A CNTNAP1 Missense Variant Is Associated with Canine Laryngeal Paralysis and Polyneuropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Selection
2.3. Sample Preparation
2.4. Axonal Size Frequency Distributions and G-Ratios
2.5. Single Nucleotide Polymorphism Array Genotyping and Imputation
2.6. Genome-Wide Association Study and Fine-Mapping
2.7. Whole-Genome Sequencing
2.8. Targeted Genotyping
2.9. Protein Predictions
2.10. Availability of Data and Material
3. Results
3.1. Phenotype
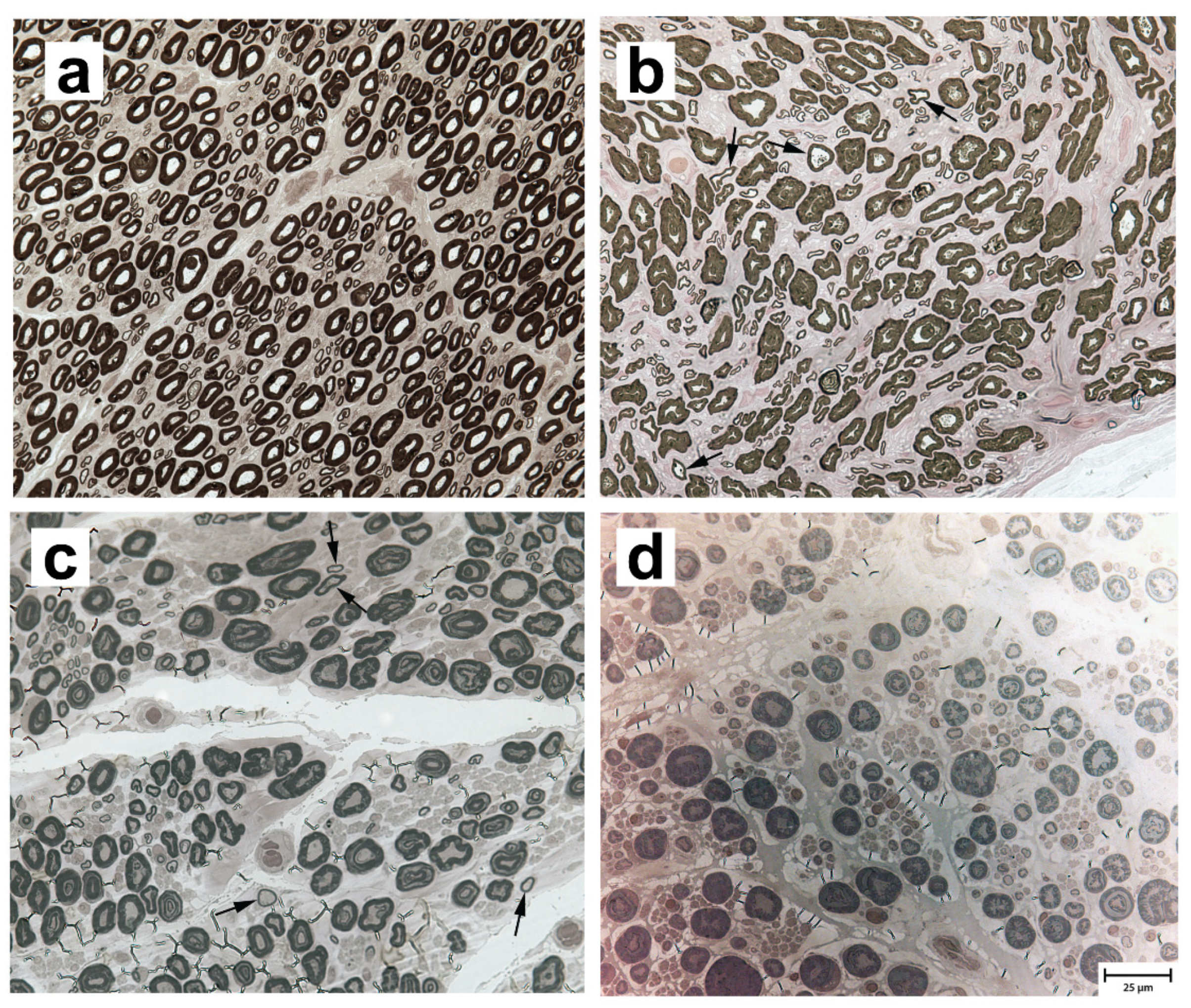
3.2. Neuropathological and Morphometric Findings
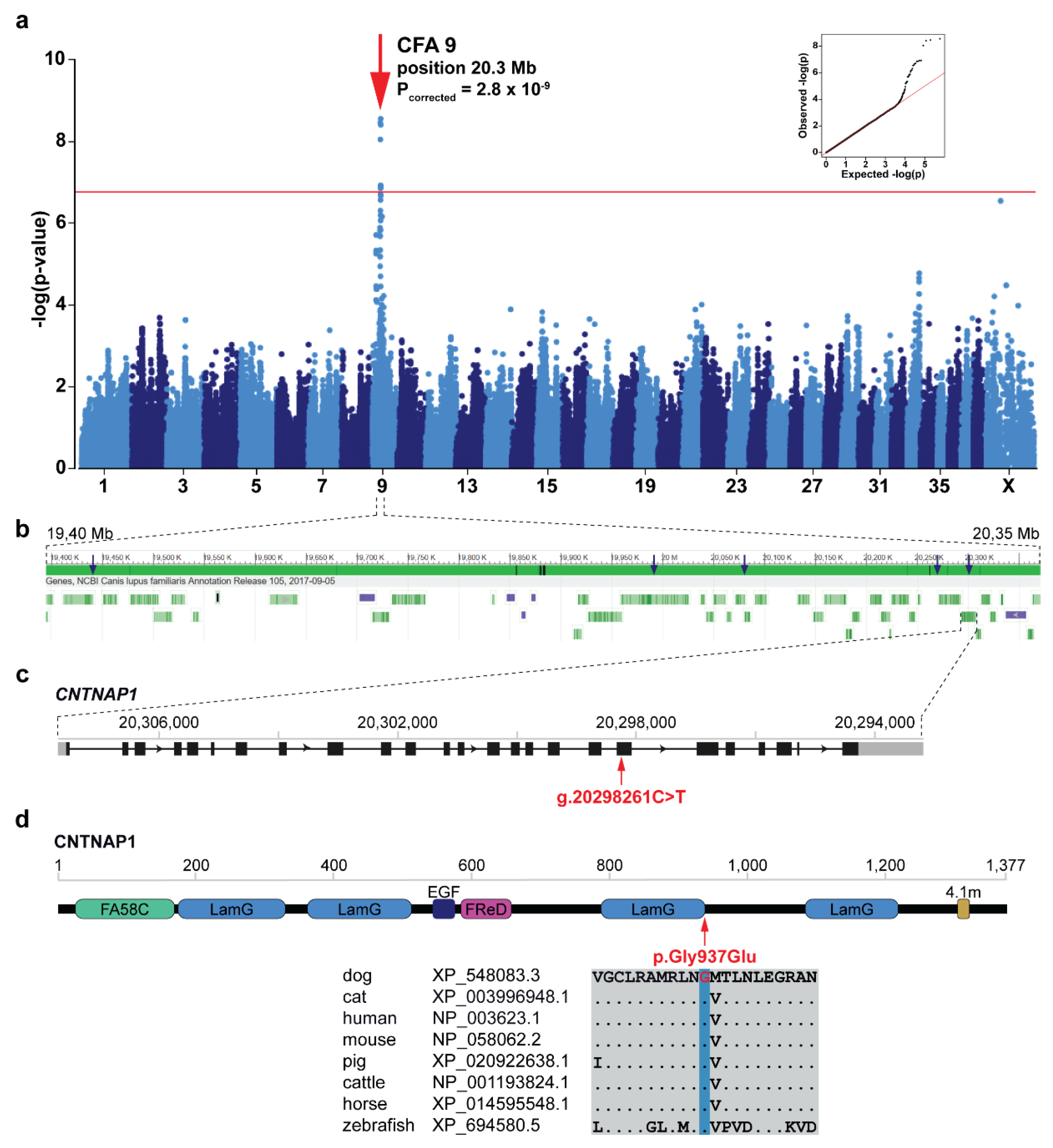
3.3. Genome-Wide Association Study and Fine-Mapping
3.4. Identification of the Candidate Causative Variant
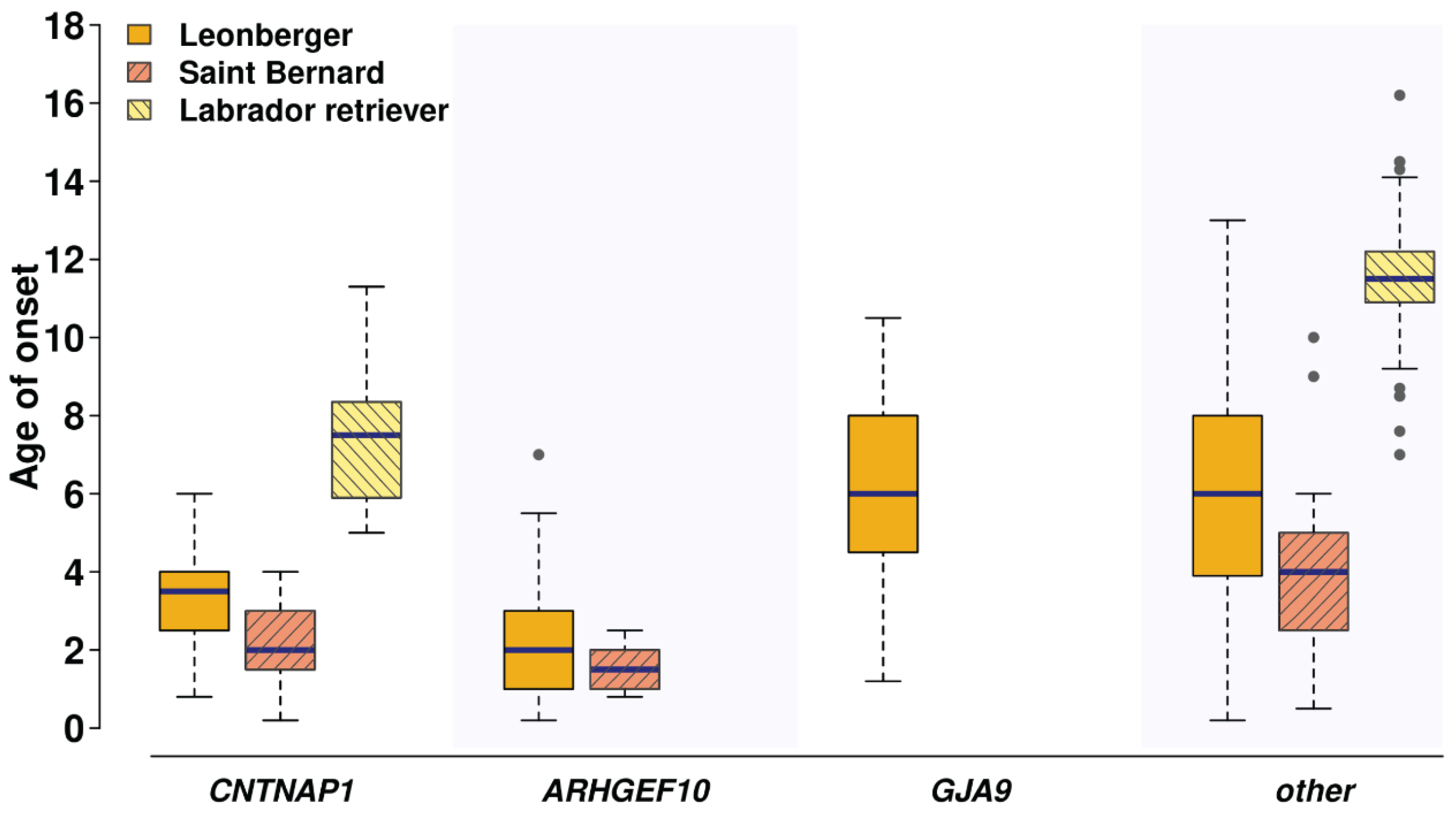
3.5. The CNTNAP1 Variant Occurs in Several Breeds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kitshoff, A.M.; Van Goethem, B.; Stegen, L.; Vandekerckhov, P.; De Rooster, H. Laryngeal paralysis in dogs: An update on recent knowledge. J. S. Afr. Vet. Assoc. 2013, 84, 1–9. [Google Scholar] [CrossRef]
- Monnet, E. Surgical Treatment of Laryngeal Paralysis. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 709–717. [Google Scholar] [CrossRef]
- Mackin, G.A. Diagnosis of patients with peripheral nerve disease. Clin. Podiatr. Med. Surg. 1994, 11, 545–569. [Google Scholar] [PubMed]
- Gabriel, A.; Poncelet, L.; Van Ham, L.; Clercx, C.; Braund, K.G.; Bhatti, S.; Detilleux, J.; Peeters, D. Laryngeal paralysis-polyneuropathy complex in young related Pyrenean mountain dogs. J. Small Anim. Pract. 2006, 47, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Granger, N. Canine inherited motor and sensory neuropathies: An updated classification in 22 breeds and comparison to Charcot-Marie-Tooth disease. Vet. J. 2011, 188, 274–285. [Google Scholar] [CrossRef] [PubMed]
- GOLPP|College of Veterinary Medicine at MSU. Available online: https://cvm.msu.edu/scs/research-initiatives/golpp (accessed on 7 September 2020).
- Letko, A.; Minor, K.M.; Jagannathan, V.; Seefried, F.R.; Mickelson, J.R.; Oliehoek, P.; Drögemüller, C. Genomic diversity and population structure of the Leonberger dog breed. Genet. Sel. Evol. 2020, 52, 61. [Google Scholar] [CrossRef]
- Ekenstedt, K.J.; Becker, D.; Minor, K.M.; Shelton, G.D.; Patterson, E.E.; Bley, T.; Oevermann, A.; Bilzer, T.; Leeb, T.; Drögemüller, C.; et al. An ARHGEF10 Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs. PLoS Genet. 2014, 10, e1004635. [Google Scholar] [CrossRef]
- Becker, D.; Minor, K.M.; Letko, A.; Ekenstedt, K.J.; Jagannathan, V.; Leeb, T.; Shelton, G.D.; Mickelson, J.R.; Drögemüller, C. A GJA9 frameshift variant is associated with polyneuropathy in Leonberger dogs. BMC Genom. 2017, 18, 662. [Google Scholar] [CrossRef]
- Wiedmer, M.; Oevermann, A.; Borer-Germann, S.E.; Gorgas, D.; Shelton, G.D.; Drögemüller, M.; Jagannathan, V.; Henke, D.; Leeb, T. A RAB3GAP1 SINE Insertion in Alaskan Huskies with Polyneuropathy, Ocular Abnormalities, and Neuronal Vacuolation (POANV) Resembling Human Warburg Micro Syndrome 1 (WARBM1). G3 Genes Genomes Genet. 2016, 6, 255–262. [Google Scholar] [CrossRef]
- Mhlanga-Mutangadura, T.; Johnson, G.S.; Schnabel, R.D.; Taylor, J.F.; Johnson, G.C.; Katz, M.L.; Shelton, G.D.; Lever, T.E.; Giuliano, E.; Granger, N.; et al. A mutation in the Warburg syndrome gene, RAB3GAP1, causes a similar syndrome with polyneuropathy and neuronal vacuolation in Black Russian Terrier dogs. Neurobiol. Dis. 2016, 86, 75–85. [Google Scholar] [CrossRef]
- Mhlanga-Mutangadura, T.; Johnson, G.S.; Ashwini, A.; Shelton, G.D.; Wennogle, S.A.; Johnson, G.C.; Kuroki, K.; O’Brien, D.P. A Homozygous RAB3GAP1:c.743delC Mutation in Rottweilers with Neuronal Vacuolation and Spinocerebellar Degeneration. J. Vet. Intern. Med. 2016, 30, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Rasouliha, S.H.; Barrientos, L.; Anderegg, L.; Klesty, C.; Lorenz, J.; Chevallier, L.; Jagannathan, V.; Rösch, S.; Leeb, T. A RAPGEF6 variant constitutes a major risk factor for laryngeal paralysis in dogs. PLoS Genet. 2019, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Minor, K.M.; Letko, A.; Becker, D.; Drögemüller, M.; Mandigers, P.J.J.; Bellekom, S.R.; Leegwater, P.A.J.; Stassen, Q.E.M.; Putschbach, K.; Fischer, A.; et al. Canine NAPEPLD-associated models of human myelin disorders. Sci. Rep. 2018, 8, 5818. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.G.; Dreger, D.L.; Rimbault, M.; Davis, B.W.; Mullen, A.B.; Carpintero-Ramirez, G.; Ostrander, E.A. Genomic Analyses Reveal the Influence of Geographic Origin, Migration, and Hybridization on Modern Dog Breed Development. Cell Rep. 2017, 19, 697–708. [Google Scholar] [CrossRef]
- Huang, M.; Hayward, J.J.; Corey, E.; Garrison, S.J.; Wagner, G.R.; Krotscheck, U.; Hayashi, K.; Schweitzer, P.A.; Lust, G.; Boyko, A.R.; et al. A novel iterative mixed model to remap three complex orthopedic traits in dogs. PLoS ONE 2017, 12, e0176932. [Google Scholar] [CrossRef]
- Browning, S.R.; Browning, B.L. Rapid and Accurate Haplotype Phasing and Missing-Data Inference for Whole-Genome Association Studies by Use of Localized Haplotype Clustering. Am. J. Hum. Genet. 2007, 81, 1084–1097. [Google Scholar] [CrossRef]
- Browning, B.L.; Browning, S.R. Genotype Imputation with Millions of Reference Samples. Am. J. Hum. Genet. 2016, 98, 116–126. [Google Scholar] [CrossRef]
- Browning, B. Conform-gt Program. Available online: https://faculty.washington.edu/browning/conform-gt.html (accessed on 22 September 2020).
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.A.M.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Zhou, X.; Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012, 44, 821–824. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Turner, S.D.; Turner, D.S. qqman: An R package for visualizing GWAS results using Q-Q and manhattan plots. bioRxiv 2014, 81, 559–575. [Google Scholar] [CrossRef]
- Jagannathan, V.; Drögemüller, C.; Leeb, T. Dog Biomedical Variant Database Consortium, (DBVDC) A comprehensive biomedical variant catalogue based on whole genome sequences of 582 dogs and eight wolves. Anim. Genet. 2019, 50, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Pejaver, V.; Urresti, J.; Lugo-Martinez, J.; Pagel, K.A.; Lin, G.N.; Nam, H.-J.; Mort, M.; Cooper, D.N.; Sebat, J.; Iakoucheva, L.M.; et al. MutPred2: Inferring the molecular and phenotypic impact of amino acid variants. bioRxiv 2017, 134981. [Google Scholar] [CrossRef]
- Choi, Y.; Chan, A.P. PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 2015, 31, 2745–2747. [Google Scholar] [CrossRef]
- Niroula, A.; Urolagin, S.; Vihinen, M. PON-P2: Prediction method for fast and reliable identification of harmful variants. PLoS ONE 2015, 10, e117380. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Low, K.; Stals, K.; Caswell, R.; Clayton-Smith, J.; Donaldson, A.; Foulds, N.; Splitt, M.; Norman, A.; Urankar, K.; Vijayakumar, K.; et al. CNTNAP1: Extending the phenotype of congenital hypomyelinating neuropathy in 6 further patients. Neuromuscul. Disord. 2017, 27, S148. [Google Scholar] [CrossRef]
- Freed, A.S.; Weiss, M.D.; Malouf, E.A.; Hisama, F.M. CNTNAP1 mutations in an adult with Charcot Marie Tooth disease. Muscle Nerve 2019, 60, E28–E30. [Google Scholar] [CrossRef]
- Sabbagh, S.; Antoun, S.; Mégarbané, A. CNTNAP1 Mutations and Their Clinical Presentations: New Case Report and Systematic Review. Case Rep. Med. 2020, 2020. [Google Scholar] [CrossRef]
- Low, K.; Stals, K.; Caswell, R.; Wakeling, M.; Clayton-Smith, J.; Donaldson, A.; Foulds, N.; Norman, A.; Splitt, M.; Urankar, K.; et al. Phenotype of CNTNAP1: A study of patients demonstrating a specific severe congenital hypomyelinating neuropathy with survival beyond infancy. Eur. J. Hum. Genet. 2018, 26, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Vallat, J.-M.; Nizon, M.; Magee, A.; Isidor, B.; Magy, L.; Péréon, Y.; Richard, L.; Ouvrier, R.; Cogné, B.; Devaux, J.; et al. Contactin-Associated Protein 1 (CNTNAP1) Mutations Induce Characteristic Lesions of the Paranodal Region. J. Neuropathol. Exp. Neurol. 2016, 75, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Meola, S.D. Brachycephalic Airway Syndrome. Top. Companion Anim. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Boyko, A.R.; Brooks, S.A.; Behan-Braman, A.; Castelhano, M.; Corey, E.; Oliveira, K.C.; Swinburne, J.E.; Todhunter, R.J.; Zhang, Z.; Ainsworth, D.M.; et al. Genomic analysis establishes correlation between growth and laryngeal neuropathy in Thoroughbreds. BMC Genom. 2014, 15, 259. [Google Scholar] [CrossRef] [PubMed]
| Cohort 1 | Dog Breed/Species | Total | Phenotype | ||
|---|---|---|---|---|---|
| LPPN-Affected 2 | LPPN Non-Affected 3 | Unknown | |||
| Discovery (n = 517) | Leonberger | 426 | 126 | 300 | 0 |
| Saint Bernard | 91 | 18 | 14 | 59 | |
| Validation (n = 1070) | Leonberger | 859 | 500 | 359 | 0 |
| Saint Bernard | 11 | 11 | 0 | 0 | |
| Labrador retriever 4 | 200 | 150 | 50 | 0 | |
| Population (n = 14,174) | 243 dog breeds | 13,798 | 0 | 0 | 13,798 |
| Unknown/mixed heritage | 314 | 0 | 0 | 314 | |
| Wolf | 58 | 0 | 0 | 58 | |
| Golden jackal | 2 | 0 | 0 | 2 | |
| Andean fox | 1 | 0 | 0 | 1 | |
| Dhole | 1 | 0 | 0 | 1 | |
| CNTNAP1 Genotypes | ||||
|---|---|---|---|---|
| Breed | LPPN Status | G/G | G/A | A/A 1 |
| Leonberger (n = 2738) | Affected (n = 434) 2 | 358 | 58 | 18 |
| LPN1/LPN2 (n = 192) 3 | 180 | 11 | 1 | |
| Non-affected (n = 659) 4 | 605 | 54 | 0 | |
| Population controls (n = 1453) | 1258 | 192 | 3 | |
| Saint Bernard (n = 305) | Affected (n = 24) 2 | 9 | 5 | 10 |
| LPN1 (n = 5) 3 | 2 | 3 | 0 | |
| Non-affected (n = 14) | 9 | 5 | 0 | |
| Population controls (n = 262) | 213 | 46 | 3 | |
| Labrador retriever (n = 1524) | Affected (n = 148) | 132 | 9 | 7 |
| Non-affected (n = 45) | 42 | 3 | 0 | |
| Population controls (n = 1331) | 1200 | 128 | 3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letko, A.; Minor, K.M.; Friedenberg, S.G.; Shelton, G.D.; Salvador, J.P.; Mandigers, P.J.J.; Leegwater, P.A.J.; Winkler, P.A.; Petersen-Jones, S.M.; Stanley, B.J.; et al. A CNTNAP1 Missense Variant Is Associated with Canine Laryngeal Paralysis and Polyneuropathy. Genes 2020, 11, 1426. https://doi.org/10.3390/genes11121426
Letko A, Minor KM, Friedenberg SG, Shelton GD, Salvador JP, Mandigers PJJ, Leegwater PAJ, Winkler PA, Petersen-Jones SM, Stanley BJ, et al. A CNTNAP1 Missense Variant Is Associated with Canine Laryngeal Paralysis and Polyneuropathy. Genes. 2020; 11(12):1426. https://doi.org/10.3390/genes11121426
Chicago/Turabian StyleLetko, Anna, Katie M. Minor, Steven G. Friedenberg, G. Diane Shelton, Jill Pesayco Salvador, Paul J. J. Mandigers, Peter A. J. Leegwater, Paige A. Winkler, Simon M. Petersen-Jones, Bryden J. Stanley, and et al. 2020. "A CNTNAP1 Missense Variant Is Associated with Canine Laryngeal Paralysis and Polyneuropathy" Genes 11, no. 12: 1426. https://doi.org/10.3390/genes11121426
APA StyleLetko, A., Minor, K. M., Friedenberg, S. G., Shelton, G. D., Salvador, J. P., Mandigers, P. J. J., Leegwater, P. A. J., Winkler, P. A., Petersen-Jones, S. M., Stanley, B. J., Ekenstedt, K. J., Johnson, G. S., Hansen, L., Jagannathan, V., Mickelson, J. R., & Drögemüller, C. (2020). A CNTNAP1 Missense Variant Is Associated with Canine Laryngeal Paralysis and Polyneuropathy. Genes, 11(12), 1426. https://doi.org/10.3390/genes11121426







