Preliminary Findings on CTG Expansion Determination in Different Tissues from Patients with Myotonic Dystrophy Type 1
Abstract
:1. Introduction
2. Materials and Methods
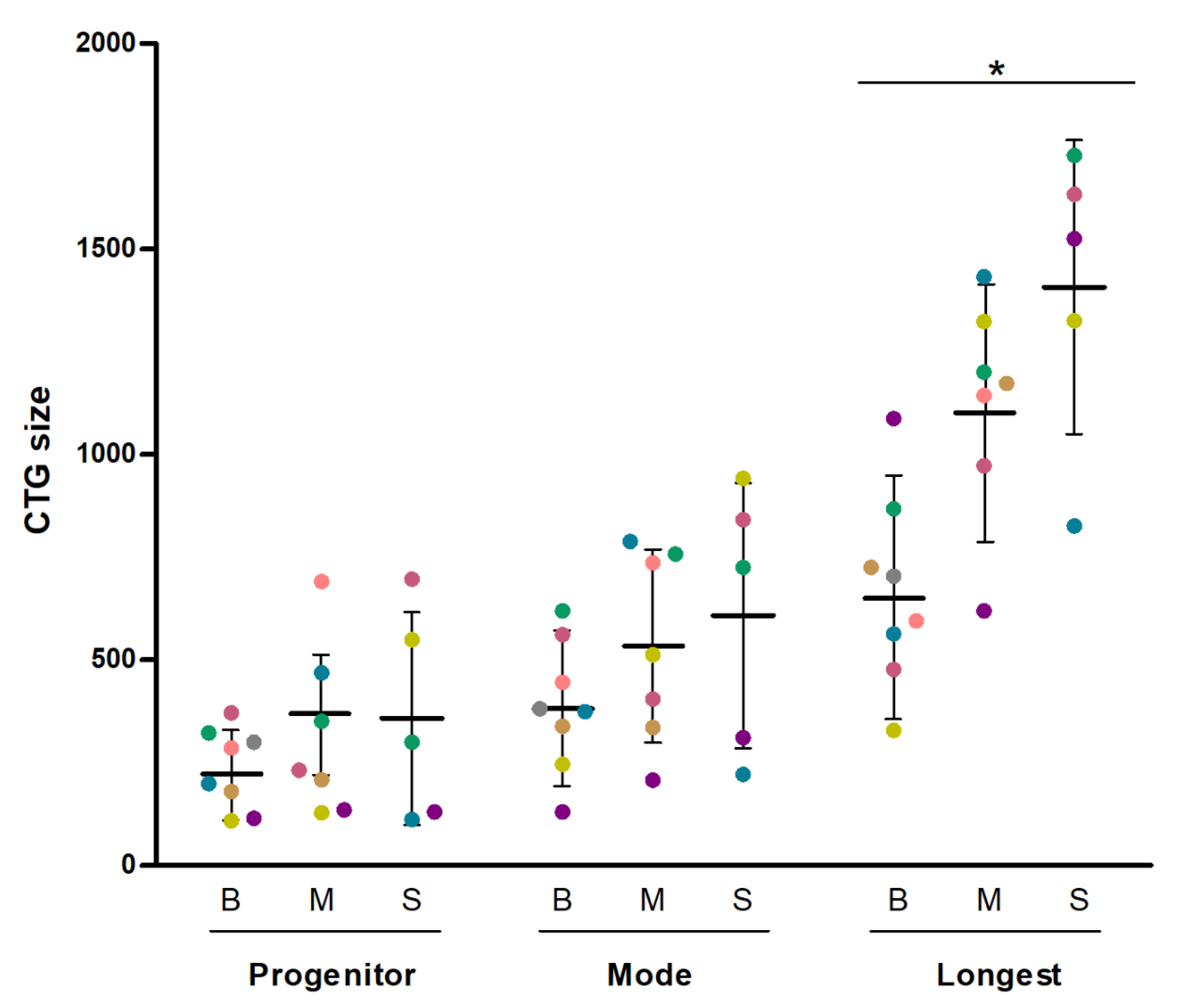
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brook, J.D.; McCurrach, M.E.; Harley, H.G.; Buckler, A.J.; Church, D.; Aburatani, H.; Hunter, K.; Stanton, V.P.; Thirion, J.-P.; Hudson, T.; et al. Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 1992, 68, 799–808. [Google Scholar] [CrossRef]
- La Spada, A.R. Trinucleotide Repeat Instability: Genetic Features and Molecular Mechanisms. Brain Pathol. 1997, 7, 943–963. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, T.; Dubel, J.R.; Harati, Y. Somatic instability of ctg repeat in myotonic dystrophy. Neurology 1993, 43, 2674–2678. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.A.; Johnson, K.; Moxley, R.T. Myotonic dystrophy patients have larger CTG expansions in skeletal muscle than in leukocytes. Ann. Neurol. 1994, 35, 104–107. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8285579 (accessed on 25 March 2020). [CrossRef] [PubMed]
- Anvret, M.; Ahlberg, G.; Grandell, U.; Hedberg, B.; Johnson, K.; Edström, L. Larger expansions of the CTG repeat in muscle compared to lymphocytes from patients with myotonic dystrophy. Hum. Mol. Genet. 1993, 2, 1397–1400. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8242063 (accessed on 25 March 2020). [CrossRef] [PubMed]
- Peterlin, B.; Logar, N.; Zidar, J. CTG repeat analysis in lymphocytes, muscles and fibroblasts in patients with myotonic dystrophy. Pflug. Arch. 1996, 431 (Suppl. 2), R199–R200. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8739333 (accessed on 25 March 2020). [CrossRef] [PubMed]
- Kim, H.J.; Na, J.-H.; Lee, Y.-M. Genotype-phenotype correlations in pediatric patients with myotonic dystrophy type 1. Korean J. Pediatr. 2019, 62, 55–61. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30304901 (accessed on 26 March 2020). [CrossRef] [Green Version]
- Hamshere, M.G.; Harley, H.; Harper, P.; Brook, J.D.; Brookfield, J.F.Y. Myotonic dystrophy: The correlation of (CTG) repeat length in leucocytes with age at onset is significant only for patients with small expansions. J. Med. Genet. 1999, 36, 59–61. [Google Scholar] [PubMed]
- Merlevede, K.; Vermander, D.; Theys, P.; Legius, E.; Ector, H.; Robberecht, W. Cardiac involvement and CTG expansion in myotonic dystrophy. J. Neurol. 2002, 249, 693–698. [Google Scholar] [CrossRef]
- Gharehbaghi-Schnell, E.B.; Finsterer, J.; Korschineck, I.; Mamoli, B.; Binder, B.R. Genotype-phenotype correlation in myotonic dystrophy. Clin. Genet. 1998, 53, 20–26. [Google Scholar] [CrossRef]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef] [Green Version]
- Cumming, S.A.; Jimenez-Moreno, C.; Okkersen, K.; Wenninger, S.; Daidj, F.; Hogarth, F.; Littleford, R.; Gorman, G.; Bassez, G.; Schoser, B.; et al. Genetic determinants of disease severity in the myotonic dystrophy type 1 OPTIMISTIC cohort. Neurology 2019, 93, e995–e1009. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31395669 (accessed on 27 March 2020). [CrossRef] [Green Version]
- Gomes-Pereira, M.; Bidichandani, S.I.; Monckton, D.G. Analysis of unstable triplet repeats using small-pool polymerase chain reaction. Methods Mol Biol. 2004, 277, 61–76. [Google Scholar]
- Ballester-Lopez, A.; Koehorst, E.; Almendrote, M.; Martínez-Piñeiro, A.; Lucente, G.; Linares-Pardo, I.; Núñez-Manchón, J.; Guanyabens, N.; Cano, A.; Lucia, A.; et al. A DM1 family with interruptions associated with atypical symptoms and late onset but not with a milder phenotype. Hum. Mutat. 2020, 41, 420–431. Available online: http://dx.doi.org/10.1002/humu.23932 (accessed on 25 March 2020). [CrossRef] [PubMed]
- Pešović, J.; Perić, S.; Brkušanin, M.; Brajušković, G.; Ević-Stojanović, V.R.; Ević, D.D.-P. Repeat interruptions modify age at onset in myotonic dystrophy type 1 by stabilizing DMPK expansions in somatic cells. Front. Genet. 2018, 9, 1–14. Available online: https://pubmed.ncbi.nlm.nih.gov/30546383/ (accessed on 22 October 2020). [CrossRef]
- Cumming, S.A.; The Scottish Myotonic Dystrophy Consortium; Hamilton, M.J.; Robb, Y.; Gregory, H.; McWilliam, C.; Cooper, A.; Adam, B.; McGhie, J.; Hamilton, G.; et al. De novo repeat interruptions are associated with reduced somatic instability and mild or absent clinical features in myotonic dystrophy type 1. Eur. J. Hum. Genet. 2018, 26, 1635–1647. Available online: http://www.nature.com/articles/s41431-018-0156-9 (accessed on 21 August 2018). [CrossRef] [PubMed]
- Coté, C.; Hiba, B.; Hebert, L.J.; Vial, C.; Remec, J.F.; Janier, M.; Puymirat, J. MRI of tibialis anterior skeletal muscle in myotonic dystrophy type 1. Can. J. Neurol. Sci. 2011, 38, 112–118. Available online: https://pubmed.ncbi.nlm.nih.gov/21156439/ (accessed on 22 October 2020). [CrossRef] [Green Version]
- Iachettini, S.; Valaperta, R.; Marchesi, A.; Perfetti, A.; Cuomo, G.; Fossati, B.; Vaienti, L.; Costa, E.; Meola, G.; Cardani, R. Tibialis anterior muscle needle biopsy and sensitive biomolecular methods: A useful tool in myotonic dystrophy type 1. Eur. J. Histochem. 2015, 59, 243–249. Available online: https://pubmed.ncbi.nlm.nih.gov/26708183/ (accessed on 23 October 2020). [CrossRef] [PubMed] [Green Version]
- Morales, F.; Couto, J.M.; Higham, C.F.; Hogg, G.; Cuenca, P.; Braida, C.; Wilson, R.H.; Adam, B.; Del Valle, G.; Brian, R.; et al. Somatic instability of the expanded CTG triplet repeat in myotonic dystrophy type 1 is a heritable quantitative trait and modifier of disease severity. Hum. Mol. Genet. 2012, 21, 3558–3567. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22595968 (accessed on 26 March 2020). [CrossRef] [Green Version]
- Chong-Nguyen, C.; Wahbi, K.; Algalarrondo, V.; Bécane, H.; Radvanyi-Hoffman, H.; Arnaud, P.; Furling, D.; Bassez, G.; Lazarus, A.; Laforet, P.; et al. Association between Mutation Size and Cardiac Involvement in Myotonic Dystrophy Type 1: An Analysis of the DM1-Heart Registry. Circ. Cardiovasc. Genet. 2017, 10, e001526. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28611030 (accessed on 26 March 2020). [CrossRef] [PubMed] [Green Version]
- Groh, W.J.; Groh, M.R.; Shen, C.; Monckton, D.G.; Bodkin, C.L.; Pascuzzi, R.M. Survival and CTG repeat expansion in adults with myotonic dystrophy type 1. Muscle Nerve 2011, 43, 648–651. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21484823 (accessed on 26 March 2020). [CrossRef]

| Patient | Sex | Age of Symptom Onset (Years) | Age at Sampling (Years) | Biceps Muscle (MRC Scale) | Myotonia (s) | 6-min Walking Distance (m) | MIRS | mRS |
|---|---|---|---|---|---|---|---|---|
| P1 | F | 15 * | 36 | 4 | 0.52 | 348 | 4 | 2 |
| P2 | M | 48 | 54 | 5 | 0.67 | 251 | 3 | 2 |
| P3 | F | 36 | 41 | 5 | 0.73 | 368 | 2 | 1 |
| P4 | F | 42 | 46 | 5 | 0.98 | 338 | 3 | 1 |
| P5 | F | 27 | 40 | 4 | NP | NP | 4 | 4 |
| P6 | M | 36 | 41 | 5 | 0.96 | 519 | 3 | 2 |
| P7 | F | 50 | 62 | 5 | NP | 436 | 2 | 1 |
| P8 | F | 35 | 38 | 5 | NP | NP | 3 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballester-Lopez, A.; Koehorst, E.; Linares-Pardo, I.; Núñez-Manchón, J.; Almendrote, M.; Lucente, G.; Arbex, A.; Puente, C.; Lucia, A.; Monckton, D.G.; et al. Preliminary Findings on CTG Expansion Determination in Different Tissues from Patients with Myotonic Dystrophy Type 1. Genes 2020, 11, 1321. https://doi.org/10.3390/genes11111321
Ballester-Lopez A, Koehorst E, Linares-Pardo I, Núñez-Manchón J, Almendrote M, Lucente G, Arbex A, Puente C, Lucia A, Monckton DG, et al. Preliminary Findings on CTG Expansion Determination in Different Tissues from Patients with Myotonic Dystrophy Type 1. Genes. 2020; 11(11):1321. https://doi.org/10.3390/genes11111321
Chicago/Turabian StyleBallester-Lopez, Alfonsina, Emma Koehorst, Ian Linares-Pardo, Judit Núñez-Manchón, Miriam Almendrote, Giuseppe Lucente, Andrea Arbex, Carles Puente, Alejandro Lucia, Darren G. Monckton, and et al. 2020. "Preliminary Findings on CTG Expansion Determination in Different Tissues from Patients with Myotonic Dystrophy Type 1" Genes 11, no. 11: 1321. https://doi.org/10.3390/genes11111321
APA StyleBallester-Lopez, A., Koehorst, E., Linares-Pardo, I., Núñez-Manchón, J., Almendrote, M., Lucente, G., Arbex, A., Puente, C., Lucia, A., Monckton, D. G., Cumming, S. A., Pintos-Morell, G., Coll-Cantí, J., Ramos-Fransi, A., Martínez-Piñeiro, A., & Nogales-Gadea, G. (2020). Preliminary Findings on CTG Expansion Determination in Different Tissues from Patients with Myotonic Dystrophy Type 1. Genes, 11(11), 1321. https://doi.org/10.3390/genes11111321







