Diverse Roles of MAX1 Homologues in Rice
Abstract
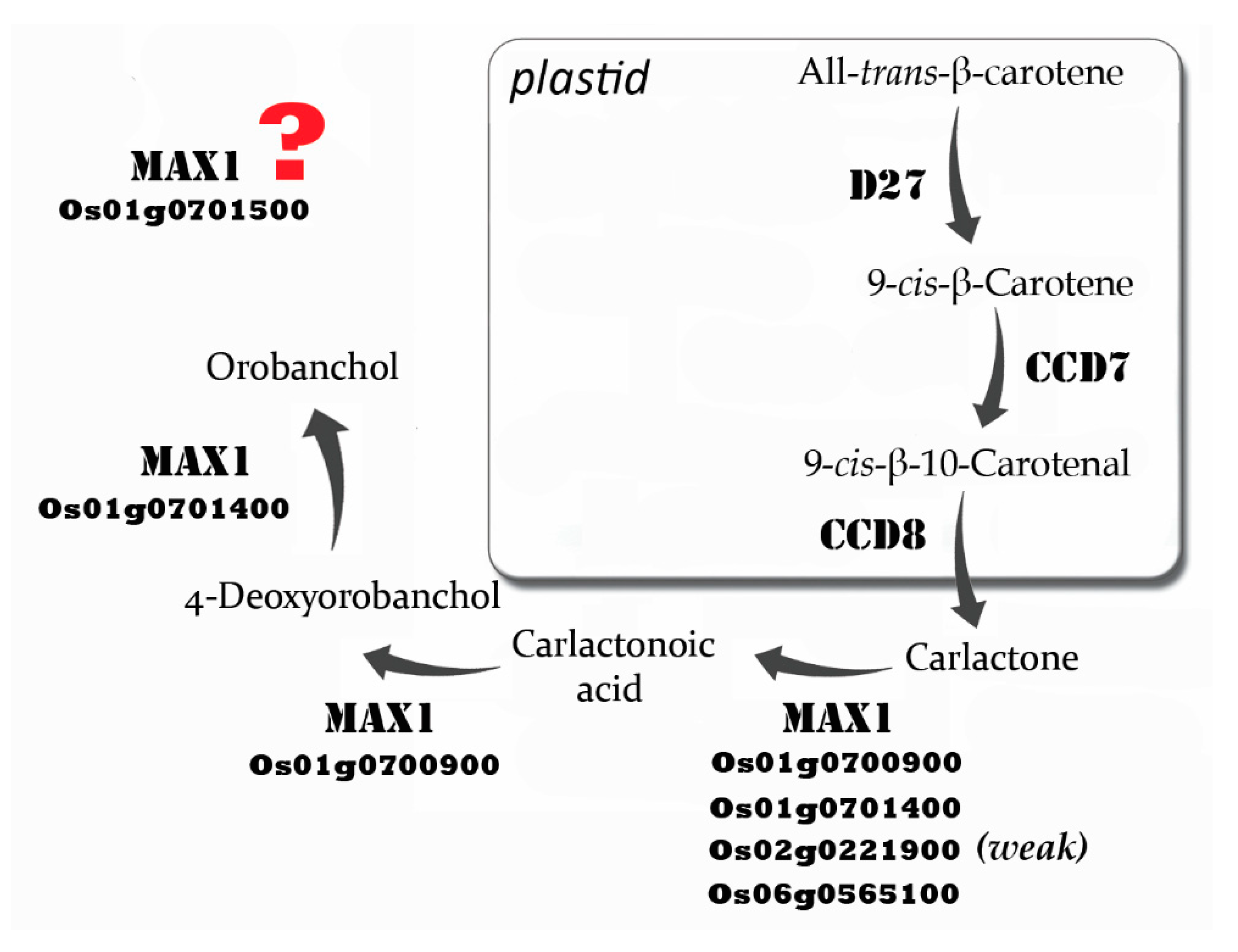
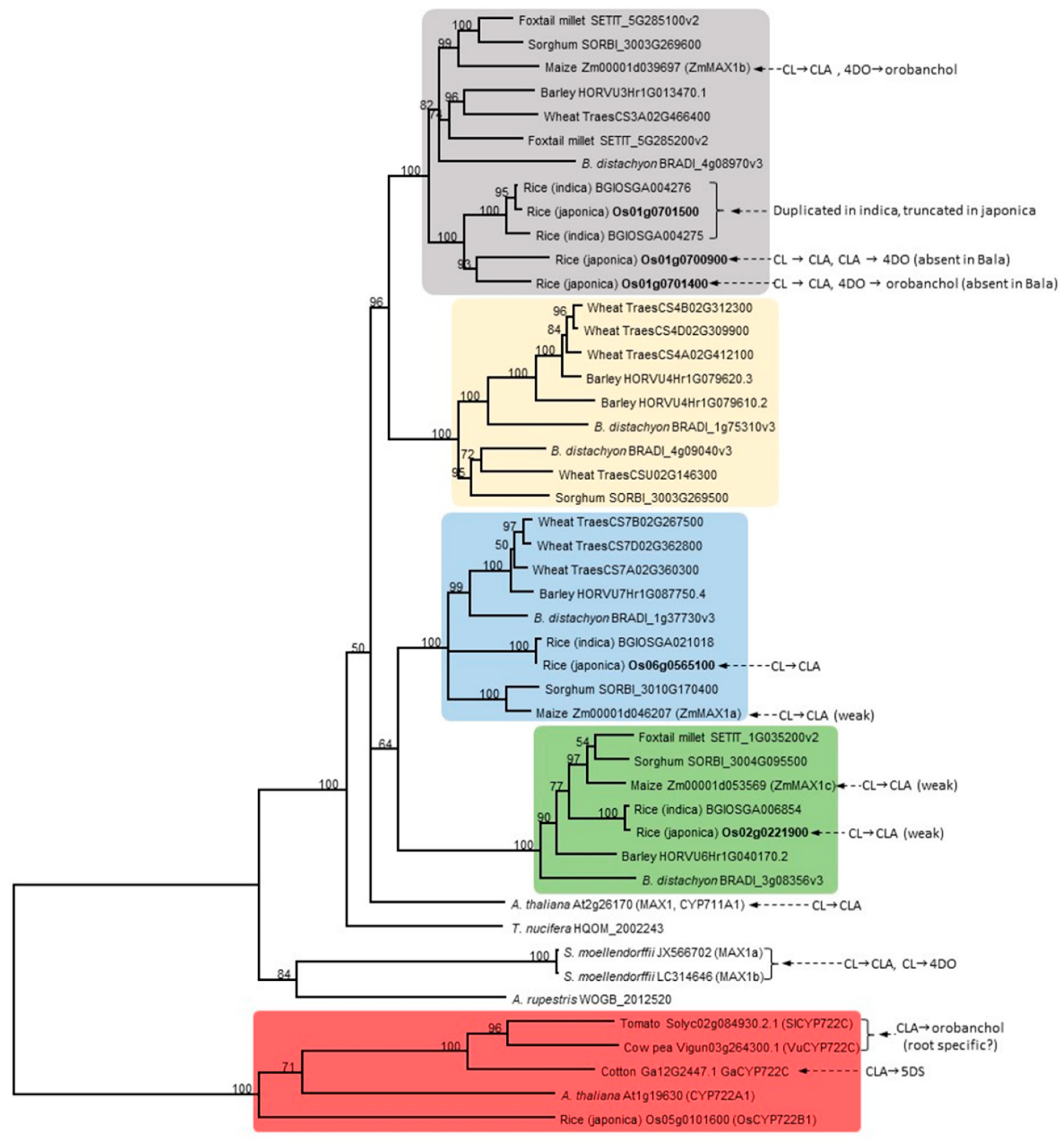
:1. Introduction
2. Materials and Methods
2.1. Rice MAX1 Sequences and Conserved Domain Identification
2.2. Searching of Transcription Factor Motifs in Promoter Region of Rice MAX1 Genes
2.3. Identification of miRNA that Regulate MAX1 Genes
2.4. Profile Expression of MAX1 Genes
2.5. Gene Co-Expression Network of Rice MAX1 Homologues
2.6. Gene Ontology of Genes Co-Expressed with Rice MAX1 Homologues
3. Results
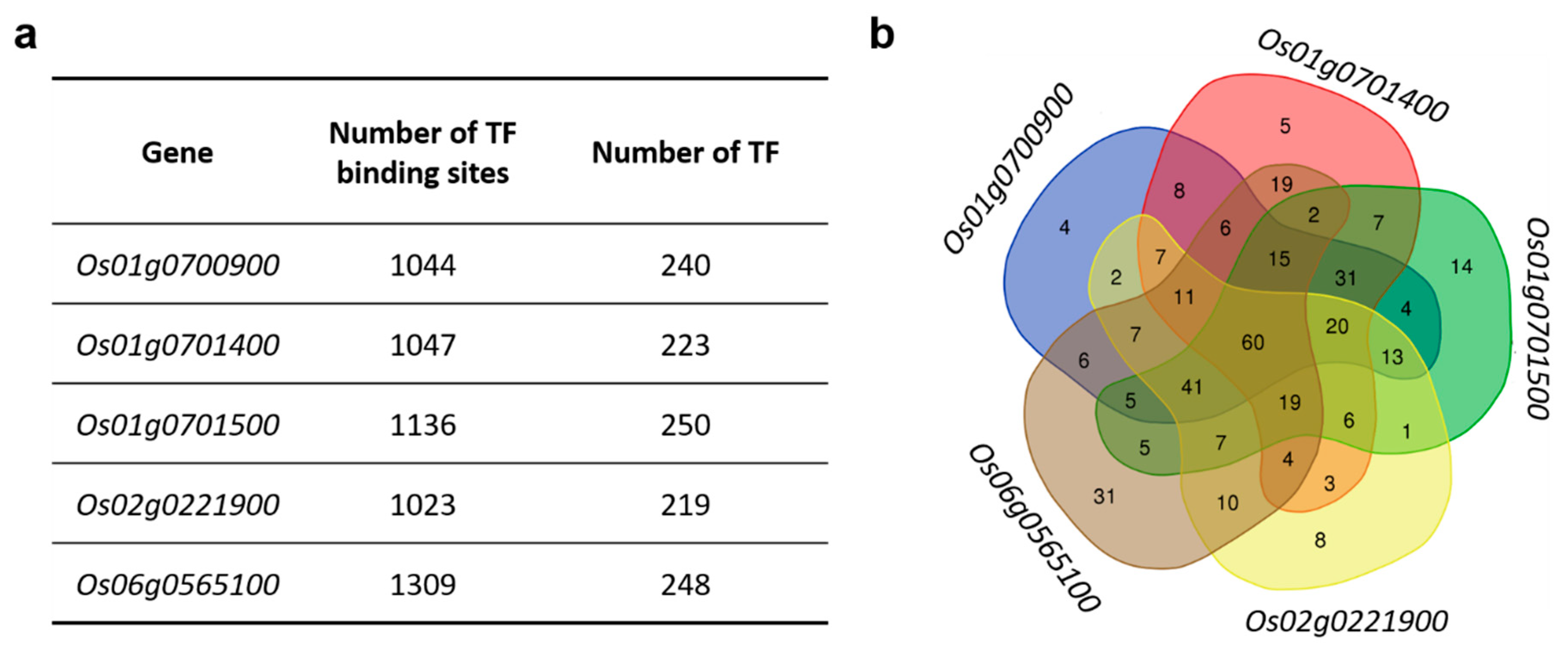
3.1. Transcription Factor Motifs That Are Present in the Promoter Region of Rice MAX1 Genes
3.2. Transcription Factors Specific to Os01g0700900
3.3. Transcription Factors Specific to Os01g0701400
3.4. Transcription Factors Specific to Os01g0701500
3.5. Transcription Factors Specific to Os02g0221900
3.6. Transcription Factors Specific to Os06g0565100
3.7. MiRNAs That May Bind Rice MAX1 Homologues
3.8. MiRNA Specific to Os01g0700900
3.9. MiRNA Specific to Os01g0701400
3.10. MiRNA Specific to Os01g0701500
3.11. MiRNA Specific to Os02g0221900
3.12. MiRNA Specific for Os06g0565100
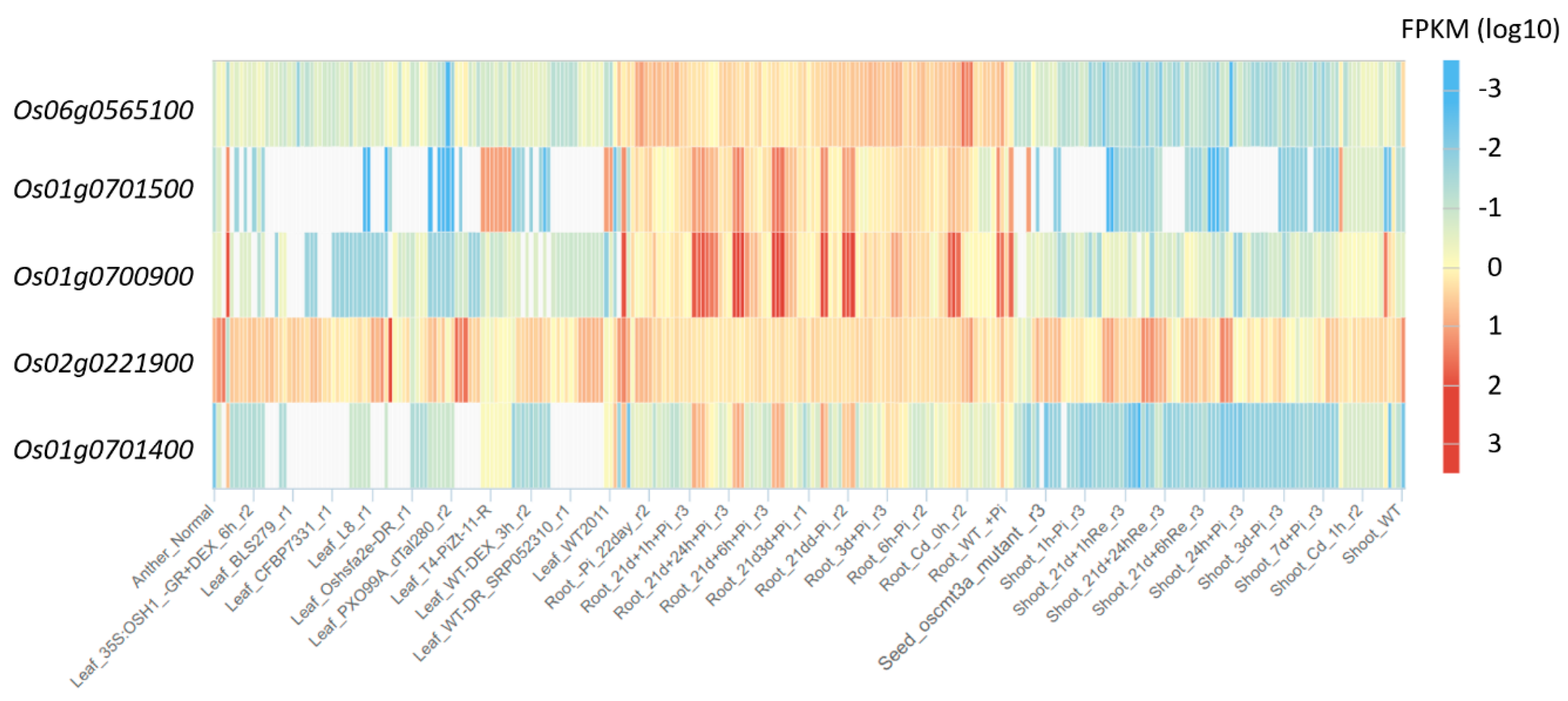
3.13. Profile Expression of MAX1 Homologues
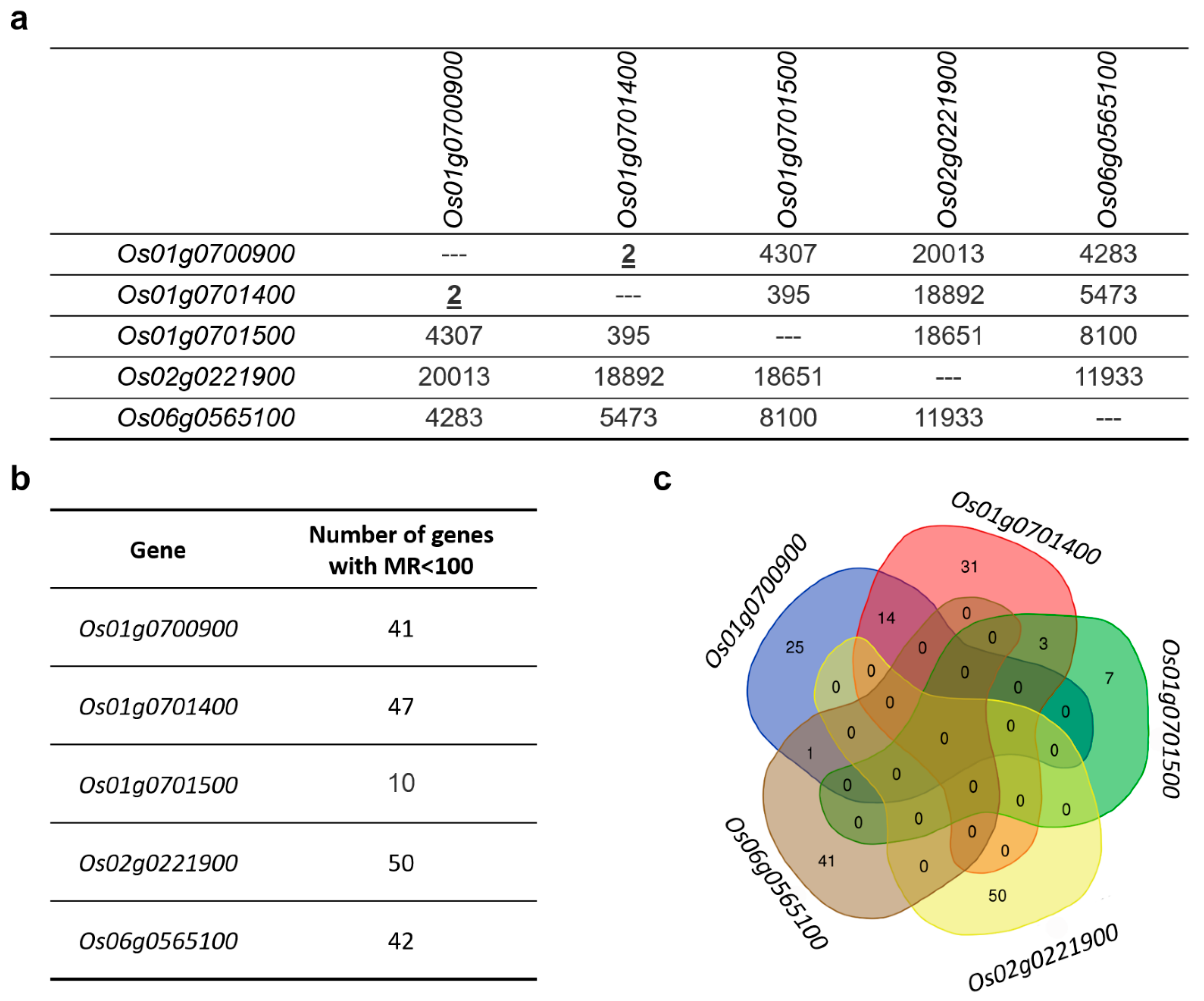
3.14. Co-Expression Gene Networks of MAX1 Homologues
3.15. Co-Expression Gene Network of Os01g0700900
3.16. Co-Expression Gene Network of Os01g0701400
3.17. Co-Expression Gene Network of Os01g0701500
3.18. Co-Expression Gene Network of Os02g0221900
3.19. Co-Expression Gene Network of Os06g0565100
3.20. Gene Ontology for Genes Co-Expressed with MAX1s
4. Discussion
4.1. TFs That Regulate Expression of MAX1 Homologues
4.2. Rice MAX1s Are Regulated by Different miRNAs
4.3. Expression Profiles of MAX1s and Their Co-Expression Gene Networks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 4DO | 4-deoxyorobanchol |
| 5DS | 5-deoxystrigol |
| ABA | abscisic acid |
| AM | arbuscular mycorrhizal |
| CCD | carotenoid cleavage dioxygenase |
| CGN | co-expression gene network |
| CL | carlactone |
| CLA | carlactonoic acid |
| D27 | DWARF27 |
| Edh1 | Early headingdate1 |
| EUI | ELONGATION OF UPPER MOST INTERNODE I |
| GAs | gibberelins |
| GEO | Gene Expression Omnibus |
| GO | Gene Ontology |
| LBO | LATERAL BRANCHING OXIDOREDUCTASE |
| LYP9 | LiangYou-Pei 9 |
| MAX1 | MORE AXILLARY GROWTH1 |
| MeCLA | methyl carlactonoate |
| Me-O-5- | methoxy-5-deoxystrigol |
| MR | Mutual Rank |
| N2Y6 | Nei-2-You 6 |
| nt | nucleotide |
| OsSWN1/2 | Oryza sativa SECONDARY WALL NAC DOMAIN PROTEIN1/2 |
| OsWS1 | Oryza sativa wax synthase isoform 1 |
| PRP | proline–rich protein |
| SL | strigolactones |
| TF | transcription factor |
References
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of Witchweed (Striga lutea Lour.): Isolation and Properties of a Potent Stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.-P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.-C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef]
- Sun, H.; Tao, J.; Gu, P.; Xu, G.; Zhang, Y. The role of strigolactones in root development. Plant Signal Behav. 2016, 11, e1110662. [Google Scholar] [CrossRef]
- Hu, Z.; Yan, H.; Yang, J.; Yamaguchi, S.; Maekawa, M.; Takamure, I.; Tsutsumi, N.; Kyozuka, J.; Nakazono, M. Strigolactones negatively regulate mesocotyl elongation in rice during germination and growth in darkness. Plant Cell Physiol. 2010, 51, 1136–1142. [Google Scholar] [CrossRef] [Green Version]
- de Saint Germain, A.; Ligerot, Y.; Dun, E.A.; Pillot, J.-P.; Ross, J.J.; Beveridge, C.A.; Rameau, C. Strigolactones stimulate internode elongation independently of gibberellins. Plant Physiol. 2013, 163, 1012–1025. [Google Scholar] [CrossRef] [Green Version]
- Agusti, J.; Herold, S.; Schwarz, M.; Sanchez, P.; Ljung, K.; Dun, E.A.; Brewer, P.B.; Beveridge, C.A.; Sieberer, T.; Sehr, E.M.; et al. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc. Natl. Acad. Sci. USA 2011, 108, 20242–20247. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Mazur, E.; Balla, J.; Gallei, M.; Kalousek, P.; Medveďová, Z.; Li, Y.; Wang, Y.; Prát, T.; Vasileva, M.; et al. Strigolactones inhibit auxin feedback on PIN-dependent auxin transport canalization. Nat. Commun. 2020, 11, 3508. [Google Scholar] [CrossRef]
- Yamada, Y.; Umehara, M. Possible Roles of Strigolactones during Leaf Senescence. Plants 2015, 4, 664–677. [Google Scholar] [CrossRef] [Green Version]
- Marzec, M.; Muszynska, A. In silico analysis of the genes encoding proteins that are involved in the biosynthesis of the RMS/MAX/D pathway revealed new roles of Strigolactones in plants. Int. J. Mol. Sci. 2015, 16, 6757–6782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzec, M. Strigolactones as Part of the Plant Defence System. Trends Plant Sci. 2016, 21, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Li, W.; Nguyen, K.H.; Fujita, M.; Tran, L.-S.P. Strigolactones in plant adaptation to abiotic stresses: An emerging avenue of plant research. Plant Cell Environ. 2018, 41, 2227–2243. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Liu, G.-W.; Emonet, A.; Kretzschmar, T.; Martinoia, E. The importance of strigolactone transport regulation for symbiotic signaling and shoot branching. Planta 2016, 243, 1351–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneyama, K.; Xie, X.; Yoneyama, K.; Kisugi, T.; Nomura, T.; Nakatani, Y.; Akiyama, K.; McErlean, C.S.P. Which are the major players, canonical or non-canonical strigolactones? J. Exp. Bot. 2018, 69, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186. [Google Scholar] [CrossRef]
- Yoneyama, K.; Akiyama, K.; Brewer, P.B.; Mori, N.; Kawano-Kawada, M.; Haruta, S.; Nishiwaki, H.; Yamauchi, S.; Xie, X.; Umehara, M.; et al. Hydroxyl carlactone derivatives are predominant strigolactones in Arabidopsis. Plant Direct 2020, 4, e00219. [Google Scholar] [CrossRef]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef] [Green Version]
- Abe, S.; Sado, A.; Tanaka, K.; Kisugi, T.; Asami, K.; Ota, S.; Kim, H.I.; Yoneyama, K.; Xie, X.; Ohnishi, T.; et al. Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc. Natl. Acad. Sci. USA 2014, 111, 18084–18089. [Google Scholar] [CrossRef] [Green Version]
- Charnikhova, T.V.; Gaus, K.; Lumbroso, A.; Sanders, M.; Vincken, J.-P.; De Mesmaeker, A.; Ruyter-Spira, C.P.; Screpanti, C.; Bouwmeester, H.J. Zealactones. Novel natural strigolactones from maize. Phytochemistry 2017, 137, 123–131. [Google Scholar] [CrossRef]
- Xie, X.; Kisugi, T.; Yoneyama, K.; Nomura, T.; Akiyama, K.; Uchida, K.; Yokota, T.; McErlean, C.S.P.; Yoneyama, K. Methyl zealactonoate, a novel germination stimulant for root parasitic weeds produced by maize. J. Pestic Sci. 2017, 42, 58–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.I.; Kisugi, T.; Khetkam, P.; Xie, X.; Yoneyama, K.; Uchida, K.; Yokota, T.; Nomura, T.; McErlean, C.S.P.; Yoneyama, K. Avenaol, a germination stimulant for root parasitic plants from Avena Strigosa. Phytochemistry 2014, 103, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bouwmeester, H.J. Structural diversity in the strigolactones. J. Exp. Bot. 2018, 69, 2219–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneyama, K.; Xie, X.; Yoneyama, K.; Takeuchi, Y. Strigolactones: Structures and biological activities. Pest Manag. Sci. 2009, 65, 467–470. [Google Scholar] [CrossRef]
- Jamil, M.; Charnikhova, T.; Cardoso, C.; Jamil, T.; Ueno, K.; Verstappen, F.; Asami, T.; Bouwmeester, H.J. Quantification of the relationship between strigolactones and Striga hermonthica infection in rice under varying levels of nitrogen and phosphorus: N and P effect on strigolactones and Striga hermonthica. Weed Res. 2011, 51, 373–385. [Google Scholar] [CrossRef]
- Xie, X.; Yoneyama, K.; Kisugi, T.; Uchida, K.; Ito, S.; Akiyama, K.; Hayashi, H.; Yokota, T.; Nomura, T.; Yoneyama, K. Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol. Plant 2013, 6, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Boyer, F.-D.; de Saint Germain, A.; Pillot, J.-P.; Pouvreau, J.-B.; Chen, V.X.; Ramos, S.; Stévenin, A.; Simier, P.; Delavault, P.; Beau, J.-M.; et al. Structure-activity relationship studies of strigolactone-related molecules for branching inhibition in garden pea: Molecule design for shoot branching. Plant Physiol. 2012, 159, 1524–1544. [Google Scholar] [CrossRef] [Green Version]
- Butt, H.; Jamil, M.; Wang, J.Y.; Al-Babili, S.; Mahfouz, M. Engineering plant architecture via CRISPR/Cas9-mediated alteration of strigolactone biosynthesis. BMC Plant Biol. 2018, 18, 174. [Google Scholar] [CrossRef] [Green Version]
- Marzec, M. Perception and Signaling of Strigolactones. Front. Plant Sci. 2016, 7, 1260. [Google Scholar] [CrossRef] [Green Version]
- Marzec, M.; Brewer, P. Binding or Hydrolysis? How Does the Strigolactone Receptor Work? Trends Plant Sci. 2019, 24, 571–574. [Google Scholar] [CrossRef]
- Booker, J.; Sieberer, T.; Wright, W.; Williamson, L.; Willett, B.; Stirnberg, P.; Turnbull, C.; Srinivasan, M.; Goddard, P.; Leyser, O. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev. Cell 2005, 8, 443–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, J.L.; Napoli, C.A.; Janssen, B.J.; Plummer, K.M.; Snowden, K.C. Analysis of the DECREASED APICAL DOMINANCE genes of petunia in the control of axillary branching. Plant Physiol. 2007, 143, 697–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewer, P.B.; Yoneyama, K.; Filardo, F.; Meyers, E.; Scaffidi, A.; Frickey, T.; Akiyama, K.; Seto, Y.; Dun, E.A.; Cremer, J.E.; et al. Lateral branching oxidoreductase acts in the final stages of strigolactone biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 6301–6306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iseki, M.; Shida, K.; Kuwabara, K.; Wakabayashi, T.; Mizutani, M.; Takikawa, H.; Sugimoto, Y. Evidence for species-dependent biosynthetic pathways for converting carlactone to strigolactones in plants. J. Exp. Bot. 2018, 69, 2305–2318. [Google Scholar] [CrossRef]
- Yoneyama, K.; Mori, N.; Sato, T.; Yoda, A.; Xie, X.; Okamoto, M.; Iwanaga, M.; Ohnishi, T.; Nishiwaki, H.; Asami, T.; et al. Conversion of carlactone to carlactonoic acid is a conserved function of MAX1 homologs in strigolactone biosynthesis. New Phytol. 2018, 218, 1522–1533. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cheng, X.; Wang, Y.; Díez-Simón, C.; Flokova, K.; Bimbo, A.; Bouwmeester, H.J.; Ruyter-Spira, C. The tomato MAX1 homolog, SlMAX1, is involved in the biosynthesis of tomato strigolactones from carlactone. New Phytol. 2018, 219, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Zhang, L.; Tang, M.; Liu, J.; Liu, H.; Yang, H.; Fan, S.; Terzaghi, W.; Wang, H.; Hua, W. Knockout of two BnaMAX1 homologs by CRISPR/Cas9-targeted mutagenesis improves plant architecture and increases yield in rapeseed (Brassica napus L.). Plant Biotechnol. J. 2020, 18, 644–654. [Google Scholar] [CrossRef] [Green Version]
- Mori, N.; Sado, A.; Xie, X.; Yoneyama, K.; Asami, K.; Seto, Y.; Nomura, T.; Yamaguchi, S.; Yoneyama, K.; Akiyama, K. Chemical identification of 18-hydroxycarlactonoic acid as an LjMAX1 product and in planta conversion of its methyl ester to canonical and non-canonical strigolactones in Lotus japonicus. Phytochemistry 2020, 174, 112349. [Google Scholar] [CrossRef]
- Zhang, Y.; van Dijk, A.D.J.; Scaffidi, A.; Flematti, G.R.; Hofmann, M.; Charnikhova, T.; Verstappen, F.; Hepworth, J.; van der Krol, S.; Leyser, O.; et al. Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat. Chem. Biol. 2014, 10, 1028–1033. [Google Scholar] [CrossRef]
- Cardoso, C.; Zhang, Y.; Jamil, M.; Hepworth, J.; Charnikhova, T.; Dimkpa, S.O.N.; Meharg, C.; Wright, M.H.; Liu, J.; Meng, X.; et al. Natural variation of rice strigolactone biosynthesis is associated with the deletion of two MAX1 orthologs. Proc. Natl. Acad. Sci. USA 2014, 111, 2379–2384. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, T.; Hamana, M.; Mori, A.; Akiyama, R.; Ueno, K.; Osakabe, K.; Osakabe, Y.; Suzuki, H.; Takikawa, H.; Mizutani, M.; et al. Direct conversion of carlactonoic acid to orobanchol by cytochrome P450 CYP722C in strigolactone biosynthesis. Sci. Adv. 2019, 5, eaax9067. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, T.; Shida, K.; Kitano, Y.; Takikawa, H.; Mizutani, M.; Sugimoto, Y. CYP722C from Gossypium arboreum catalyzes the conversion of carlactonoic acid to 5-deoxystrigol. Planta 2020, 251, 97. [Google Scholar] [CrossRef]
- Challis, R.J.; Hepworth, J.; Mouchel, C.; Waites, R.; Leyser, O. A role for more axillary growth1 (MAX1) in evolutionary diversity in strigolactone signaling upstream of MAX2. Plant Physiol. 2013, 161, 1885–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, T.; Shinde, H.; Shiotani, N.; Yamamoto, S.; Mizutani, M.; Takikawa, H.; Sugimoto, Y. Conversion of methyl carlactonoate to heliolactone in sunflower. Nat. Prod. Res. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.H.; Siu-Ting, K.; Taylor, A.; O’Connell, M.J.; Bennett, T. Strigolactone synthesis is ancestral in land plants, but canonical strigolactone signalling is a flowering plant innovation. BMC Biol. 2019, 17, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Zou, D.; Sang, J.; Xu, X.; Yin, H.; Li, M.; Wu, S.; Hu, S.; Hao, L.; Zhang, Z. Rice Expression Database (RED): An integrated RNA-Seq-derived gene expression database for rice. J. Genet Genom. 2017, 44, 235–241. [Google Scholar] [CrossRef]
- Sato, Y.; Namiki, N.; Takehisa, H.; Kamatsuki, K.; Minami, H.; Ikawa, H.; Ohyanagi, H.; Sugimoto, K.; Itoh, J.-I.; Antonio, B.A.; et al. RiceFREND: A platform for retrieving coexpressed gene networks in rice. Nucleic Acids Res. 2013, 41, D1214–D1221. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Liu, Y.; Yan, H.; You, Q.; Yi, X.; Du, Z.; Xu, W.; Su, Z. agriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef]
- da Maia, L.C.; Cadore, P.R.B.; Benitez, L.C.; Danielowski, R.; Braga, E.J.B.; Fagundes, P.R.R.; Magalhães, A.M.; Costa de Oliveira, A. Transcriptome profiling of rice seedlings under cold stress. Funct. Plant Biol. 2017, 44, 419. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lee, A.-R.; Park, S.-Y.; Jin, S.-H.; Lee, J.; Ham, T.-H.; Park, Y.; Zhao, W.-G.; Kwon, S.-W. Genome-wide association study reveals candidate genes related to low temperature tolerance in rice (Oryza sativa) during germination. 3 Biotech 2018, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Kitazumi, A.; Pabuayon, I.C.M.; Ohyanagi, H.; Fujita, M.; Osti, B.; Shenton, M.R.; Kakei, Y.; Nakamura, Y.; Brar, D.S.; Kurata, N.; et al. Potential of Oryza officinalis to augment the cold tolerance genetic mechanisms of Oryza sativa by network complementation. Sci. Rep. 2018, 8, 16346. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-J.; Ahn, H.; Jung, I.; Rhee, S.; Kim, S.; Kwon, H.-B. Novel drought-responsive regulatory coding and non-coding transcripts from Oryza sativa L. Genes Genom. 2016, 38, 949–960. [Google Scholar] [CrossRef]
- Ahn, H.; Jung, I.; Shin, S.-J.; Park, J.; Rhee, S.; Kim, J.-K.; Jung, W.; Kwon, H.-B.; Kim, S. Transcriptional Network Analysis Reveals Drought Resistance Mechanisms of AP2/ERF Transgenic Rice. Front. Plant Sci. 2017, 8, 1044. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Pan, Y.; Zhao, X.; Zhu, L.; Fu, B.; Li, Z. Genome-wide temporal-spatial gene expression profiling of drought responsiveness in rice. BMC Genom. 2011, 12, 149. [Google Scholar] [CrossRef] [Green Version]
- Chung, P.J.; Jung, H.; Choi, Y.D.; Kim, J.-K. Genome-wide analyses of direct target genes of four rice NAC-domain transcription factors involved in drought tolerance. BMC Genom. 2018, 19, 40. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-W.; Jeong, H.-J.; Jung, K.-H. Integrating omics analysis of salt stress-responsive genes in rice. Genes Genom. 2015, 37, 645–655. [Google Scholar] [CrossRef]
- Mito, T.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M.; Matsui, K. Generation of chimeric repressors that confer salt tolerance in Arabidopsis and rice. Plant Biotechnol. J. 2011, 9, 736–746. [Google Scholar] [CrossRef]
- Hossain, M.R. Salinity Tolerance and Transcriptomics in Rice. Ph.D. Dissertation, University of Birmingham, Birmingham, UK, 2014. [Google Scholar]
- Das, C.K.; Bastia, D.; Swain, S.; Mahapatra, S. Computational analysis of genes encoding for molecular determinants of arsenic tolerance in rice (Oryza sativa L.) to engineer low arsenic content varieties. ORYZA Int. J. Rice 2018, 55, 248. [Google Scholar] [CrossRef]
- Ogawa, I.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Time course analysis of gene regulation under cadmium stress in rice. Plant Soil 2009, 325, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-C.; Kan, C.-C.; Hung, T.-H.; Hsieh, P.-H.; Wang, S.-Y.; Hsieh, W.-Y.; Hsieh, M.-H. Identification of early ammonium nitrate-responsive genes in rice roots. Sci. Rep. 2017, 7, 16885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, P.-H.; Kan, C.-C.; Wu, H.-Y.; Yang, H.-C.; Hsieh, M.-H. Early molecular events associated with nitrogen deficiency in rice seedling roots. Sci. Rep. 2018, 8, 12207. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Hanada, K.; Shimizu, M.; Seki, M.; Nakanishi, H.; Nishizawa, N.K. Transcriptomic analysis of rice in response to iron deficiency and excess. Rice 2014, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, S. Genome-wide view of the expression profiles of NAC-domain genes in response to infection by rice viruses. In Omics Technologies and Crop Improvement; Benkeblia, N., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 127–152. [Google Scholar]
- Wang, C.; Tariq, R.; Ji, Z.; Wei, Z.; Zheng, K.; Mishra, R.; Zhao, K. Transcriptome analysis of a rice cultivar reveals the differentially expressed genes in response to wild and mutant strains of Xanthomonas oryzae pv. oryzae. Sci. Rep. 2019, 9, 3757. [Google Scholar] [CrossRef] [Green Version]
- Tezuka, D.; Kawamata, A.; Kato, H.; Saburi, W.; Mori, H.; Imai, R. The rice ethylene response factor OsERF83 positively regulates disease resistance to Magnaporthe oryzae. Plant Physiol. Biochem. 2019, 135, 263–271. [Google Scholar] [CrossRef]
- Lavarenne, J.; Gonin, M.; Guyomarc’h, S.; Rouster, J.; Champion, A.; Sallaud, C.; Laplaze, L.; Gantet, P.; Lucas, M. Inference of the gene regulatory network acting downstream of CROWN ROOTLESS 1 in rice reveals a regulatory cascade linking genes involved in auxin signaling, crown root initiation, and root meristem specification and maintenance. Plant J. 2019, 100, 954–968. [Google Scholar] [CrossRef]
- Matsubara, K.; Yano, M. Genetic and Molecular Dissection of Flowering Time Control in Rice. In Rice Genomics, Genetics and Breeding; Sasaki, T., Ashikari, M., Eds.; Springer: Singapore, 2018; pp. 177–190. ISBN 978-981-10-7460-8. [Google Scholar]
- Das, S.P.; Deb, D.; Dey, N. Micromorphic and Molecular Studies of Floral Organs of a Multiple Seeded Rice (Oryza sativa L.). Plant Mol. Biol. Rep. 2018, 36, 764–775. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, H.; Lee, S.; Lee, J.; Kim, S.-J.; Park, J.-W.; Woo, H.R.; Lim, P.O.; An, G.; Nam, H.G.; et al. Molecular bases for differential aging programs between flag and second leaves during grain-filling in rice. Sci. Rep. 2017, 7, 8792. [Google Scholar] [CrossRef] [Green Version]
- Yamatani, H.; Sato, Y.; Masuda, Y.; Kato, Y.; Morita, R.; Fukunaga, K.; Nagamura, Y.; Nishimura, M.; Sakamoto, W.; Tanaka, A.; et al. NYC4, the rice ortholog of Arabidopsis THF1, is involved in the degradation of chlorophyll—Protein complexes during leaf senescence. Plant J. 2013, 74, 652–662. [Google Scholar] [CrossRef]
- Sugimoto, K.; Marzougi, S.; Yano, M. Genetic Control of Seed Dormancy in Rice. In γ Field Symposia; Institute of Radiation Breeding, NIAS: Ohmiya, Japan, 2009. [Google Scholar]
- Chu, Y.; Xu, N.; Wu, Q.; Yu, B.; Li, X.; Chen, R.; Huang, J. Rice transcription factor OsMADS57 regulates plant height by modulating gibberellin catabolism. Rice 2019, 12, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.D.; Moon, S.; Nguyen, V.N.T.; Gho, Y.; Chandran, A.K.N.; Soh, M.-S.; Song, J.T.; An, G.; Oh, S.A.; Park, S.K.; et al. Genome-wide identification and analysis of rice genes preferentially expressed in pollen at an early developmental stage. Plant Mol. Biol. 2016, 92, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Fujita, M.; Takahashi, H.; Nakazono, M.; Tsutsumi, N.; Kurata, N. Transcriptome analysis of developing ovules in rice isolated by laser microdissection. Plant Cell Physiol. 2013, 54, 750–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Zhang, F.; Wang, H.; Wang, W.; Zhao, F.; Li, Z.; Sun, C.; Chen, F.; Xu, F.; Chang, S.; et al. Ef-cd locus shortens rice maturity duration without yield penalty. Proc. Natl. Acad. Sci. USA 2019, 116, 18717–18722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, H.; Taoka, K.; Shimamoto, K. Regulation of flowering in rice: Two florigen genes, a complex gene network, and natural variation. Curr. Opin. Plant Biol. 2011, 14, 45–52. [Google Scholar] [CrossRef]
- Hori, K.; Matsubara, K.; Yano, M. Genetic control of flowering time in rice: Integration of Mendelian genetics and genomics. Theor. Appl. Genet. 2016, 129, 2241–2252. [Google Scholar] [CrossRef]
- Kitomi, Y.; Itoh, J.-I.; Uga, Y. Genetic Mechanisms Involved in the Formation of Root System Architecture. In Rice Genomics, Genetics and Breeding; Sasaki, T., Ashikari, M., Eds.; Springer: Singapore, 2018; pp. 241–274. ISBN 978-981-10-7460-8. [Google Scholar]
- Lee, D.-K.; Jung, H.; Jang, G.; Jeong, J.S.; Kim, Y.S.; Ha, S.-H.; Do Choi, Y.; Kim, J.-K. Overexpression of the OsERF71 Transcription Factor Alters Rice Root Structure and Drought Resistance. Plant Physiol. 2016, 172, 575–588. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Indoliya, Y.; Chauhan, A.S.; Singh, S.P.; Singh, A.P.; Dwivedi, S.; Tripathi, R.D.; Chakrabarty, D. Nitric oxide mediated transcriptional modulation enhances plant adaptive responses to arsenic stress. Sci. Rep. 2017, 7, 3592. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, L.; Wang, W.; Zhao, X.; Zhu, L.; Fu, B.; Li, Z. Genome-wide gene expression profiling of introgressed indica rice alleles associated with seedling cold tolerance improvement in a japonica rice background. BMC Genom. 2012, 13, 461. [Google Scholar] [CrossRef]
- Tula, S.; Ansari, M.W.; Babu, A.P.; Pushpalatha, G.; Sreenu, K.; Sarla, N.; Tuteja, N.; Rai, V. Physiological Assessment and Allele Mining in Rice Cultivars for Salinity and Drought Stress Tolerance. Vegetos 2013, 26, 219. [Google Scholar] [CrossRef]
- Mohanty, B.; Kitazumi, A.; Cheung, C.Y.M.; Lakshmanan, M.; de Los Reyes, B.G.; Jang, I.-C.; Lee, D.-Y. Identification of candidate network hubs involved in metabolic adjustments of rice under drought stress by integrating transcriptome data and genome-scale metabolic network. Plant Sci. 2016, 242, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Burgess, P.; Huang, B. Transcriptional regulation of hormone-synthesis and signaling pathways by overexpressing cytokinin-synthesis contributes to improved drought tolerance in creeping bentgrass. Physiol. Plant 2017, 161, 235–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oono, Y.; Kawahara, Y.; Yazawa, T.; Kanamori, H.; Kuramata, M.; Yamagata, H.; Hosokawa, S.; Minami, H.; Ishikawa, S.; Wu, J.; et al. Diversity in the complexity of phosphate starvation transcriptomes among rice cultivars based on RNA-Seq profiles. Plant Mol. Biol. 2013, 83, 523–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, B.; Takahashi, H.; de Los Reyes, B.G.; Wijaya, E.; Nakazono, M.; Lee, D.-Y. Transcriptional regulatory mechanism of alcohol dehydrogenase 1-deficient mutant of rice for cell survival under complete submergence. Rice 2016, 9, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croissant-Sych, Y.; Okita, T.W. Identification of positive and negative regulatory cis-elements of the rice glutelin Gt3 promoter. Plant Sci. 1996, 116, 27–35. [Google Scholar] [CrossRef]
- Mohanty, B.; Krishnan, S.P.T.; Swarup, S.; Bajic, V.B. Detection and preliminary analysis of motifs in promoters of anaerobically induced genes of different plant species. Ann. Bot. 2005, 96, 669–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, E.; Wallner, A.; Rimbault, I.; Barrachina, C.; Klonowska, A.; Moulin, L.; Czernic, P. Monitoring of Rice Transcriptional Responses to Contrasted Colonizing Patterns of Phytobeneficial Burkholderia s.l. Reveals a Temporal Shift in JA Systemic Response. Front. Plant Sci. 2019, 10, 1141. [Google Scholar] [CrossRef]
- Le, C.T.T.; Brumbarova, T.; Ivanov, R.; Stoof, C.; Weber, E.; Mohrbacher, J.; Fink-Straube, C.; Bauer, P. ZINC FINGER OF ARABIDOPSIS THALIANA12 (ZAT12) Interacts with FER-LIKE IRON DEFICIENCY-INDUCED TRANSCRIPTION FACTOR (FIT) Linking Iron Deficiency and Oxidative Stress Responses. Plant Physiol. 2016, 170, 540–557. [Google Scholar] [CrossRef]
- Kan, C.-C.; Chung, T.-Y.; Juo, Y.-A.; Hsieh, M.-H. Glutamine rapidly induces the expression of key transcription factor genes involved in nitrogen and stress responses in rice roots. BMC Genom. 2015, 16, 731. [Google Scholar] [CrossRef] [Green Version]
- Fujino, K.; Matsuda, Y. Genome-wide analysis of genes targeted by qLTG3-1 controlling low-temperature germinability in rice. Plant Mol. Biol. 2010, 72, 137–152. [Google Scholar] [CrossRef]
- Cui, Y.; Li, M.; Yin, X.; Song, S.; Xu, G.; Wang, M.; Li, C.; Peng, C.; Xia, X. OsDSSR1, a novel small peptide, enhances drought tolerance in transgenic rice. Plant Sci. 2018, 270, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Yu, C.; Ai, L.; Zhou, Y.; Duan, L. Gene Expression Profiles Deciphering the Pathways of Coronatine Alleviating Water Stress in Rice (Oryza sativa L.) Cultivar Nipponbare (Japonica). Int. J. Mol. Sci. 2019, 20, 2543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuteja, N.; Sahoo, R.K.; Huda, K.M.K.; Tula, S.; Tuteja, R. OsBAT1 Augments Salinity Stress Tolerance by Enhancing Detoxification of ROS and Expression of Stress-Responsive Genes in Transgenic Rice. Plant Mol. Biol. Rep. 2015, 33, 1192–1209. [Google Scholar] [CrossRef]
- Huang, T.-L.; Huang, L.-Y.; Fu, S.-F.; Trinh, N.-N.; Huang, H.-J. Genomic profiling of rice roots with short- and long-term chromium stress. Plant Mol. Biol. 2014, 86, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Li, Y.; Yang, J.; Li, Y. Comparative transcriptional profiling of two rice genotypes carrying SUB1A-1 but exhibiting differential tolerance to submergence. Funct. Plant Biol. 2012, 39, 449. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Tanaka, W.; Sakamoto, T.; Kurata, T.; Hirano, H.-Y. Genetic Enhancer Analysis Reveals that FLORAL ORGAN NUMBER2 and OsMADS3 Co-operatively Regulate Maintenance and Determinacy of the Flower Meristem in Rice. Plant Cell Physiol. 2017, 58, 893–903. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, T.; Takanashi, H.; Mogi, M.; Takahashi, H.; Kikuchi, S.; Yano, K.; Okamoto, T.; Fujita, M.; Kurata, N.; Tsutsumi, N. Distinct gene expression profiles in egg and synergid cells of rice as revealed by cell type-specific microarrays. Plant Physiol. 2011, 155, 881–891. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Yoshida, K.; Mitsuda, N.; Ohme-Takagi, M.; Takamizo, T. Male-sterile and cleistogamous phenotypes in tall fescue induced by chimeric repressors of SUPERWOMAN1 and OsMADS58. Plant Sci. 2012, 183, 183–189. [Google Scholar] [CrossRef]
- Kuwano, M.; Masumura, T.; Yoshida, K.T. A novel endosperm transfer cell-containing region-specific gene and its promoter in rice. Plant Mol. Biol. 2011, 76, 47–56. [Google Scholar] [CrossRef]
- Ke, S.; Liu, X.-J.; Luan, X.; Yang, W.; Zhu, H.; Liu, G.; Zhang, G.; Wang, S. Genome-wide transcriptome profiling provides insights into panicle development of rice (Oryza sativa L.). Gene 2018, 675, 285–300. [Google Scholar] [CrossRef]
- Kim, J.S.; Chae, S.; Jun, K.M.; Pahk, Y.-M.; Lee, T.-H.; Chung, P.J.; Kim, Y.-K.; Nahm, B.H. Genome-wide identification of grain filling genes regulated by the OsSMF1 transcription factor in rice. Rice 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piao, W.; Kim, E.-Y.; Han, S.-H.; Sakuraba, Y.; Paek, N.-C. Rice Phytochrome B (OsPhyB) Negatively Regulates Dark- and Starvation-Induced Leaf Senescence. Plants 2015, 4, 644–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Sakuraba, Y.; Lee, T.; Kim, K.-W.; An, G.; Lee, H.Y.; Paek, N.-C. Mutation of Oryza sativa CORONATINE INSENSITIVE 1b (OsCOI1b) delays leaf senescence. J. Integr. Plant Biol. 2015, 57, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Aya, K.; Morinaka, Y.; Nagamatsu, S.; Sato, Y.; Antonio, B.A.; Namiki, N.; Nagamura, Y.; Matsuoka, M. Survey of Genes Involved in Rice Secondary Cell Wall Formation Through a Co-Expression Network. Plant Cell Physiol. 2013, 54, 1803–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, K.; Kondo, M.; Aya, K.; Miyao, A.; Sato, Y.; Antonio, B.A.; Namiki, N.; Nagamura, Y.; Matsuoka, M. Identification of Transcription Factors Involved in Rice Secondary Cell Wall Formation. Plant Cell Physiol. 2013, 54, 1791–1802. [Google Scholar] [CrossRef]
- Yoshida, K.; Sakamoto, S.; Kawai, T.; Kobayashi, Y.; Sato, K.; Ichinose, Y.; Yaoi, K.; Akiyoshi-Endo, M.; Sato, H.; Takamizo, T.; et al. Engineering the Oryza sativa cell wall with rice NAC transcription factors regulating secondary wall formation. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Zhong, R.; Lee, C.; McCarthy, R.L.; Reeves, C.K.; Jones, E.G.; Ye, Z.-H. Transcriptional activation of secondary wall biosynthesis by rice and maize NAC and MYB transcription factors. Plant Cell Physiol. 2011, 52, 1856–1871. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Li, D.; Mao, D.; Liu, X.; Ji, C.; Li, X.; Zhao, X.; Cheng, Z.; Chen, C.; Zhu, L. Overexpression of microRNA319 impacts leaf morphogenesis and leads to enhanced cold tolerance in rice (Oryza sativa L.). Plant Cell Environ. 2013, 36, 2207–2218. [Google Scholar] [CrossRef]
- Jin, Y.; Pan, W.; Zheng, X.; Cheng, X.; Liu, M.; Ma, H.; Ge, X. OsERF101, an ERF family transcription factor, regulates drought stress response in reproductive tissues. Plant Mol. Biol. 2018, 98, 51–65. [Google Scholar] [CrossRef]
- Kortz, A.; Hochholdinger, F.; Yu, P. Cell Type-Specific Transcriptomics of Lateral Root Formation and Plasticity. Front. Plant Sci. 2019, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.K.; Vadde, R.; Gouda, G.; Donde, R.; Kumar, J.; Nayak, S.; Behera, L. The impact of natural selection on gene associated with panicle number formation in Oryza sativa. Can. J. Biotechnol. 2017, 1, 198. [Google Scholar] [CrossRef]
- Fu, Z.; Yu, J.; Cheng, X.; Zong, X.; Xu, J.; Chen, M.; Li, Z.; Zhang, D.; Liang, W. The Rice Basic Helix-Loop-Helix Transcription Factor tdr interacting protein2 Is a Central Switch in Early Anther Development. Plant Cell 2014, 26, 1512–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyoshi, K.; Kagaya, Y.; Ogawa, Y.; Nagato, Y.; Hattori, T. Temporal and spatial expression pattern of the OSVP1 and OSEM genes during seed development in rice. Plant Cell Physiol. 2002, 43, 307–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minakuchi, K.; Kameoka, H.; Yasuno, N.; Umehara, M.; Luo, L.; Kobayashi, K.; Hanada, A.; Ueno, K.; Asami, T.; Yamaguchi, S.; et al. FINE CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant Cell Physiol. 2010, 51, 1127–1135. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.R.; Casiana, M.V.C.; Fu, B.Y.; Zhu, L.H.; Zhou, Y.L.; Li, Z.K. Different patterns of gene expression in rice varieties undergoing a resistant or susceptible interaction with the bacterial leaf streak pathogen. Afr. J. Biotechnol. 2011, 10, 14419–14438. [Google Scholar] [CrossRef] [Green Version]
- Brusamarello-Santos, L.C.C.; Pacheco, F.; Aljanabi, S.M.M.; Monteiro, R.A.; Cruz, L.M.; Baura, V.A.; Pedrosa, F.O.; Souza, E.M.; Wassem, R. Differential gene expression of rice roots inoculated with the diazotroph Herbaspirillum seropedicae. Plant Soil 2012, 356, 113–125. [Google Scholar] [CrossRef]
- Yi, S.Y.; Lee, H.Y.; Kim, H.A.; Lim, C.J.; Kim, W.B.; Jang, H.A.; Jeon, J.-S.; Kwon, S.-Y. Microarray Analysis of bacterial blight resistance 1 mutant rice infected with Xanthomonas oryzae pv. oryzae. Plant Breed. Biotechnol. 2013, 1, 354–365. [Google Scholar] [CrossRef] [Green Version]
- Lilly, J.; Subramanian, B. Gene network mediated by WRKY13 to regulate resistance against sheath infecting fungi in rice (Oryza sativa L.). Plant Sci. 2019, 280, 269–282. [Google Scholar] [CrossRef]
- He, H.; Zhu, S.; Jiang, Z.; Ji, Y.; Wang, F.; Zhao, R.; Bie, T. Comparative mapping of powdery mildew resistance gene Pm21 and functional characterization of resistance-related genes in wheat. Theor. Appl. Genet. 2016, 129, 819–829. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Li, H.; Zhang, H.; Miao, X. Identification of transcription factors potential related to brown planthopper resistance in rice via microarray expression profiling. BMC Genom. 2012, 13, 687. [Google Scholar] [CrossRef] [Green Version]
- Yuexiong, Z.; Gang, Q.; Qianqian, M.; Minyi, W.; Xinghai, Y.; Zengfeng, M.; Haifu, L.; Chi, L.; Zhenjing, L.; Fang, L.; et al. Identification of Major Locus Bph35 Resistance to Brown Planthopper in Rice. Rice Sci. 2020, 27, 237–245. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, S.; Yang, T.; Zeng, Z.; Huang, Z.; Liu, Q.; Wang, X.; Leach, J.; Leung, H.; Liu, B. Global transcriptional profiling of a cold-tolerant rice variety under moderate cold stress reveals different cold stress response mechanisms. Physiol. Plant 2015, 154, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Aoki, N.; Matsumura, H.; Okamura, M.; Ohsugi, R.; Shimono, H. Cooling water before panicle initiation increases chilling-induced male sterility and disables chilling-induced expression of genes encoding OsFKBP65 and heat shock proteins in rice spikelets. Plant Cell Environ. 2015, 38, 1255–1274. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, J.; Song, K.; Sun, Y.; Qin, Q.; Xue, Y. Transcriptome analysis of rice (Oryza sativa L.) shoots responsive to cadmium stress. Sci. Rep. 2019, 9, 10177. [Google Scholar] [CrossRef] [Green Version]
- Lakshmanan, M.; Mohanty, B.; Lim, S.-H.; Ha, S.-H.; Lee, D.-Y. Metabolic and transcriptional regulatory mechanisms underlying the anoxic adaptation of rice coleoptile. AoB PLANTS 2014, 6. [Google Scholar] [CrossRef] [Green Version]
- Kottapalli, K.R.; Satoh, K.; Rakwal, R.; Shibato, J.; Doi, K.; Nagata, T.; Kikuchi, S. Combining in silico mapping and arraying: An approach to identifying common candidate genes for submergence tolerance and resistance to bacterial leaf blight in rice. Mol. Cells 2007, 24, 394–408. [Google Scholar]
- Wu, J.; Yu, C.; Hunag, L.; Wu, M.; Liu, B.; Liu, Y.; Song, G.; Liu, D.; Gan, Y. Overexpression of MADS-box transcription factor OsMADS25 enhances salt stress tolerance in Rice and Arabidopsis. Plant Growth Regul. 2020, 90, 163–171. [Google Scholar] [CrossRef]
- Finatto, T.; de Oliveira, A.C.; Chaparro, C.; da Maia, L.C.; Farias, D.R.; Woyann, L.G.; Mistura, C.C.; Soares-Bresolin, A.P.; Llauro, C.; Panaud, O.; et al. Abiotic stress and genome dynamics: Specific genes and transposable elements response to iron excess in rice. Rice 2015, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.K.; Sevanthi, V.A.M.; Chaudhary, S.; Tyagi, P.; Venkadesan, S.; Rani, M.; Mandal, P.K. Transcriptome Analysis of Two Rice Varieties Contrasting for Nitrogen Use Efficiency under Chronic N Starvation Reveals Differences in Chloroplast and Starch Metabolism-Related Genes. Genes 2018, 9, 206. [Google Scholar] [CrossRef] [Green Version]
- Fujishiro, Y.; Agata, A.; Ota, S.; Ishihara, R.; Takeda, Y.; Kunishima, T.; Ikeda, M.; Kyozuka, J.; Hobo, T.; Kitano, H. Comprehensive panicle phenotyping reveals that qSrn7/FZP influences higher-order branching. Sci. Rep. 2018, 8, 12511. [Google Scholar] [CrossRef]
- Jung, H.; Chung, P.J.; Park, S.-H.; Redillas, M.C.F.R.; Kim, Y.S.; Suh, J.-W.; Kim, J.-K. Overexpression of OsERF48 causes regulation of OsCML16, a calmodulin-like protein gene that enhances root growth and drought tolerance. Plant Biotechnol. J. 2017, 15, 1295–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Yu, H.; Xiong, G.; Wang, J.; Jiao, Y.; Liu, G.; Jing, Y.; Meng, X.; Hu, X.; Qian, Q.; et al. Genome-wide binding analysis of the transcription activator ideal plant architecture1 reveals a complex network regulating rice plant architecture. Plant Cell 2013, 25, 3743–3759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wan, L.; Zhang, L.; Zhang, Z.; Zhang, H.; Quan, R.; Zhou, S.; Huang, R. An ethylene response factor OsWR1 responsive to drought stress transcriptionally activates wax synthesis related genes and increases wax production in rice. Plant Mol. Biol. 2012, 78, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W.; El-Kereamy, A.; Zhu, T.; Beatty, P.H.; Good, A.G.; Bi, Y.-M.; Rothstein, S.J. The APETALA-2-like transcription factor OsAP2-39 controls key interactions between abscisic acid and gibberellin in rice. PLoS Genet. 2010, 6, e1001098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangrauthia, S.K.; Bhogireddy, S.; Agarwal, S.; Prasanth, V.V.; Voleti, S.R.; Neelamraju, S.; Subrahmanyam, D. Genome-wide changes in microRNA expression during short and prolonged heat stress and recovery in contrasting rice cultivars. J. Exp. Bot. 2017, 68, 2399–2412. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, F.; Li, J.; Chen, J.-P.; Zhang, H.-M. Integrative Analysis of the microRNAome and Transcriptome Illuminates the Response of Susceptible Rice Plants to Rice Stripe Virus. PLoS ONE 2016, 11, e0146946. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Xu, X.H.; Wu, X.; Wang, Y.; Lu, X.; Sun, H.; Xie, X. Genome-wide identification of microRNAs and their targets in wild type and phyB mutant provides a key link between microRNAs and the phyB-mediated light signaling pathway in rice. Front. Plant Sci. 2015, 6, 372. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-I.; Santi, C.; Jobet, E.; Lacut, E.; El Kholti, N.; Karlowski, W.M.; Verdeil, J.-L.; Breitler, J.C.; Périn, C.; Ko, S.-S.; et al. Complex regulation of two target genes encoding SPX-MFS proteins by rice miR827 in response to phosphate starvation. Plant Cell Physiol. 2010, 51, 2119–2131. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Huang, W.; Ying, Y.; Li, S.; Secco, D.; Tyerman, S.; Whelan, J.; Shou, H. Functional characterization of the rice SPX-MFS family reveals a key role of OsSPX-MFS1 in controlling phosphate homeostasis in leaves. New Phytol. 2012, 196, 139–148. [Google Scholar] [CrossRef]
- Xia, K.; Ou, X.; Gao, C.; Tang, H.; Jia, Y.; Deng, R.; Xu, X.; Zhang, M. OsWS1 involved in cuticular wax biosynthesis is regulated by osa-miR1848. Plant Cell Environ. 2015, 38, 2662–2673. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Bai, H.; Liu, C.; Chen, E.; Chen, Q.; Zhuang, J.; Shen, B. Genome-wide analysis of microRNAs and their target genes related to leaf senescence of rice. PLoS ONE 2014, 9, e114313. [Google Scholar] [CrossRef] [Green Version]
- Xia, K.; Ou, X.; Tang, H.; Wang, R.; Wu, P.; Jia, Y.; Wei, X.; Xu, X.; Kang, S.-H.; Kim, S.-K.; et al. Rice microRNA osa-miR1848 targets the obtusifoliol 14α-demethylase gene OsCYP51G3 and mediates the biosynthesis of phytosterols and brassinosteroids during development and in response to stress. New Phytol. 2015, 208, 790–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Liu, X.; Yuan, L.; Wu, K.; Duan, J.; Wang, X.; Yang, L. Transcriptional profiling in cadmium-treated rice seedling roots using suppressive subtractive hybridization. Plant Physiol. Biochem. 2012, 50, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; Kim, G.-J.; Kim, K.-W.; Chu, S.-H.; Shin, J.-D.; Lee, Y.-J.; Park, Y.-J.; Park, S.-W. Functional Haplotype and eQTL Analyses of Genes Affecting Cadmium Content in Cultivated Rice. Rice 2019, 12, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, W.-C.; Chen, Y.-A.; Hsiung, Y.-C.; Fu, S.-F.; Chou, C.-H.; Trinh, N.N.; Chen, Y.-C.; Huang, H.-J. Autotoxicity mechanism of Oryza sativa: Transcriptome response in rice roots exposed to ferulic acid. BMC Genom. 2013, 14, 351. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zheng, L.; He, F.; Zhao, F.-J.; Shen, Z.; Zheng, L. Transcriptional and physiological analyses identify a regulatory role for hydrogen peroxide in the lignin biosynthesis of copper-stressed rice roots. Plant Soil 2015, 387, 323–336. [Google Scholar] [CrossRef]
- Chujo, T.; Miyamoto, K.; Shimogawa, T.; Shimizu, T.; Otake, Y.; Yokotani, N.; Nishizawa, Y.; Shibuya, N.; Nojiri, H.; Yamane, H.; et al. OsWRKY28, a PAMP-responsive transrepressor, negatively regulates innate immune responses in rice against rice blast fungus. Plant Mol. Biol. 2013, 82, 23–37. [Google Scholar] [CrossRef]
- Hu, L.; Liang, W.; Yin, C.; Cui, X.; Zong, J.; Wang, X.; Hu, J.; Zhang, D. Rice MADS3 Regulates ROS Homeostasis during Late Anther Development. Plant Cell 2011, 23, 515–533. [Google Scholar] [CrossRef] [Green Version]
- Aung, M.S.; Masuda, H.; Kobayashi, T.; Nishizawa, N.K. Physiological and transcriptomic analysis of responses to different levels of iron excess stress in various rice tissues. Soil Sci. Plant Nutr. 2018, 64, 370–385. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamaguchi, T.; Nakayama, K.; Koike, S. Effects of High Nitrogen Supply on the Susceptibility to Coolness at the Young Microspore Stage in Rice (Oryza sativa L.): Gene Expression Analysis in Mature Anthers. Plant Prod. Sci. 2009, 12, 271–277. [Google Scholar] [CrossRef]
- Yang, S.; Hao, D.; Song, Z.; Yang, G.; Wang, L.; Su, Y. RNA-Seq analysis of differentially expressed genes in rice under varied nitrogen supplies. Gene 2015, 555, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Brunings, A.M.; Datnoff, L.E.; Ma, J.F.; Mitani, N.; Nagamura, Y.; Rathinasabapathi, B.; Kirst, M. Differential gene expression of rice in response to silicon and rice blast fungus Magnaporthe oryzae. Ann. Appl. Biol. 2009, 155, 161–170. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L.; Tang, Z.; Shi, X.; Ma, J.; Cui, R. Transcriptome analysis of roots from resistant and susceptible rice varieties infected with Hirschmanniella mucronata. FEBS Open Bio 2019, 9, 1968–1982. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Sharma, V.; Katara, P. Comparative transcriptomics of rice and exploitation of target genes for blast infection. Agri Gene 2016, 1, 143–150. [Google Scholar] [CrossRef]
- Lakra, N.; Kaur, C.; Singla-Pareek, S.L.; Pareek, A. Mapping the ‘early salinity response’ triggered proteome adaptation in contrasting rice genotypes using iTRAQ approach. Rice 2019, 12, 3. [Google Scholar] [CrossRef]
- Phule, A.S.; Barbadikar, K.M.; Maganti, S.M.; Seguttuvel, P.; Subrahmanyam, D.; Babu, M.B.B.P.; Kumar, P.A. RNA-seq reveals the involvement of key genes for aerobic adaptation in rice. Sci. Rep. 2019, 9, 5235. [Google Scholar] [CrossRef] [Green Version]
- Bertini, L.; Palazzi, L.; Proietti, S.; Pollastri, S.; Arrigoni, G.; Polverino de Laureto, P.; Caruso, C. Proteomic Analysis of MeJa-Induced Defense Responses in Rice against Wounding. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [Green Version]
- Shiono, K.; Yamauchi, T.; Yamazaki, S.; Mohanty, B.; Malik, A.I.; Nagamura, Y.; Nishizawa, N.K.; Tsutsumi, N.; Colmer, T.D.; Nakazono, M. Microarray analysis of laser-microdissected tissues indicates the biosynthesis of suberin in the outer part of roots during formation of a barrier to radial oxygen loss in rice (Oryza sativa). J. Exp. Bot. 2014, 65, 4795–4806. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Li, C.; Wu, Z.; Jia, Y.; Wang, H.; Sun, S.; Mao, C.; Wang, X. Abscisic Acid Regulates Auxin Homeostasis in Rice Root Tips to Promote Root Hair Elongation. Front. Plant Sci. 2017, 8, 1121. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, R.; Chu, Y.; Huang, J.; Jin, L.; Wang, G.; Huang, J. Overexpression of RCc3 improves root system architecture and enhances salt tolerance in rice. Plant Physiol. Biochem. 2018, 130, 566–576. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Chi, W.-C.; Trinh, N.N.; Huang, L.-Y.; Chen, Y.-C.; Cheng, K.-T.; Huang, T.-L.; Lin, C.-Y.; Huang, H.-J. Transcriptome profiling and physiological studies reveal a major role for aromatic amino acids in mercury stress tolerance in rice seedlings. PLoS ONE 2014, 9, e95163. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Chang, C.; Ma, L.; Nasir, F.; Zhang, J.; Li, W.; Tran, L.-S.P.; Tian, C. Comparative study of the mycorrhizal root transcriptomes of wild and cultivated rice in response to the pathogen Magnaporthe oryzae. Rice 2019, 12, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbeek, R.E.M.; Van Buyten, E.; Alam, M.Z.; De Vleesschauwer, D.; Van Bockhaven, J.; Asano, T.; Kikuchi, S.; Haeck, A.; Demeestere, K.; Gheysen, G.; et al. Jasmonate-Induced Defense Mechanisms in the Belowground Antagonistic Interaction between Pythium arrhenomanes and Meloidogyne graminicola in Rice. Front. Plant Sci. 2019, 10, 1515. [Google Scholar] [CrossRef]
- Yokotani, N.; Tsuchida-Mayama, T.; Ichikawa, H.; Mitsuda, N.; Ohme-Takagi, M.; Kaku, H.; Minami, E.; Nishizawa, Y. OsNAC111, a blast disease-responsive transcription factor in rice, positively regulates the expression of defense-related genes. Mol. Plant Microbe Interact. 2014, 27, 1027–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Shi, S.; Nasir, F.; Chang, C.; Li, W.; Tran, L.-S.P.; Tian, C. Comparative analysis of the root transcriptomes of cultivated and wild rice varieties in response to Magnaporthe oryzae infection revealed both common and species-specific pathogen responses. Rice 2018, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Al-Bader, N.; Meier, A.; Geniza, M.; Gongora, Y.S.; Oard, J.; Jaiswal, P. Loss of Premature Stop Codon in the Wall-Associated Kinase 91 (OsWAK91) Gene Confers Sheath Blight Disease Resistance in Rice. bioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, J.; Yang, Y.; Zhou, J.; Qiu, Y.; Yu, C.; Cheng, Y.; Yan, C.; Chen, J. Characterization of a Novel NBS-LRR Gene Involved in Bacterial Blight Resistance in Rice. Plant Mol. Biol. Rep. 2013, 31, 649–656. [Google Scholar] [CrossRef]
- Li, W.Q.; Jia, Y.L.; Liu, F.Q.; Wang, F.Q.; Fan, F.J.; Wang, J.; Zhu, J.Y.; Xu, Y.; Zhong, W.G.; Yang, J. Genome-wide identification and characterization of long non-coding RNAs responsive to Dickeya zeae in rice. RSC Adv. 2018, 8, 34408–34417. [Google Scholar] [CrossRef] [Green Version]
- Chung, P.J.; Jung, H.; Jeong, D.-H.; Ha, S.-H.; Choi, Y.D.; Kim, J.-K. Transcriptome profiling of drought responsive noncoding RNAs and their target genes in rice. BMC Genom. 2016, 17, 563. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Yu, G.; Wang, L.; Li, W.; He, R.; Wang, B.; Huang, S.; Cheng, X. A mitochondrial phosphate transporter, McPht gene, confers an acclimation regulation of the transgenic rice to phosphorus deficiency. J. Integr. Agric. 2018, 17, 1932–1945. [Google Scholar] [CrossRef] [Green Version]
- Jeong, K.; Baten, A.; Waters, D.L.E.; Pantoja, O.; Julia, C.C.; Wissuwa, M.; Heuer, S.; Kretzschmar, T.; Rose, T.J. Phosphorus remobilization from rice flag leaves during grain filling: An RNA-seq study. Plant Biotechnol. J. 2017, 15, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, Y.; Yue, W.; Wang, S.; Li, S.; Wang, M.; Zhao, Y.; Wang, C.; Mao, C.; Whelan, J.; Shou, H. Two h-Type Thioredoxins Interact with the E2 Ubiquitin Conjugase PHO2 to Fine-Tune Phosphate Homeostasis in Rice. Plant Physiol. 2017, 173, 812–824. [Google Scholar] [CrossRef] [Green Version]
- Takehisa, H.; Sato, Y. Transcriptome monitoring visualizes growth stage-dependent nutrient status dynamics in rice under field conditions. Plant J. 2019, 97, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Obara, M.; Takeda, T.; Hayakawa, T.; Yamaya, T. Mapping quantitative trait loci controlling root length in rice seedlings grown with low or sufficient supply using backcross recombinant lines derived from a cross between Oryza sativa L. and Oryza glaberrima Steud. Soil Sci. Plant Nutr. 2011, 57, 80–92. [Google Scholar] [CrossRef]
- Shin, S.-Y.; Jeong, J.S.; Lim, J.Y.; Kim, T.; Park, J.H.; Kim, J.-K.; Shin, C. Transcriptomic analyses of rice (Oryza sativa) genes and non-coding RNAs under nitrogen starvation using multiple omics technologies. BMC Genom. 2018, 19, 532. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.-J.; Tsai, Y.-C.; Wu, H.-P.; Huang, L.-T.; Chen, Y.-C.; Chen, Y.-F.; Wu, C.-C.; Tseng, Y.-T.; Hsing, Y.-I.C. Both Hd1 and Ehd1 are important for artificial selection of flowering time in cultivated rice. Plant Sci. 2016, 242, 187–194. [Google Scholar] [CrossRef]
- Yabuki, Y.; Ohashi, M.; Imagawa, F.; Ishiyama, K.; Beier, M.P.; Konishi, N.; Umetsu-Ohashi, T.; Hayakawa, T.; Yamaya, T.; Kojima, S. A temporal and spatial contribution of asparaginase to asparagine catabolism during development of rice grains. Rice 2017, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Zuo, Z.; Qiu, J.-L. Identification and Characterization of ABA-Responsive MicroRNAs in Rice. J Genet Genom. 2015, 42, 393–402. [Google Scholar] [CrossRef]
- Yoshida, Y.; Miyamoto, K.; Yamane, H.; Nishizawa, Y.; Minami, E.; Nojiri, H.; Okada, K. OsTGAP1 is responsible for JA-inducible diterpenoid phytoalexin biosynthesis in rice roots with biological impacts on allelopathic interaction. Physiol. Plant 2017, 161, 532–544. [Google Scholar] [CrossRef]
- Kim, Y.-O.; Kang, H. Comparative expression analysis of genes encoding metallothioneins in response to heavy metals and abiotic stresses in rice (Oryza sativa) and Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2018, 82, 1656–1665. [Google Scholar] [CrossRef]
- Kuramata, M.; Abe, T.; Kawasaki, A.; Ebana, K.; Shibaya, T.; Yano, M.; Ishikawa, S. Genetic diversity of arsenic accumulation in rice and QTL analysis of methylated arsenic in rice grains. Rice 2013, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.-L.; Nguyen, Q.T.T.; Fu, S.-F.; Lin, C.-Y.; Chen, Y.-C.; Huang, H.-J. Transcriptomic changes and signalling pathways induced by arsenic stress in rice roots. Plant Mol. Biol. 2012, 80, 587–608. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Liu, A.; Zou, J.; Zhou, X.; Xiang, J.; Rerksiri, W.; Peng, Y.; Xiong, X.; Chen, X. Expression profile in rice panicle: Insights into heat response mechanism at reproductive stage. PLoS ONE 2012, 7, e49652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawaki, N.; Tsujimoto, R.; Shigyo, M.; Konishi, M.; Toki, S.; Fujiwara, T.; Yanagisawa, S. A nitrate-inducible GARP family gene encodes an auto-repressible transcriptional repressor in rice. Plant Cell Physiol. 2013, 54, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Di, D.; Li, G.; Kronzucker, H.J.; Shi, W. Spatio-temporal dynamics in global rice gene expression (Oryza sativa L.) in response to high ammonium stress. J. Plant Physiol. 2017, 212, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Redillas, M.C.F.R.; Jeong, J.S.; Kim, Y.S.; Jung, H.; Bang, S.W.; Choi, Y.D.; Ha, S.-H.; Reuzeau, C.; Kim, J.-K. The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol. J. 2012, 10, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Kitamoto, H.K.; Nakamura, A.; Fukuda, A.; Tanaka, Y. Rice shaker potassium channel OsKAT1 confers tolerance to salinity stress on yeast and rice cells. Plant Physiol. 2007, 144, 1978–1985. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-L.; Yang, S.-H.; Lv, M.; Ding, S.-W.; Li, J.-Y.; Xu, C.-L.; Xie, H. RNA-Seq revealed that infection with white tip nematodes could downregulate rice photosynthetic genes. Funct. Integr. Genom. 2020, 20, 367–381. [Google Scholar] [CrossRef]
- Nasir, F.; Tian, L.; Shi, S.; Chang, C.; Ma, L.; Gao, Y.; Tian, C. Strigolactones positively regulate defense against Magnaporthe oryzae in rice (Oryza sativa). Plant Physiol. Biochem. 2019, 142, 106–116. [Google Scholar] [CrossRef]
- Liu, T.; Kim, D.W.; Niitsu, M.; Maeda, S.; Watanabe, M.; Kamio, Y.; Berberich, T.; Kusano, T. Polyamine oxidase 7 is a terminal catabolism-type enzyme in Oryza sativa and is specifically expressed in anthers. Plant Cell Physiol. 2014, 55, 1110–1122. [Google Scholar] [CrossRef] [Green Version]
- You, C.; Chen, L.; He, H.; Wu, L.; Wang, S.; Ding, Y.; Ma, C. iTRAQ-based proteome profile analysis of superior and inferior Spikelets at early grain filling stage in japonica Rice. BMC Plant Biol. 2017, 17, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohashi, M.; Ishiyama, K.; Kojima, S.; Konishi, N.; Sasaki, K.; Miyao, M.; Hayakawa, T.; Yamaya, T. Outgrowth of Rice Tillers Requires Availability of Glutamine in the Basal Portions of Shoots. Rice 2018, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Funabiki, A.; Furukawa, K.; Komori, N.; Koike, M.; Tokuji, Y.; Takamure, I.; Kato, K. Genome-wide analysis and expression profiling of half-size ABC protein subgroup G in rice in response to abiotic stress and phytohormone treatments. Mol. Genet. Genom. 2012, 287, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Soga, K.; Hoson, T.; Kotake, T.; Kojima, M.; Sakakibara, H.; Yamazaki, T.; Higashibata, A.; Ishioka, N.; Shimazu, T.; et al. Persistence of plant hormone levels in rice shoots grown under microgravity conditions in space: Its relationship to maintenance of shoot growth. Physiol. Plantarum. 2017, 161, 285–293. [Google Scholar] [CrossRef]
- Ruyter-Spira, C.; Al-Babili, S.; van der Krol, S.; Bouwmeester, H. The biology of strigolactones. Trends Plant Sci. 2013, 18, 72–83. [Google Scholar] [CrossRef]
- Marzec, M. Strigolactones and Gibberellins: A New Couple in the Phytohormone World? Trends Plant Sci. 2017, 22, 813–815. [Google Scholar] [CrossRef]
- Meshi, T.; Iwabuchi, M. Plant transcription factors. Plant Cell Physiol. 1995, 36, 1405–1420. [Google Scholar]
- Nakagawa, H.; Ohkura, E.; Ohmiya, K.; Hattori, T. The seed-specific transcription factor VP1 (OSVP1) is expressed in rice suspension-cultured cells. Plant Cell Physiol. 1996, 37, 355–362. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Visentin, I.; Pagliarani, C.; Deva, E.; Caracci, A.; Turečková, V.; Novák, O.; Lovisolo, C.; Schubert, A.; Cardinale, F. A novel strigolactone-miR156 module controls stomatal behaviour during drought recovery. Plant Cell Environ. 2020, 43, 1613–1624. [Google Scholar] [CrossRef]
- Haider, I.; Andreo-Jimenez, B.; Bruno, M.; Bimbo, A.; Floková, K.; Abuauf, H.; Ntui, V.O.; Guo, X.; Charnikhova, T.; Al-Babili, S.; et al. The interaction of strigolactones with abscisic acid during the drought response in rice. J. Exp. Bot. 2018, 69, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Daszkowska-Golec, A.; Collin, A.; Melzer, M.; Eggert, K.; Szarejko, I. Barley strigolactone signaling mutant hvd14.d reveals the role of strigolactones in ABA-dependent response to drought. Plant Cell Environ. 2020. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, P.; Douglas, A.; Price, A.H.; Norton, G.J. Biallelic and Genome Wide Association Mapping of Germanium Tolerant Loci in Rice (Oryza sativa L.). PLoS ONE 2015, 10, e0137577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proebsting, W.M.; Hedden, P.; Lewis, M.J.; Croker, S.J.; Proebsting, L.N. Gibberellin concentration and transport in genetic lines of pea: Effects of grafting. Plant Physiol. 1992, 100, 1354–1360. [Google Scholar] [CrossRef]
- Regnault, T.; Davière, J.-M.; Wild, M.; Sakvarelidze-Achard, L.; Heintz, D.; Carrera Bergua, E.; Lopez Diaz, I.; Gong, F.; Hedden, P.; Achard, P. The gibberellin precursor GA12 acts as a long-distance growth signal in Arabidopsis. Nat. Plants 2015, 1, 15073. [Google Scholar] [CrossRef] [PubMed]
- Katyayini, N.U.; Rinne, P.L.H.; Tarkowská, D.; Strnad, M.; van der Schoot, C. Dual Role of Gibberellin in Perennial Shoot Branching: Inhibition and Activation. Front. Plant Sci. 2020, 11, 736. [Google Scholar] [CrossRef]
- Wheeldon, C.D.; Bennett, T. There and back again: An evolutionary perspective on long-distance coordination of plant growth and development. Semin. Cell Dev. Biol. 2020. [Google Scholar] [CrossRef]



| Os 01g0700900 | Os 01g0701400 | Os 01g0701500 | Os 02g0221900 | Os 06g0565100 | |
|---|---|---|---|---|---|
| abiotic stresses | |||||
| iron status | 1 | 0 | 1 | 0 | 4 |
| nitrogen status | 2 | 0 | 2 | 0 | 11 |
| phosphorus status | 0 | 1 | 0 | 0 | 2 |
| arsenic | 1 | 1 | 0 | 0 | 9 |
| cadmium | 2 | 0 | 1 | 0 | 8 |
| chromium | 0 | 0 | 1 | 1 | 0 |
| cold | 4 | 2 | 1 | 0 | 7 |
| drought | 2 | 1 | 3 | 1 | 20 |
| salt | 2 | 1 | 3 | 0 | 7 |
| submergence | 0 | 1 | 1 | 0 | 3 |
| biotic stresses | |||||
| bacteria | 1 | 1 | 1 | 0 | 11 |
| viruses | 1 | 0 | 2 | 0 | 0 |
| fungi | 1 | 0 | 2 | 0 | 17 |
| insects | 0 | 0 | 0 | 0 | 11 |
| developmental processes | |||||
| plant height | 1 | 0 | 0 | 0 | 0 |
| shoot architecture | 0 | 0 | 0 | 0 | 6 |
| root development | 1 | 1 | 2 | 1 | 6 |
| flower development | 1 | 1 | 3 | 2 | 2 |
| seed development | 0 | 0 | 0 | 2 | 0 |
| seed dormancy | 1 | 0 | 0 | 1 | 0 |
| leaf senescence | 1 | 0 | 1 | 0 | 0 |
| secondary wall formation | 0 | 0 | 4 | 0 | 0 |
| wax synthesis | 0 | 0 | 0 | 0 | 1 |
| hormonal cross talk | |||||
| abscisic acid | 0 | 1 | 0 | 0 | 1 |
| ethylene | 0 | 1 | 0 | 0 | 1 |
| gibberelins | 1 | 0 | 0 | 0 | 1 |
| Os 01g0700900 | Os 01g0701400 | Os 01g0701500 | Os 02g0221900 | Os 06g0565100 | |
|---|---|---|---|---|---|
| abiotic stresses | |||||
| phosphorus status | miR827 | ||||
| zinc deficiency | miR528-5p | ||||
| cold | miR528-5p | ||||
| drought | miR528-5p | ||||
| heat | miR2055 | miR166d-5p | miR166b-5p miR528-5p miR5519 | miR1848 | |
| biotic stresses | |||||
| bacteria | miR166b-5p | ||||
| viruses | miR1432-3p | miR166d-5p miR2097-3p | |||
| fungi | miR2103 | ||||
| developmental processes | |||||
| flower development | miR5514 | miR528-5p | |||
| leaf senescence | miR1848 | ||||
| light signalling | miR1430 | ||||
| wax synthesis | miR1848 | ||||
| hormonal cross talk | |||||
| abscisic acid | miR528-5p | ||||
| brassinosteroids | miR1848 | ||||
| Os 01g0700900 | Os 01g0701400 | Os 01g0701500 | Os 02g0221900 | Os 06g0565100 | |
|---|---|---|---|---|---|
| abiotic stresses | |||||
| phosphorus status | miR827 | ||||
| zinc deficiency | miR528-5p | ||||
| cold | miR528-5p | ||||
| drought | miR528-5p | ||||
| heat | miR2055 | miR166d-5p | miR166b-5p miR528-5p miR5519 | miR1848 | |
| biotic stresses | |||||
| bacteria | miR166b-5p | ||||
| viruses | miR1432-3p | miR166d-5p miR2097-3p | |||
| fungi | miR2103 | ||||
| developmental processes | |||||
| flower development | miR5514 | miR528-5p | |||
| leaf senescence | miR1848 | ||||
| light signalling | miR1430 | ||||
| wax synthesis | miR1848 | ||||
| hormonal cross talk | |||||
| abscisic acid | miR528-5p | ||||
| brassinosteroids | miR1848 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzec, M.; Situmorang, A.; Brewer, P.B.; Brąszewska, A. Diverse Roles of MAX1 Homologues in Rice. Genes 2020, 11, 1348. https://doi.org/10.3390/genes11111348
Marzec M, Situmorang A, Brewer PB, Brąszewska A. Diverse Roles of MAX1 Homologues in Rice. Genes. 2020; 11(11):1348. https://doi.org/10.3390/genes11111348
Chicago/Turabian StyleMarzec, Marek, Apriadi Situmorang, Philip B. Brewer, and Agnieszka Brąszewska. 2020. "Diverse Roles of MAX1 Homologues in Rice" Genes 11, no. 11: 1348. https://doi.org/10.3390/genes11111348
APA StyleMarzec, M., Situmorang, A., Brewer, P. B., & Brąszewska, A. (2020). Diverse Roles of MAX1 Homologues in Rice. Genes, 11(11), 1348. https://doi.org/10.3390/genes11111348






