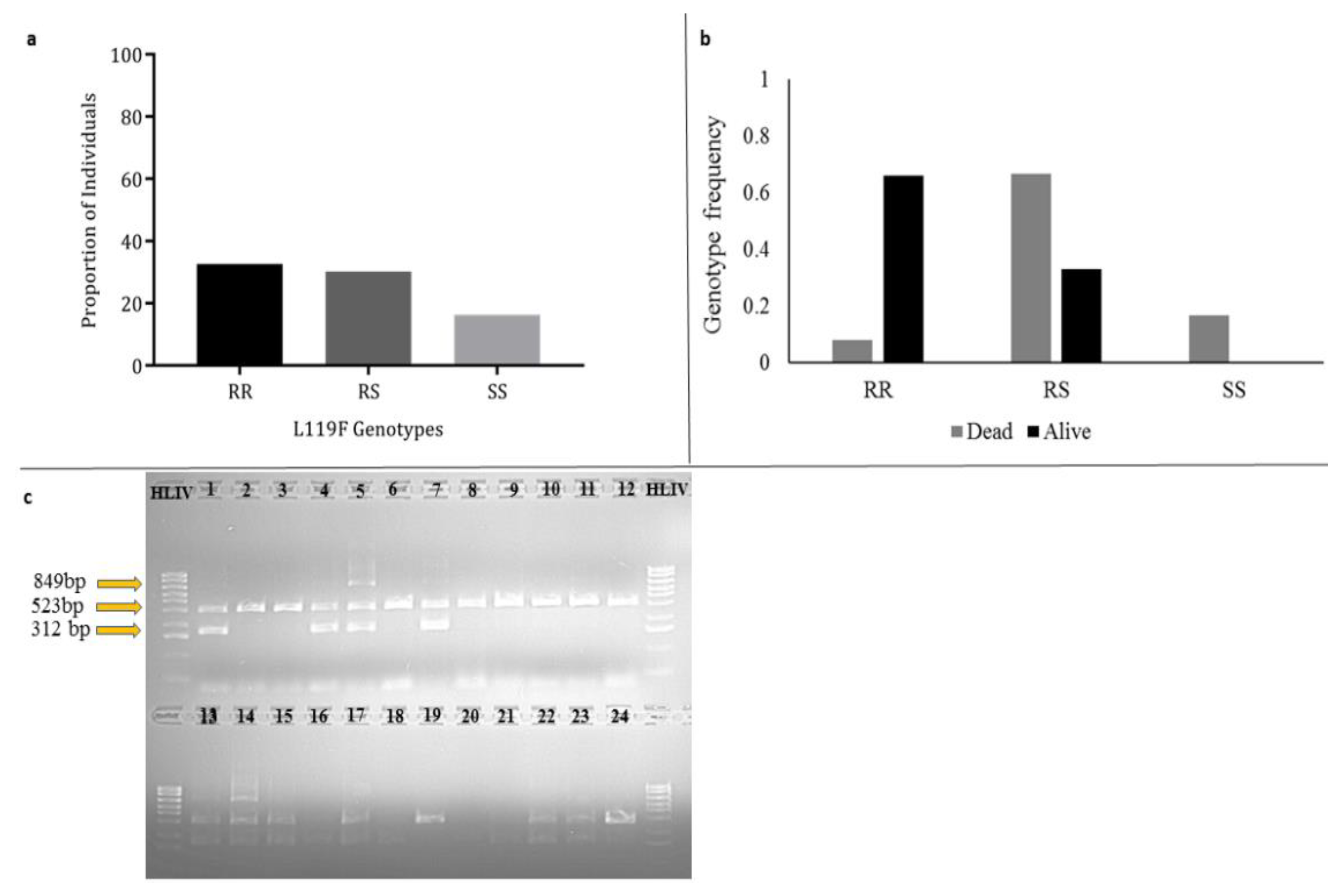
3.7. Investigating the Role of L119F-GSTe2 Mutation in DDT Resistance
3.8. Polymorphism Analysis of the ace-1 Fragment Encompassing the G119S and N485I Resistance Mutations
3.9. Polymorphism Analysis of the Voltage-Gated Sodium Channel Encompassing the L1014F/S Knockdown Resistance Mutation
4. Discussion
4.1. Evidence of Unusually High Transmission Capability by Gajerar Giwa An. funestus
4.2. Evidence of Multiple Resistance in the Gajerar Giwa An. funestus
4.3. A Low-Efficacy of Long-Lasting Insecticidal Nets Is a Serious Challenge to Malaria Control
4.4. Insecticide Resistance Mechanism Is Driven by Metabolic Resistance Mechanisms in Gajerar Giwa An. funestus Population
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. World Malaria Report; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Alonso, P.; Noor, A.M. The global fight against malaria is at crossroads. Lancet 2017, 390, 2532–2534. [Google Scholar] [CrossRef]
- WHO. World Malaria Report; World Health Organisation: Geneva, Switzerland, 2017; ISBN 978-92-4-156552-3. [Google Scholar]
- Ohiri, K.; Ukoha, N.K.; Nwangwu, C.W.; Chima, C.C.; Ogundeji, Y.K.; Rone, A.; Reich, M.R. An Assessment of Data Availability, Quality, and Use in Malaria Program Decision Making in Nigeria. Health Syst. Reform 2016, 2, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.; Mukhtar, M.M.; Datti, J.A.; Irving, H.; Kusimo, M.O.; Tchapga, W.; Lawal, N.; Sambo, F.I.; Wondji, C.S. Temporal escalation of Pyrethroid Resistance in the major malaria vector Anopheles coluzzii from Sahelo-Sudanian Region of northern Nigeria. Sci. Rep. 2019, 9, 7395. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.; Manu, Y.A.; Tukur, Z.; Irving, H.; Wondji, C.S. High frequency of kdr L1014F is associated with pyrethroid resistance in Anopheles coluzzii in Sudan savannah of northern Nigeria. BMC Infect. Dis. 2014, 14, 441. [Google Scholar] [CrossRef][Green Version]
- Oduola, A.O.; Abba, E.; Adelaja, O.; Taiwo-Ande, A.; Poloma-Yoriyo, K.; Samson-Awolola, T. Widespread Report of Multiple Insecticide Resistance in Anopheles gambiae s.l. Mosquitoes in Eight Communities in Southern Gombe, North-Eastern Nigeria. J. Arthropod-Borne Dis. 2019, 13, 50–61. [Google Scholar] [CrossRef]
- Bruce-Chwatt, L.; Haworth, J. Annual Report of the Malarial Control Pilot Project in Western Sokoto (Northern Nigeria) 1955–56. Int. J. Pest Manag. A 1957, 3, 18–22. [Google Scholar] [CrossRef]
- WHO. A Detailed Examination of An. funestus from Southern and Northern Nigeria; World Health Organization: Geneva, Switzerland, 1959. [Google Scholar]
- Service, M. Some basic entomological factors concerned with the transmission and control of malaria in Northern Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1965, 59, 291–296. [Google Scholar] [CrossRef]
- Service, M.W. The behaviour of malaria vectors in huts sprayed with DDT and with a mixture of DDT and malathion in Northern Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1964, 58, 72–79. [Google Scholar] [CrossRef]
- Molineaux, L.; Gramiccia, G. The Garki Project: Research on the Epidemiology and Control of Malaria in the Sudan Savanna of West Africa; World Health Organisation: Geneva, Switzerland, 1980. [Google Scholar]
- Bruce-Chwatt, L.J. Malaria in Nigeria. Bull. World Health Organ. 1951, 4, 301. [Google Scholar]
- Gillies, M.T.; De Meillon, B. The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region). S. Afr. Inst. Med. Res. 1968, 54l, 1–343. [Google Scholar]
- Coetzee, M.; Fontenille, D. Advances in the study of Anopheles funestus, a major vector of malaria in Africa. Insect Biochem. Mol. Boil. 2004, 34, 599–605. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Technical Strategy for Malaria 2016–2030; World Health Organisation: Geneva, Switzerland, 2015; ISBN 978 92 4 156499 1. [Google Scholar]
- Awolola, T.S.; Oyewole, I.; Koekemoer, L.; Coetzee, M. Identification of three members of the Anopheles funestus (Diptera: Culicidae) group and their role in malaria transmission in two ecological zones in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Djouaka, R.; Atoyebi, S.; Tchigossou, G.M.; Riveron, J.M.; Irving, H.; Akoton, R.; Kusimo, M.O.; Bakare, A.A.; Wondji, C.S. Evidence of a multiple insecticide resistance in the malaria vector Anopheles funestus in South West Nigeria. Malar. J. 2016, 15, 565. [Google Scholar] [CrossRef] [PubMed]
- President’s Malaria Initiative. Seventh Annual Report to Congress. Available online: http://pmi.gov/countries/mops/fy13/nigeria_mop-fy13.pdf (accessed on 25 October 2018).
- Riveron, J.M.; Yunta, C.; Ibrahim, S.S.; Djouaka, R.; Irving, H.; Menze, B.D.; Ismail, H.; Hemingway, J.; Ranson, H.; Albert, A.; et al. A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Boil. 2014, 15, R27. [Google Scholar] [CrossRef]
- Martínez-Torres, D.; Chandre, F.; Williamson, M.S.; Darriet, F.; Bergé, J.B.; Devonshire, A.L.; Guillet, P.; Pasteur, N.; Pauron, D. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol. Boil. 1998, 7, 179–184. [Google Scholar] [CrossRef]
- Ranson, H.; Jensen, B.; Vulule, J.M.; Wang, X.; Hemingway, J.; Collins, F. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol. Boil. 2000, 9, 491–497. [Google Scholar] [CrossRef]
- Weill, M.; Malcolm, C.; Chandre, F.; Mogensen, K.; Berthomieu, A.; Marquine, M.; Raymond, M. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol. Boil. 2004, 13, 1–7. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Ndula, M.; Riveron, J.M.; Irving, H.; Wondji, C.S. The P450 CYP6Z1 confers carbamate/pyrethroid cross-resistance in a major African malaria vector beside a novel carbamate-insensitive N485I acetylcholinesterase-1 mutation. Mol. Ecol. 2016, 25, 3436–3452. [Google Scholar] [CrossRef]
- Andrada, A.; Herrera, S.; Inyang, U.; Mohammed, A.B.; Uhomoibhi, P.; Yé, Y. A subnational profiling analysis reveals regional differences as the main predictor of ITN ownership and use in Nigeria. Malar. J. 2019, 18, 185. [Google Scholar] [CrossRef]
- Morgan, J.C.; Irving, H.; Okedi, L.M.; Steven, A.; Wondji, C.S. Pyrethroid Resistance in an Anopheles funestus Population from Uganda. PLoS ONE 2010, 5, e11872. [Google Scholar] [CrossRef]
- Gillies, M.; Coetzee, M. A supplement to anophelinae of Africa south of Sahara (Afro-tropical region). S. Afr. Inst. Med. Res. 1987, 55, 1–143. [Google Scholar]
- Koekemoer, L.L.; Kamau, L.; Coetzee, M.; Hunt, R.H. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am. J. Trop. Med. Hyg. 2002, 66, 804–811. [Google Scholar] [CrossRef]
- Livak, K.J. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics 1984, 107, 611–634. [Google Scholar]
- WHO. Malaria Entomology and Vector Control. Guide for Participants; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- WHO. A Toolkit for Integrated Vector Management in Sub-Saharan Africa; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Kent, R.J.; Thuma, P.E.; Mharakurwa, S.; Norris, D.E. Seasonality, Blood Feeding Behavior, and Transmission of Plasmodium falciparum by Anopheles Arabiensis after an Extended Drought in Southern Zambia. Am. J. Trop. Med. Hyg. 2007, 76, 267–274. [Google Scholar] [CrossRef]
- Bass, C.; Nikou, D.; Blagborough, A.M.; Vontas, J.; Sinden, R.; Williamson, M.S.; Field, L. PCR-based detection of Plasmodium in Anopheles mosquitoes: A comparison of a new high-throughput assay with existing methods. Malar. J. 2008, 7, 177. [Google Scholar] [CrossRef]
- Snounou, G.; Viriyakosol, S.; Zhu, X.P.; Jarra, W.; Pinheiro, L.; Rosário, V.E.D.; Thaithong, S.; Brown, K. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 1993, 61, 315–320. [Google Scholar] [CrossRef]
- WHO. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Hunt, R.H.; Brooke, B.D.; Pillay, C.; Koekemoer, L.L.; Coetzee, M. Laboratory selection for and characteristics of pyrethroid resistance in the malaria vector Anopheles funestus. Med. Vet. Entomol. 2005, 19, 271–275. [Google Scholar] [CrossRef]
- Riveron, J.M.; Chiumia, M.; Menze, B.D.; Barnes, K.G.; Irving, H.; Ibrahim, S.S.; Weedall, G.; Mzilahowa, T.; Wondji, C.S. Rise of multiple insecticide resistance in Anopheles funestus in Malawi: A major concern for malaria vector control. Malar. J. 2015, 14, 344. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect P450 inhibitors and insecticides: Challenges and opportunities. Pest Manag. Sci. 2014, 71, 793–800. [Google Scholar] [CrossRef]
- Tchouakui, M.; Chiang, M.-C.; Ndo, C.; Kuicheu, C.K.; Amvongo-Adjia, N.; Wondji, M.J.; Tchoupo, M.; Kusimo, M.O.; Riveron, J.M.; Wondji, C.S. A marker of glutathione S-transferase-mediated resistance to insecticides is associated with higher Plasmodium infection in the African malaria vector Anopheles funestus. Sci. Rep. 2019, 9, 5772. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 18, 95–98. [Google Scholar]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Boil. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Djouaka, R.; Irving, H.; Tukur, Z.; Wondji, C.S. Exploring Mechanisms of Multiple Insecticide Resistance in a Population of the Malaria Vector Anopheles funestus in Benin. PLoS ONE 2011, 6, e27760. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Orsborne, J.; Furuya-Kanamori, L.; Jeffries, C.L.; Kristan, M.; Mohammed, A.R.; Afrane, Y.A.; O’Reilly, K.; Massad, E.; Drakeley, C.; Walker, T.; et al. Using the human blood index to investigate host biting plasticity: A systematic review and meta-regression of the three major African malaria vectors. Malar. J. 2018, 17, 479. [Google Scholar] [CrossRef]
- Riveron, J.M.; Huijben, S.; Tchapga, W.; Tchouakui, M.; Wondji, M.J.; Tchoupo, M.; Irving, H.; Cuamba, N.; Maquina, M.; Paaijmans, K.; et al. Escalation of Pyrethroid Resistance in the Malaria Vector Anopheles funestus Induces a Loss of Efficacy of Piperonyl Butoxide–Based Insecticide-Treated Nets in Mozambique. J. Infect. Dis. 2019, 220, 467–475. [Google Scholar] [CrossRef]
- Riveron, J.M.; Osae, M.; Egyir-Yawson, A.; Irving, H.; Ibrahim, S.S.; Wondji, C.S. Multiple insecticide resistance in the major malaria vector Anopheles funestus in southern Ghana: Implications for malaria control. Parasites Vectors 2016, 9, 504. [Google Scholar] [CrossRef]
- Samb, B.; Konate, L.; Irving, H.; Riveron, J.M.; Dia, I.; Faye, O.; Wondji, C.S. Investigating molecular basis of lambda-cyhalothrin resistance in an Anopheles funestus population from Senegal. Parasites Vectors 2016, 9, 449. [Google Scholar] [CrossRef]
- Menze, B.D.; Riveron, J.M.; Ibrahim, S.S.; Irving, H.; Antonio-Nkondjio, C.; Awono-Ambene, P.H.; Wondji, C.S. Multiple Insecticide Resistance in the Malaria Vector Anopheles funestus from Northern Cameroon Is Mediated by Metabolic Resistance Alongside Potential Target Site Insensitivity Mutations. PLoS ONE 2016, 11, e0163261. [Google Scholar] [CrossRef]
- Menze, B.; Kouamo, M.; Wondji, M.J.; Tchapga, W.; Tchoupo, M.; Kusimo, M.O.; Mouhamadou, C.S.; Riveron, J.; Wondji, C.S. An Experimental Hut Evaluation of PBO-Based and Pyrethroid-Only Nets against the Malaria Vector Anopheles funestus Reveals a Loss of Bed Nets Efficacy Associated with GSTe2 Metabolic Resistance. Genes 2020, 11, 143. [Google Scholar] [CrossRef]
- Riveron, J.M.; Watsenga, F.; Irving, H.; Irish, S.R.; Wondji, C.S. High Plasmodium Infection Rate and Reduced Bed Net Efficacy in Multiple Insecticide-Resistant Malaria Vectors in Kinshasa, Democratic Republic of Congo. J. Infect. Dis. 2017, 217, 320–328. [Google Scholar] [CrossRef]
- Menze, B.D.; Wondji, M.J.; Tchapga, W.; Tchoupo, M.; Riveron, J.M.; Wondji, C.S. Bionomics and insecticides resistance profiling of malaria vectors at a selected site for experimental hut trials in central Cameroon. Malar. J. 2018, 17, 317. [Google Scholar] [CrossRef]
- Fadel, A.N.; Ibrahim, S.S.; Tchouakui, M.; Terence, E.; Wondji, M.J.; Tchoupo, M.; Wanji, S.; Wondji, C.S. A combination of metabolic resistance and high frequency of the 1014F kdr mutation is driving pyrethroid resistance in Anopheles coluzzii population from Guinea savanna of Cameroon. Parasites Vectors 2019, 12, 263. [Google Scholar] [CrossRef]
- Tchouakui, M.; Fossog, B.T.; Ngannang, V.B.; Djonabaye, D.; Tchapga, W.; Njiokou, F.; Wondji, C.S. Investigation of the influence of a glutathione S-transferase metabolic resistance to pyrethroids/DDT on mating competitiveness in males of the African malaria vector, Anopheles funestus. Wellcome Open Res. 2019, 4, 13. [Google Scholar] [CrossRef]
- Irving, H.; Wondji, C.S. Investigating knockdown resistance (kdr) mechanism against pyrethroids/DDT in the malaria vector Anopheles funestus across Africa. BMC Genet. 2017, 18, 76. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.S.; Mukhtar, M.M.; Irving, H.; Riveron, J.M.; Fadel, A.N.; Tchapga, W.; Hearn, J.; Muhammad, A.; Sarkinfada, F.; Wondji, C.S. Exploring the Mechanisms of Multiple Insecticide Resistance in a Highly Plasmodium-Infected Malaria Vector Anopheles funestus Sensu Stricto from Sahel of Northern Nigeria. Genes 2020, 11, 454. https://doi.org/10.3390/genes11040454
Ibrahim SS, Mukhtar MM, Irving H, Riveron JM, Fadel AN, Tchapga W, Hearn J, Muhammad A, Sarkinfada F, Wondji CS. Exploring the Mechanisms of Multiple Insecticide Resistance in a Highly Plasmodium-Infected Malaria Vector Anopheles funestus Sensu Stricto from Sahel of Northern Nigeria. Genes. 2020; 11(4):454. https://doi.org/10.3390/genes11040454
Chicago/Turabian StyleIbrahim, Sulaiman S., Muhammad M. Mukhtar, Helen Irving, Jacob M. Riveron, Amen N. Fadel, Williams Tchapga, Jack Hearn, Abdullahi Muhammad, Faruk Sarkinfada, and Charles S. Wondji. 2020. "Exploring the Mechanisms of Multiple Insecticide Resistance in a Highly Plasmodium-Infected Malaria Vector Anopheles funestus Sensu Stricto from Sahel of Northern Nigeria" Genes 11, no. 4: 454. https://doi.org/10.3390/genes11040454
APA StyleIbrahim, S. S., Mukhtar, M. M., Irving, H., Riveron, J. M., Fadel, A. N., Tchapga, W., Hearn, J., Muhammad, A., Sarkinfada, F., & Wondji, C. S. (2020). Exploring the Mechanisms of Multiple Insecticide Resistance in a Highly Plasmodium-Infected Malaria Vector Anopheles funestus Sensu Stricto from Sahel of Northern Nigeria. Genes, 11(4), 454. https://doi.org/10.3390/genes11040454







