Domesticated gag Gene of Drosophila LTR Retrotransposons Is Involved in Response to Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Drosophila Strains and Treatments
2.2. RNA Isolation and Quantitative RT-PCR
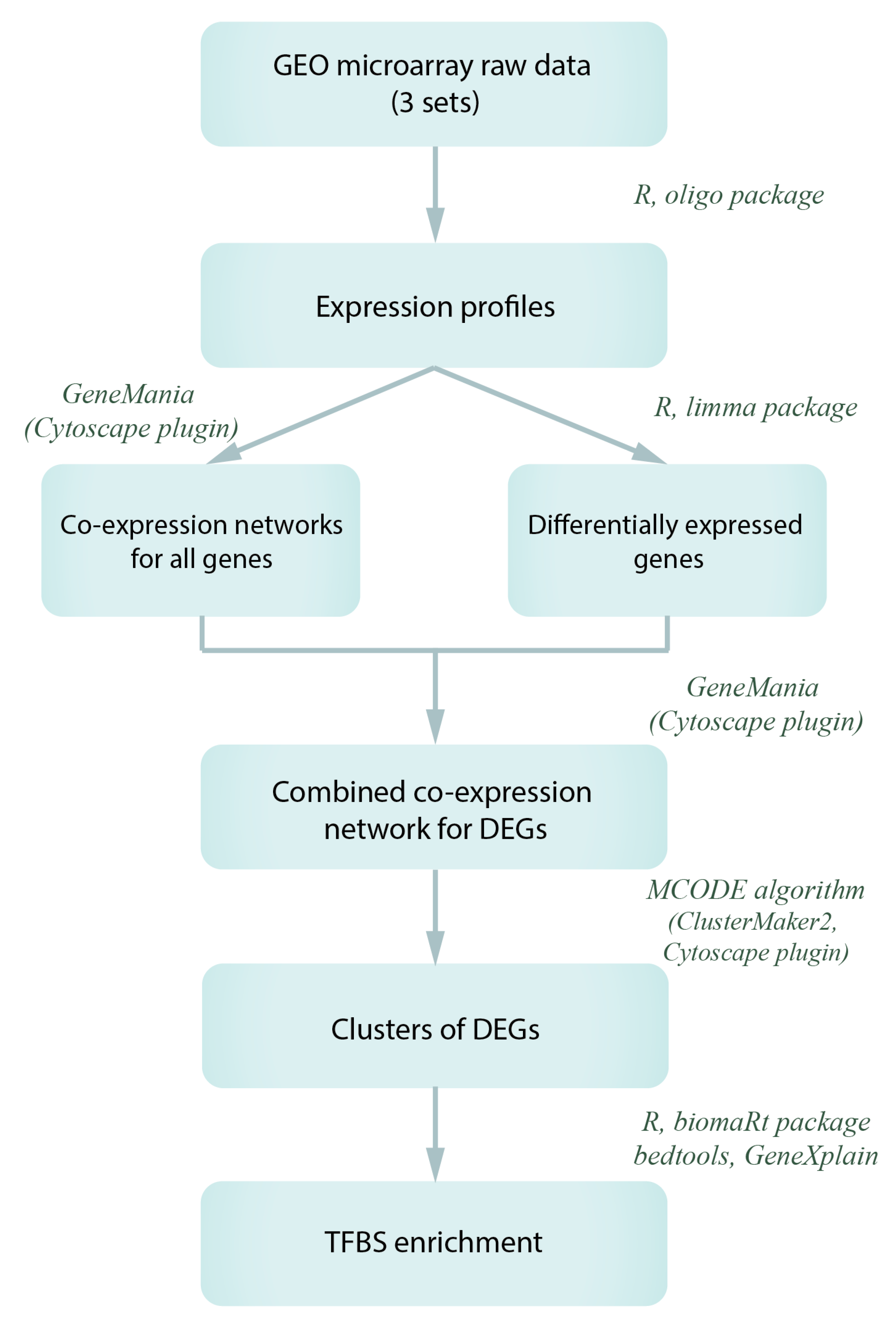
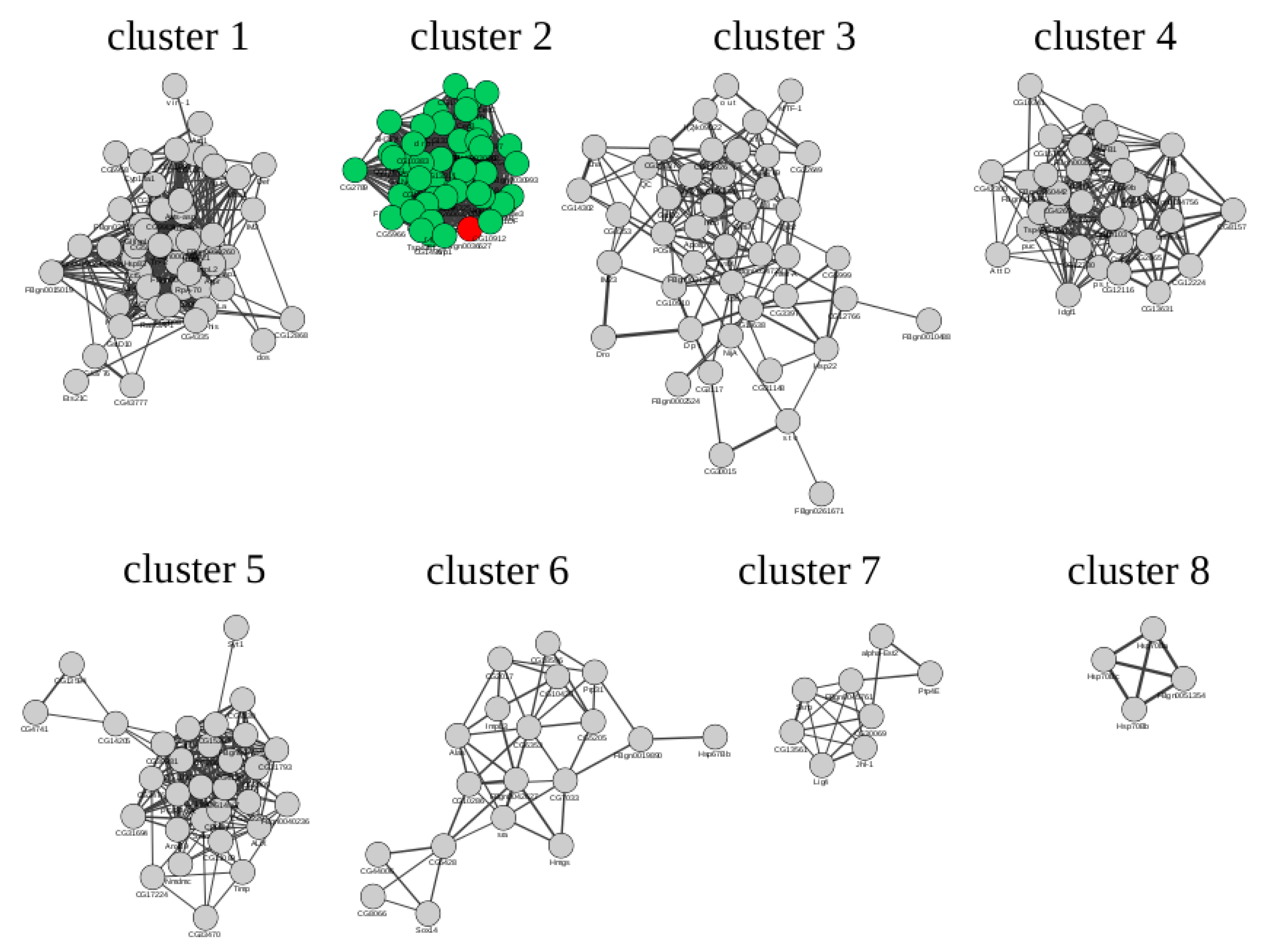
2.3. Microarray Data Reprocessing and Co-Expression Network Analysis
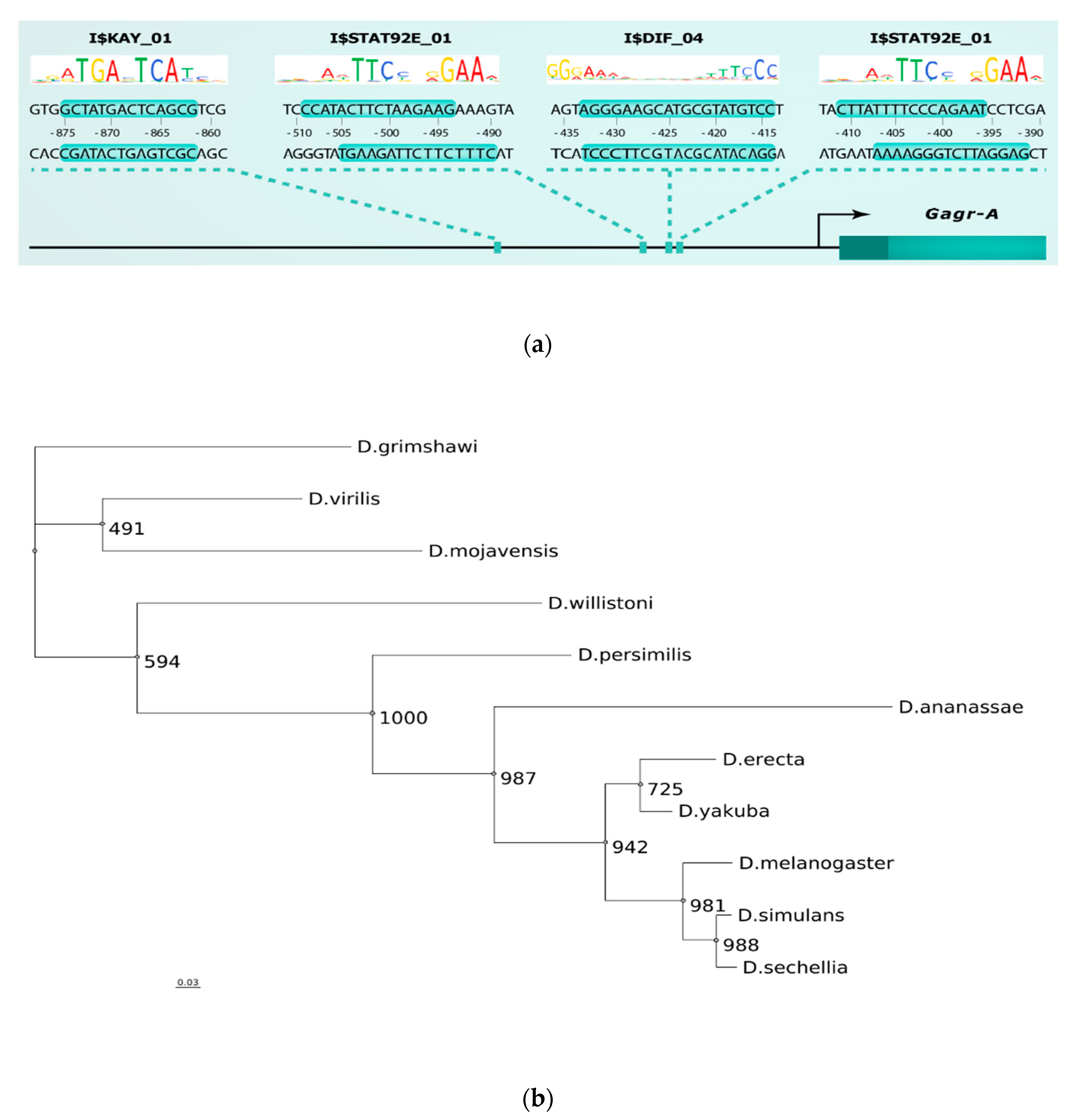
2.4. TFBS Enrichment Analysis and Search for Potential Transcription Regulators of Gagr and Co-Expressed Genes
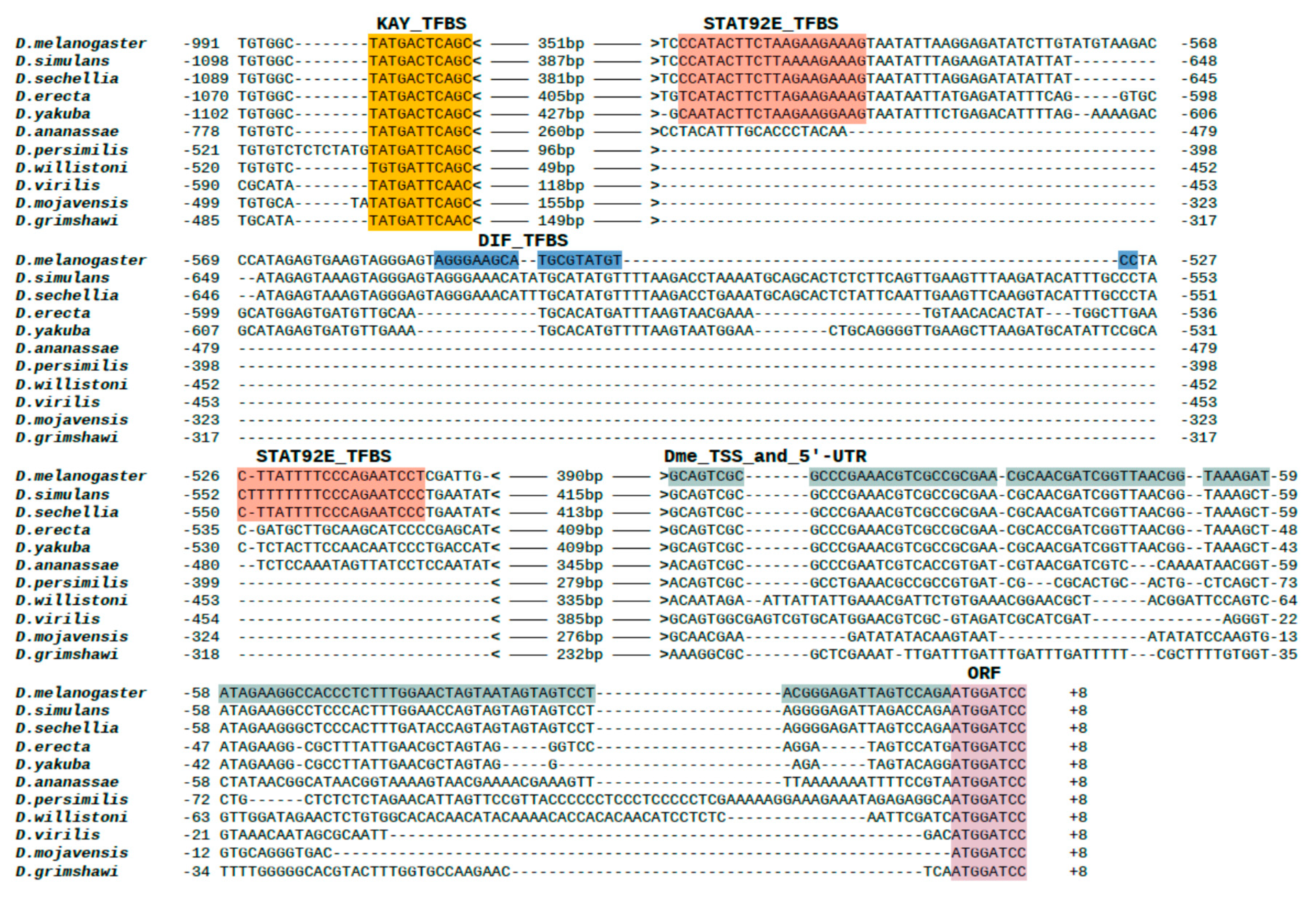
2.5. Alignment of The Promoter Regions of The Gagr Gene of Various Drosophila Species and Phylogenetic Analysis
3. Results
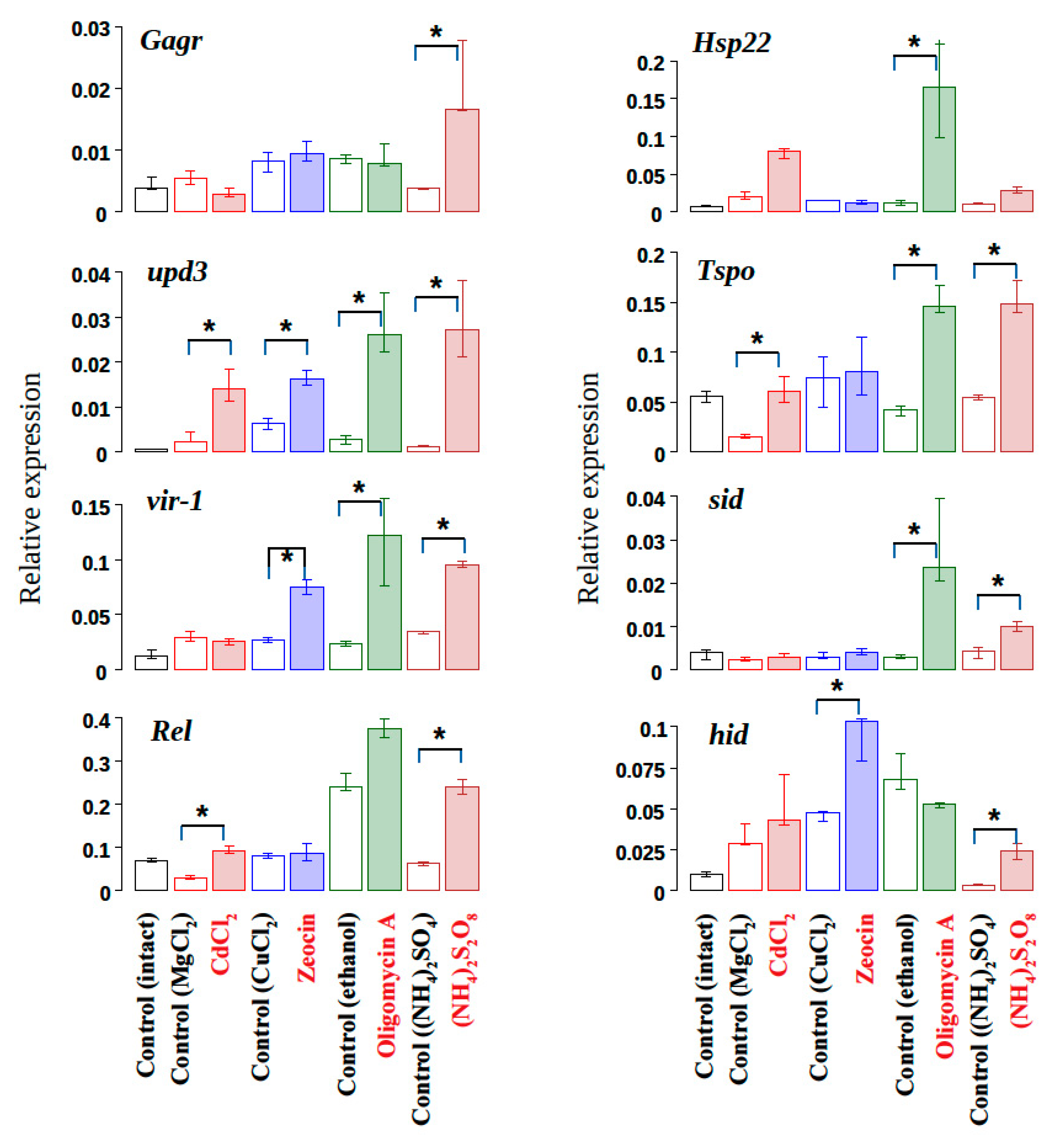
3.1. The Influence of Exogenous Stress Factors Causing Cell Homeostasis Disturbance on The Expression of The Gagr Gene in D. melanogaster Imago
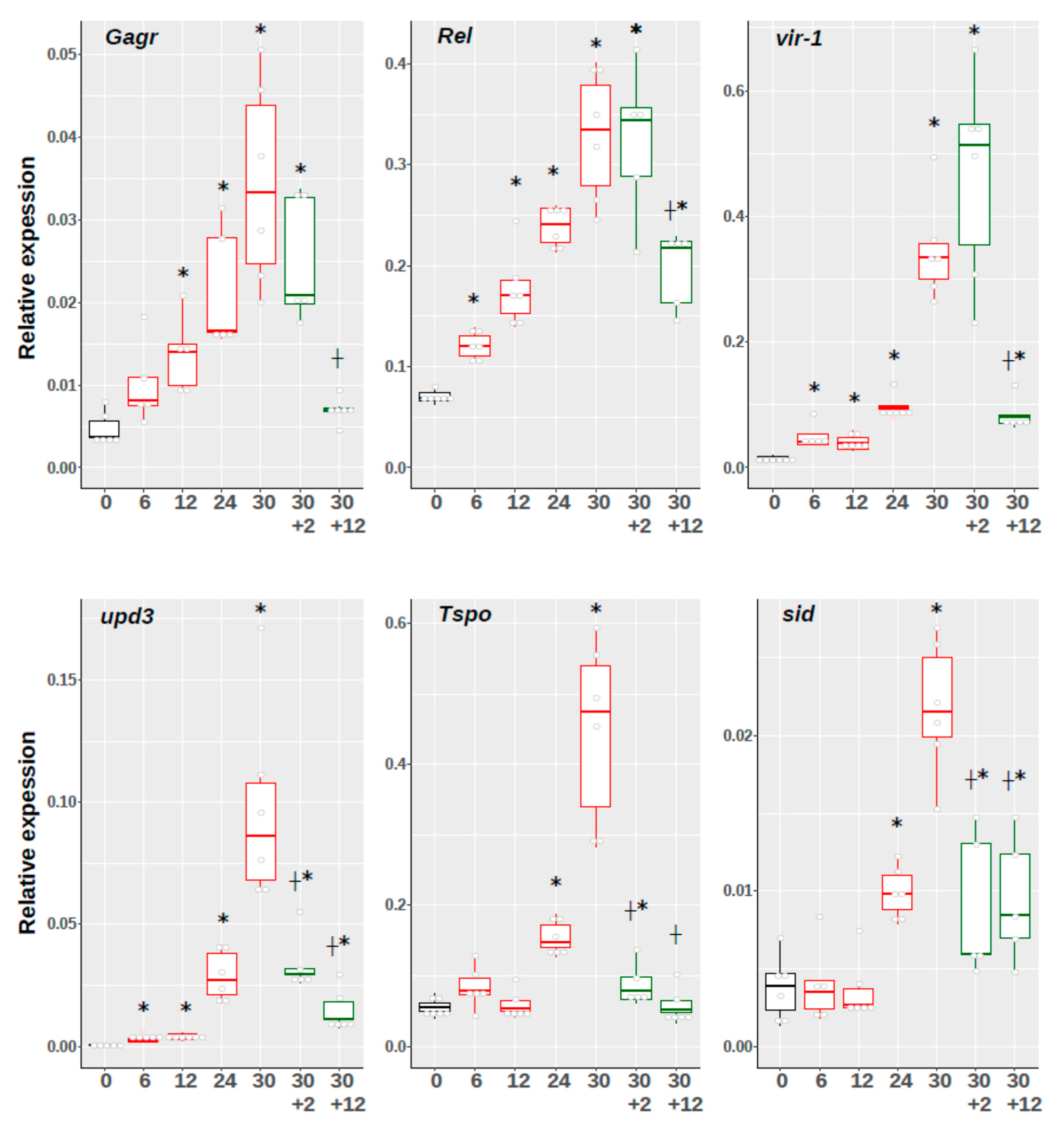
3.2. Investigation of The Temporal Dynamics of Expression of Gagr Gene and Stress Response Effector Genes Upon Exposure to Ammonium Persulfate in D. melanogaster Imago
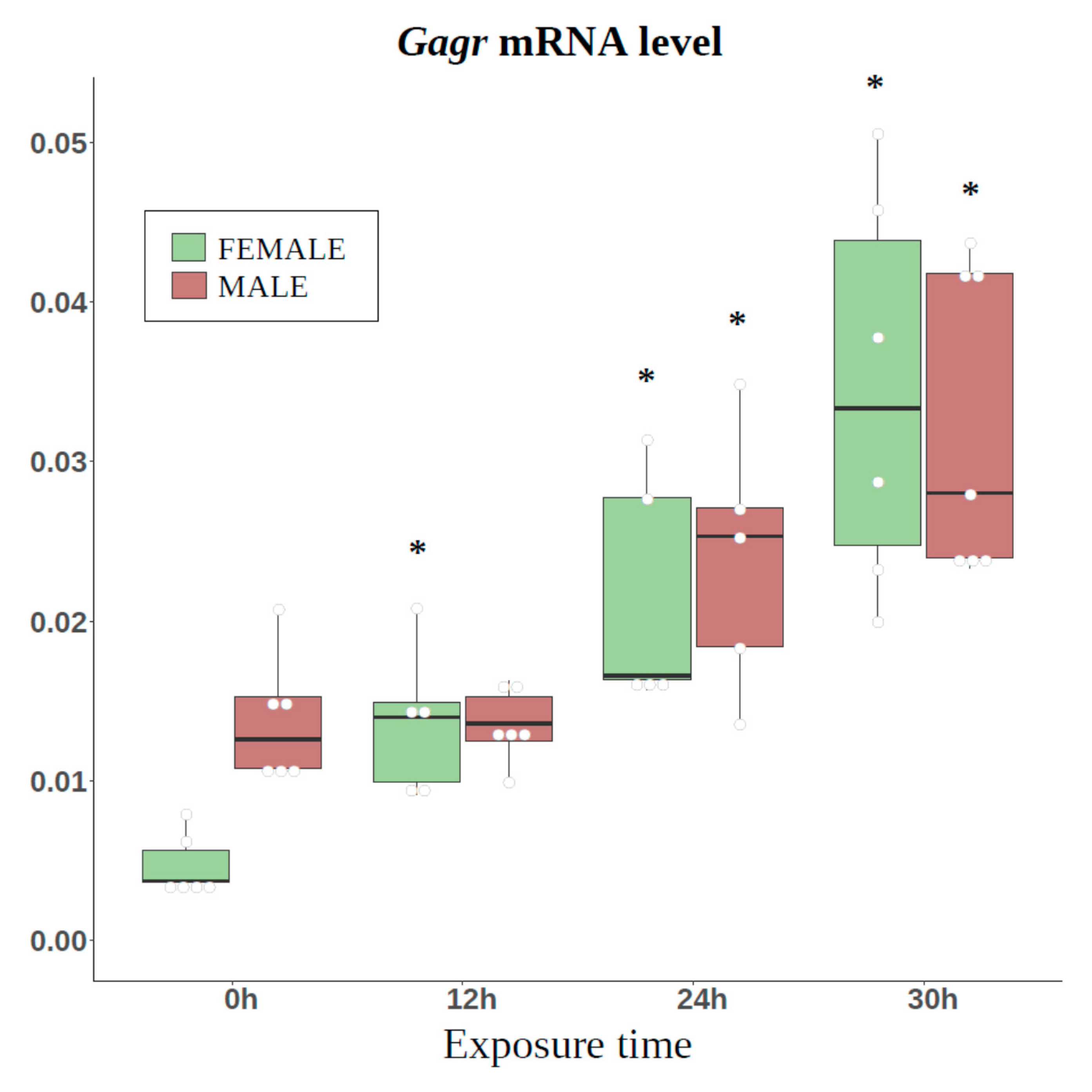
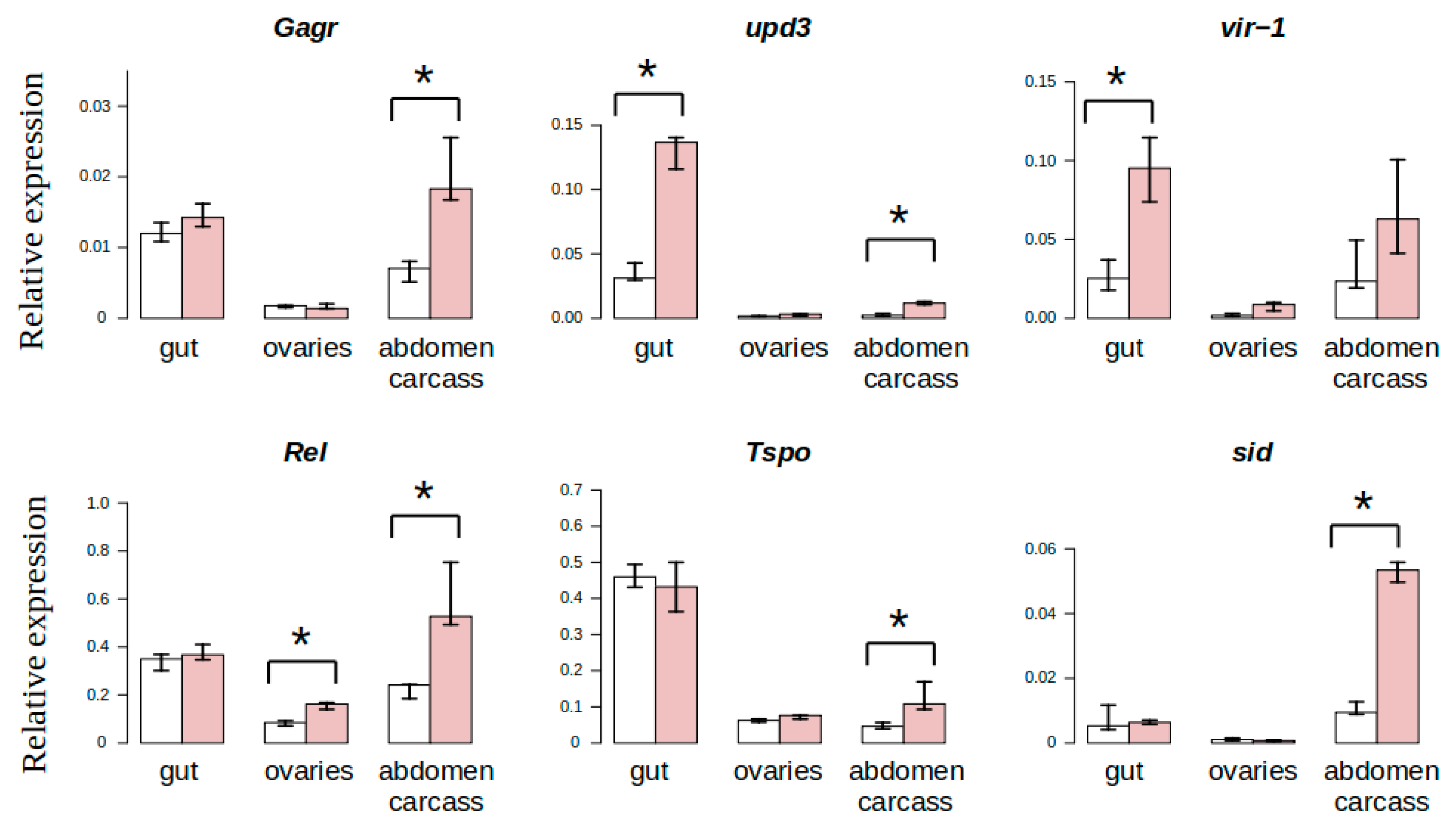
3.3. Study of Tissue-Specific Activation of Gagr Gene Expression in D. melanogaster Females Induced by Ammonium Persulfate
3.4. Search for Potential Regulators of Stress-Induced Activation of Expression of The Gagr Gene in D. melanogaster Imago
3.5. Phylogenetic Analysis of Promoter Regions of The Gagr Gene in Various Species of The Genus Drosophila
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Volff, J.-N. Turning junk into gold: Domestication of transposable elements and the creation of new genes in eukaryotes. Bioessays 2006, 28, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Fedoroff, N.V. Presidential address. Transposable elements, epigenetics, and genome evolution. Science 2012, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Kaneko-Ishino, T.; Ishino, F. The role of genes domesticated from LTR retrotransposons and retroviruses in mammals. Front Microbiol. 2012, 3, 262. [Google Scholar] [CrossRef] [PubMed]
- Schrader, L.; Schmitz, J. The impact of transposable elements in adaptive evolution. Mol. Ecol. 2019, 28, 1537–1549. [Google Scholar] [CrossRef]
- Miller, W.J.; Mcdonald, J.F.; Pinsker, W. Molecular domestication of mobile elements. Genetica 1997, 261–270. [Google Scholar] [CrossRef]
- Volff, J.-N. Gene and protein evolution. Preface. Genome Dyn. 2007, 3, VII. [Google Scholar]
- Kokosar, J.; Kordis, D. Genesis and regulatory wiring of retroelement-derived domesticated genes: A phylogenomic perspective. Mol. Biol. Evol. 2013, 30, 1015–1031. [Google Scholar] [CrossRef]
- Ono, R.; Kuroki, Y.; Naruse, M.; Ishii, M.; Iwasaki, S.; Toyoda, A.; Fujiyama, A.; Shaw, G.; Renfree, M.B. Identification of tammar wallaby SIRH12, derived from a marsupial-specific retrotransposition event. DNA Res. 2011, 18, 211–219. [Google Scholar] [CrossRef]
- Emera, D.; Wagner, G.P. Transposable element recruitments in the mammalian placenta: Impacts and mechanisms. Brief. Funct. Genom. 2012, 11, 267–276. [Google Scholar] [CrossRef]
- Henke, C.; Strissel, P.L.; Schubert, M.-T.; Mitchell, M.; Stolt, C.C.; Faschingbauer, F.; Beckmann, M.W.; Strick, R. Selective expression of sense and antisense transcripts of the sushi-ichi-related retrotransposon--derived family during mouse placentogenesis. Retrovirology 2015, 12, 9. [Google Scholar] [CrossRef]
- Pang, S.W.; Lahiri, C.; Poh, C.L.; Tan, K.O. PNMA family: Protein interaction network and cell signalling pathways implicated in cancer and apoptosis. Cell Signal. 2018, 45, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.; Stone, J.R.; Maki, J.L.; Collins, T.; Wagner, G. Mammalian SCAN domain dimer is a domain-swapped homolog of the HIV capsid C-terminal domain. Mol. Cell 2005, 17, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Emerson, R.O.; Thomas, J.H. Gypsy and the birth of the SCAN domain. J. Virol. 2011, 85, 12043–12052. [Google Scholar] [CrossRef] [PubMed]
- Benit, L.; De Parseval, N.; Casella, J.F.; Callebaut, I.; Cordonnier, A.; Heidmann, T. Cloning of a new murine endogenous retrovirus, MuERV-L, with strong similarity to the human HERV-L element and with a gag coding sequence closely related to the Fv1 restriction gene. J. Virol. 1997, 71, 5652–5657. [Google Scholar] [CrossRef]
- Yap, M.W.; Colbeck, E.; Ellis, S.A.; Stoye, J.P. Evolution of the retroviral restriction gene Fv1: Inhibition of non-MLV retroviruses. PLoS Pathog. 2014, 10, e1003968. [Google Scholar] [CrossRef]
- Best, S.; Le Tissier, P.; Towers, G.; Stoye, J.P. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 1996, 382, 826–829. [Google Scholar] [CrossRef]
- Nefedova, L.N.; Kuzmin, I.V.; Makhnovskii, P.A.; Kim, A.I. Domesticated retroviral GAG gene in Drosophila: New functions for an old gene. Virology 2014, 450–451, 196–204. [Google Scholar] [CrossRef]
- Silverman, N.; Zhou, R.; Erlich, R.L.; Hunter, M.; Bernstein, E.; Schneider, D.; Maniatis, T. Immune activation of NF-kappaB and JNK requires Drosophila TAK1. J. Biol. Chem. 2003, 278, 48928–48934. [Google Scholar]
- Kemp, C.; Mueller, S.; Goto, A.; Barbier, V.; Paro, S.; Bonnay, F.; Dostert, C.; Troxler, L.; Hetru, C.; Meignin, C.; et al. Broad RNA interference-mediated antiviral immunity and virus-specific inducible responses in Drosophila. J. Immunol. 2013, 190, 650–658. [Google Scholar] [CrossRef]
- Guruharsha, K.G.; Rual, J.-F.; Zhai, B.; Mintseris, J.; Vaidya, P.; Vaidya, N.; Beekman, C.; Wong, C.; Rhee, D.Y.; Cenaj, O.; et al. A protein complex network of Drosophila melanogaster. Cell 2011, 147, 690–703. [Google Scholar] [CrossRef]
- Ashton-Beaucage, D.; Udell, C.M.; Gendron, P.; Sahmi, M.; Lefrancois, M.; Baril, C.; Guenier, A.S.; Duchaine, J.; Lamarre, D.; Lemieux, S.; et al. A functional screen reveals an extensive layer of transcriptional and splicing control underlying RAS/MAPK signaling in Drosophila. PLoS Biol. 2014, 12, e1001809. [Google Scholar]
- Ko, H.S.; Uehara, T.; Nomura, Y. Role of ubiquilin associated with protein-disulfide isomerase in the endoplasmic reticulum in stress-induced apoptotic cell death. J. Biol. Chem. 2002, 277, 35386–35392. [Google Scholar] [CrossRef] [PubMed]
- Grek, C.; Townsend, D.M. Protein disulfide isomerase superfamily in disease and the regulation of apoptosis. endoplasmic reticulum. Stress. Dis. 2014, 1, 4–17. [Google Scholar]
- Majzoub, K.; Hafirassou, M.L.; Meignin, C.; Goto, A.; Marzi, S.; Fedorova, A.; Verdier, Y.; Vinh, J.; Hoffmann, J.A.; Martin, F.; et al. RACK1 controls IRES-mediated translation of viruses. Cell 2014, 159, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Eilers, M.; Oppliger, W.; Schatz, G. Both ATP and an energized inner membrane are required to import a purified precursor protein into mitochondria. EMBO J. 1987, 6, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Haynes, C.M.; Fiorese, C.J.; Lin, Y.-F. Evaluating and responding to mitochondrial dysfunction: The mitochondrial unfolded-protein response and beyond. Trends Cell Biol. 2013, 23, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, H.; Heo, H.-R.; Kim, J.W.; Kim, H.-R.; Hong, Y.; Yang, S.-R.; Han, S.-S.; Lee, S.-J.; Kim, W.-J.; et al. Cadmium-induced ER stress and inflammation are mediated through C/EBP-DDIT3 signaling in human bronchial epithelial cells. Exp. Mol. Med. 2017, 49, e372. [Google Scholar] [CrossRef]
- Liu, J.; Luo, L.-F.; Wang, D.-L.; Wang, W.-X.; Zhu, J.-L.; Li, Y.-C.; Chen, N.-Z.; Huang, H.-L.; Zhang, W.-C. Cadmium induces ovarian granulosa cell damage by activating PERK-eIF2alpha-ATF4 through endoplasmic reticulum stress. Biol. Reprod. 2019, 100, 292–299. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Zhang, S.-L.; Liu, Z.-Y.; Tian, Y.; Sun, Q. Cadmium toxicity induces ER stress and apoptosis via impairing energy homoeostasis in cardiomyocytes. Biosci. Rep. 2015, 35, e00214. [Google Scholar] [CrossRef]
- Chen, Z.J.; Chen, J.X.; Wu, L.K.; Li, B.Y.; Tian, Y.F.; Xian, M.; Huang, Z.P.; Yu, R.A. Induction of Endoplasmic reticulum stress by cadmium and its regulation on Nrf2 Signaling pathway in kidneys of rats. Biomed. Environ. Sci. 2019, 32, 1–10. [Google Scholar]
- Shao, C.-C.; Li, N.; Zhang, Z.-W.; Su, J.; Li, S.; Li, J.-L.; Xu, S.-W. Cadmium supplement triggers endoplasmic reticulum stress response and cytotoxicity in primary chicken hepatocytes. Ecotoxicol. Environ. Saf. 2014, 106, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Chankova, S.G.; Dimova, E.; Dimitrova, M.; Bryant, P.E. Induction of DNA double-strand breaks by zeocin in Chlamydomonas reinhardtii and the role of increased DNA double-strand breaks rejoining in the formation of an adaptive response. Radiat. Environ. Biophys. 2007, 46, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Lottersberger, F.; Buonomo, S.B.; Sfeir, A.; de Lange, T. 53BP1 regulates DSB repair using Rif1 to control 5’ end resection. Science 2013, 339, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Koschmann, J.; Bhar, A.; Stegmaier, P.; Kel, A.E.; Wingender, E. “Upstream analysis”: An Integrated promoter-pathway analysis approach to causal interpretation of microarray data. Microarrays 2015, 4, 270–286. [Google Scholar] [CrossRef]
- Makhnovskii, P.A.; Kuzmin, I.V.; Nefedova, L.N.; Kim, A.I. Functional analysis of Grp and Iris, the gag and env domesticated errantivirus genes, in the Drosophila melanogaster genome. Mol. Biol. 2016, 50, 435–444. [Google Scholar] [CrossRef]
- Santabarbara-Ruiz, P.; Lopez-Santillan, M.; Martinez-Rodriguez, I.; Binagui-Casas, A.; Perez, L.; Milan, M.; Corominas, M.; Serras, F. ROS-induced JNK and p38 signaling is required for unpaired cytokine activation during Drosophila regeneration. PLoS Genet. 2015, 11, e1005595. [Google Scholar] [CrossRef]
- Bunker, B.D.; Nellimoottil, T.T.; Boileau, R.M.; Classen, A.K.; Bilder, D. The transcriptional response to tumorigenic polarity loss in Drosophila. Elife 2015, 4. [Google Scholar] [CrossRef]
- Dostert, C.; Jouanguy, E.; Irving, P.; Troxler, L.; Galiana-Arnoux, D.; Hetru, C.; Hoffmann, J.A.; Imler, J.L. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat. Immunol. 2005, 6, 946–953. [Google Scholar] [CrossRef]
- Paradkar, P.N.; Trinidad, L.; Voysey, R.; Duchemin, J.-B.; Walker, P.J. Secreted Vago restricts West Nile virus infection in Culex mosquito cells by activating the Jak-STAT pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 18915–18920. [Google Scholar] [CrossRef]
- Carpenter, J.; Hutter, S.; Baines, J.F.; Roller, J.; Saminadin-Peter, S.S.; Parsch, J.; Jiggins, F.M. The transcriptional response of Drosophila melanogaster to infection with the sigma virus (Rhabdoviridae). PLoS ONE 2009, 4, e6838. [Google Scholar] [CrossRef]
- Gruenewald, C.; Botella, J.A.; Bayersdorfer, F.; Navarro, J.A.; Schneuwly, S. Hyperoxia-induced neurodegeneration as a tool to identify neuroprotective genes in Drosophila melanogaster. Free Radic. Biol. Med. 2009, 46, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Meadows, S.; Sharp, L.; Jan, L.Y.; Jan, Y.N. Genome-wide study of aging and oxidative stress response in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2000, 97, 13726–13731. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Brady, H.; Ruocco, M.G.; Sun, H.; Williams, D.; Lee, S.J.; Kato, T., Jr.; Richards, N.; Chan, K.; Mercurio, F.; et al. Targeting of TAK1 by the NF-kappa B protein Relish regulates the JNK-mediated immune response in Drosophila. Genes Dev. 2004, 18, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Morrow, G.; Samson, M.; Michaud, S.; Tanguay, R.M. Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB J. 2004, 18, 598–599. [Google Scholar] [CrossRef]
- Lin, R.; Angelin, A.; Da Settimo, F.; Martini, C.; Taliani, S.; Zhu, S.; Wallace, D.C. Genetic analysis of dTSPO, an outer mitochondrial membrane protein, reveals its functions in apoptosis, longevity, and Ab42-induced neurodegeneration. Aging Cell 2014, 13, 507–518. [Google Scholar] [CrossRef]
- Seong, C.-S.; Varela-Ramirez, A.; Tang, X.; Anchondo, B.; Magallanes, D.; Aguilera, R.J. Cloning and characterization of a novel Drosophila stress induced DNase. PLoS ONE 2014, 9, e103564. [Google Scholar] [CrossRef][Green Version]
- Shlevkov, E.; Morata, G. A dp53/JNK-dependant feedback amplification loop is essential for the apoptotic response to stress in Drosophila. Cell Death Differ. 2012, 19, 451–460. [Google Scholar] [CrossRef]
- Silva, J.M.; Wong, A.; Carelli, V.; Cortopassi, G.A. Inhibition of mitochondrial function induces an integrated stress response in oligodendroglia. Neurobiol. Dis. 2009, 34, 357–365. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Sánchez-Aragó, M.; Cuezva, J.M. AMPK and GCN2-ATF4 signal the repression of mitochondria in colon cancer cells. Biochem. J. 2012, 444, 249–259. [Google Scholar] [CrossRef]
- Argue, K.J.; Neckameyer, W.S. Altering the sex determination pathway in Drosophila fat body modifies sex-specific stress responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R82–R92. [Google Scholar] [CrossRef]
- Palmer, W.H.; Medd, N.C.; Beard, P.M.; Obbard, D.J. Isolation of a natural DNA virus of Drosophila melanogaster, and characterisation of host resistance and immune responses. PLoS Pathog. 2018, 14, e1007050. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, X.; Feng, Q. Fat Body Biology in the Last Decade. Annu. Rev. Entomol. 2019, 64, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, K.J.; Kierdorf, K.; Pouchelon, C.A.; Vivancos, V.; Dionne, M.S.; Geissmann, F. Macrophage-derived upd3 cytokine causes impaired glucose homeostasis and reduced lifespan in Drosophila fed a lipid-rich diet. Immunity 2015, 42, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Landis, G.; Shen, J.; Tower, J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging 2012, 4, 768–789. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, H.D. Drosophila as a model for unfolded protein response research. BMB Rep. Korea (South) 2015, 48, 445–453. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, X.; Ryoo, H.D.; Jasper, H. Integration of UPRER and oxidative stress signaling in the control of intestinal stem cell proliferation. PLoS Genet. 2014, 10, e1004568. [Google Scholar] [CrossRef]
- Ahmed-de-Prado, S.; Diaz-Garcia, S.; Baonza, A. JNK and JAK/STAT signalling are required for inducing loss of cell fate specification during imaginal wing discs regeneration in Drosophila melanogaster. Dev. Biol. 2018, 441, 31–41. [Google Scholar] [CrossRef]
- Malik, H.S.; Henikoff, S. Positive selection of Iris, a retroviral envelope-derived host gene in Drosophila melanogaster. PLoS Genet. 2005, 1, e44. [Google Scholar] [CrossRef]
| Stress Agent | Expected Biological Effect | Treatment Control |
|---|---|---|
| Ammonium persulfate, 0.1 M | Oxidative stress | Ammonium sulfate, 0.1 M (as a control of salt stress and of ammonium ions) |
| Oligomycin A, 1 mM (ethanol solution) | Disruption of mitochondrial proteostasis, inhibition of mitochondrial ATP synthase [25,26] | Ethanol, ~5% |
| Cadmium chloride, 0.1 M | ER stress, unfolded protein response [27,28,29,30,31] | Magnesium chloride, 0.1 M (as a divalent metal salt) |
| Zeocin, 10 mM (chelated with copper (II) ions) | Initiation of double-strand DNA breaks [32,33] | Copper (II) chloride, 15 mM |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Gagr | AACTTCGATGGCAGTGATCC | GCTCATTTGTCGCGTGAAGA |
| vir-1 | CGTAAAGAGTGCCATCAT | CGTGTTCCTGCTCCAAATCT |
| Relish | TCATACACACCGCCAAGAAG | CTGTCTCCTGATGCAGTTCC |
| upd3 | AACGGCCAGAACCAGGAATC | GAGAGGGCAAACTGGGACAT |
| hid | GGAAGGAAGCGGATAAGGACA | CCCGATGAACTCGACGCTAC |
| sid | GGAAGTGTTCAAGCGAATTG | AGCAGATACAACGTCTGGTG |
| Tspo | CTAGCAGCCACGCTAAGTC | GTTCGATAGGTCGGAAAGC |
| Hsp22 | CTTTCACGCCTTCTTCCAC | GTGAGTTTGTAGCCATCCTTG |
| alphaTub84D | GTGCATGTTGTCCAACACCAC | AGAACTCTCCCTCCTCCATA |
| RpL40 | CTGCGTGGTGGTATCATTG | CAGGTTGTTGGTGTGTCC |
| EloB | GCACAAACATACACACTCACG | TTTCCTACTTCGCTTGCACC |
| Gene | Description |
|---|---|
| upd3 | Target of JNK signaling cascade and transcription factor AP-1 [36]; ligand that activates Jak-STAT signaling pathway [37]; one of key cytokines in response to various stress factors. |
| vir-1 | Prospective target of Jak-STAT signaling pathway [38,39]. Expression of vir-1 is activated in response to viral infection [19,38,40] and oxidative stress [41,42]. |
| Rel | NFkB transcription factor. Stress-induced activation of Rel expression depends on JNK signaling [43]. |
| Hsp22 | Chaperone; involved in oxidative stress reactions in mitochondria [44] |
| Tspo | Mitochondrial protein; positive regulator of apoptosis; plays important role in oxidative stress [45] |
| Sid | DNase gene activated by oxidative stress [46] |
| Hid | Target of transcription factor foxo; positive regulator of apoptosis [38]; p53 cascade target [47] |
| PWM_ID | TF | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | Cluster 6 |
|---|---|---|---|---|---|---|---|
| I$KAY_01 | Kay | 1.24 | 2.84 | 0.70 | 0.76 | 1.10 | 0.57 |
| I$KAY_02 | Kay | 0.65 | 0.60 | 0.75 | 1.01 | 0.54 | 0.51 |
| I$JRA_01 | Jra | 0.59 | 0.66 | 0.72 | 1.03 | 0.51 | 0.54 |
| I$ATF2_01 | Atf2 | 0.67 | 0.74 | 0.95 | 1.03 | 0.56 | 0.48 |
| I$ATF6_02 | Atf6 | 0.73 | 0.63 | 0.70 | 1.09 | 0.52 | 0.56 |
| I$CREBA_02 | CrebA | 0.81 | 0.70 | 0.73 | 1.19 | 0.53 | 0.57 |
| I$GATAE_01 | GATAe | 0.64 | 1.17 | 1.22 | 0.65 | 0.72 | 0.48 |
| I$GRN_01 | Grn | 0.63 | 1.68 | 1.13 | 0.69 | 0.68 | 0.40 |
| I$PNR_02 | Pnr | 0.63 | 1.01 | 0.77 | 0.68 | 0.56 | 0.49 |
| I$PNR_03 | Pnr | 0.63 | 1.60 | 1.15 | 0.66 | 0.67 | 0.42 |
| I$SRP_01 | Srp | 0.63 | 1.70 | 1.12 | 0.71 | 0.68 | 0.40 |
| I$MTTFA_01 | Tfam | 0.60 | 1.63 | 1.27 | 0.62 | 0.66 | 0.44 |
| I$DIF_01 | Dif | 0.57 | 1.78 | 0.81 | 0.69 | 0.59 | 0.27 |
| I$DIF_02 | Dif | 0.60 | 1.52 | 0.85 | 0.66 | 0.61 | 0.27 |
| I$DIF_03 | Dif | 0.52 | 1.02 | 0.93 | 0.68 | 1.17 | 0.45 |
| I$REL_01 | Rel | 0.60 | 1.38 | 0.69 | 0.67 | 0.72 | 0.30 |
| I$STAT_01 | Stat92E | 0.56 | 1.16 | 0.63 | 0.73 | 0.37 | 0.48 |
| I$STAT92E_01 | Stat92E | 0.61 | 1.08 | 0.63 | 0.53 | 0.53 | 0.38 |
| I$CNC_01 | Cnc | 0.59 | 0.61 | 0.73 | 0.70 | 0.60 | 1.10 |
| I$HSF_05 | Hsf | 0.57 | 0.74 | 0.67 | 0.64 | 0.48 | 1.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhnovskii, P.; Balakireva, Y.; Nefedova, L.; Lavrenov, A.; Kuzmin, I.; Kim, A. Domesticated gag Gene of Drosophila LTR Retrotransposons Is Involved in Response to Oxidative Stress. Genes 2020, 11, 396. https://doi.org/10.3390/genes11040396
Makhnovskii P, Balakireva Y, Nefedova L, Lavrenov A, Kuzmin I, Kim A. Domesticated gag Gene of Drosophila LTR Retrotransposons Is Involved in Response to Oxidative Stress. Genes. 2020; 11(4):396. https://doi.org/10.3390/genes11040396
Chicago/Turabian StyleMakhnovskii, Pavel, Yevheniia Balakireva, Lidia Nefedova, Anton Lavrenov, Ilya Kuzmin, and Alexander Kim. 2020. "Domesticated gag Gene of Drosophila LTR Retrotransposons Is Involved in Response to Oxidative Stress" Genes 11, no. 4: 396. https://doi.org/10.3390/genes11040396
APA StyleMakhnovskii, P., Balakireva, Y., Nefedova, L., Lavrenov, A., Kuzmin, I., & Kim, A. (2020). Domesticated gag Gene of Drosophila LTR Retrotransposons Is Involved in Response to Oxidative Stress. Genes, 11(4), 396. https://doi.org/10.3390/genes11040396




