Systems Level Analysis and Identification of Pathways and Key Genes Associated with Delirium
Abstract
1. Introduction
2. Materials and Methods
2.1. Genes Associated with Delirium
2.2. Construction of a Human Protein−Protein Interaction (PPI) Network
2.3. PPI Subnetworks among Proteins Encoded by Delirium-Associated Genes
2.4. PPI Subnetworks Simulation
2.5. Pathway and GO Enrichment Analysis
2.6. Psychiatric Disorder Association Analysis
2.7. Enrichment Analysis with Genes Located in HARs
3. Results
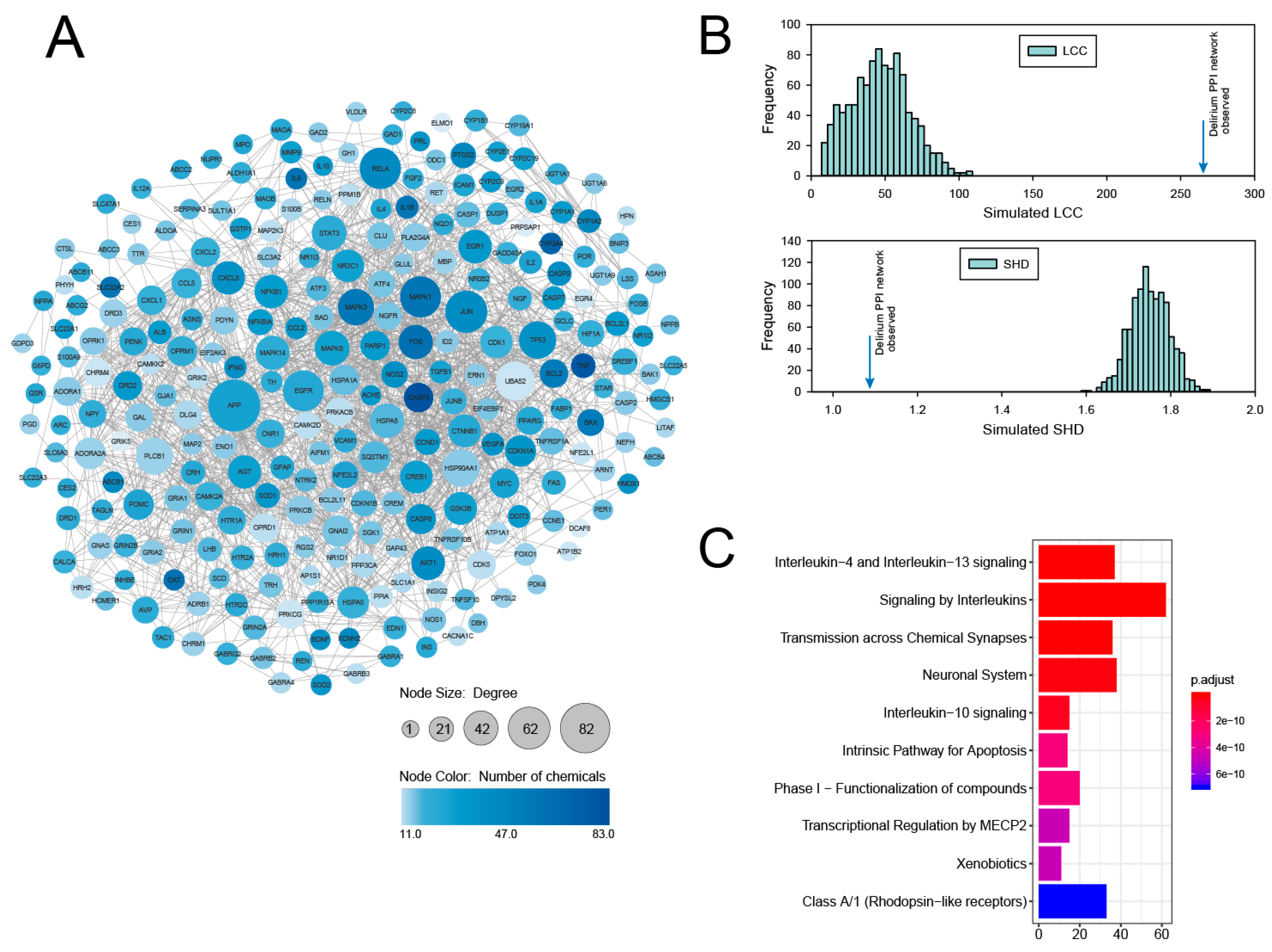
3.1. PPI Network Analysis of Delirium-Associated Genes Derived from Chemical–Gene Interactions in the CTD
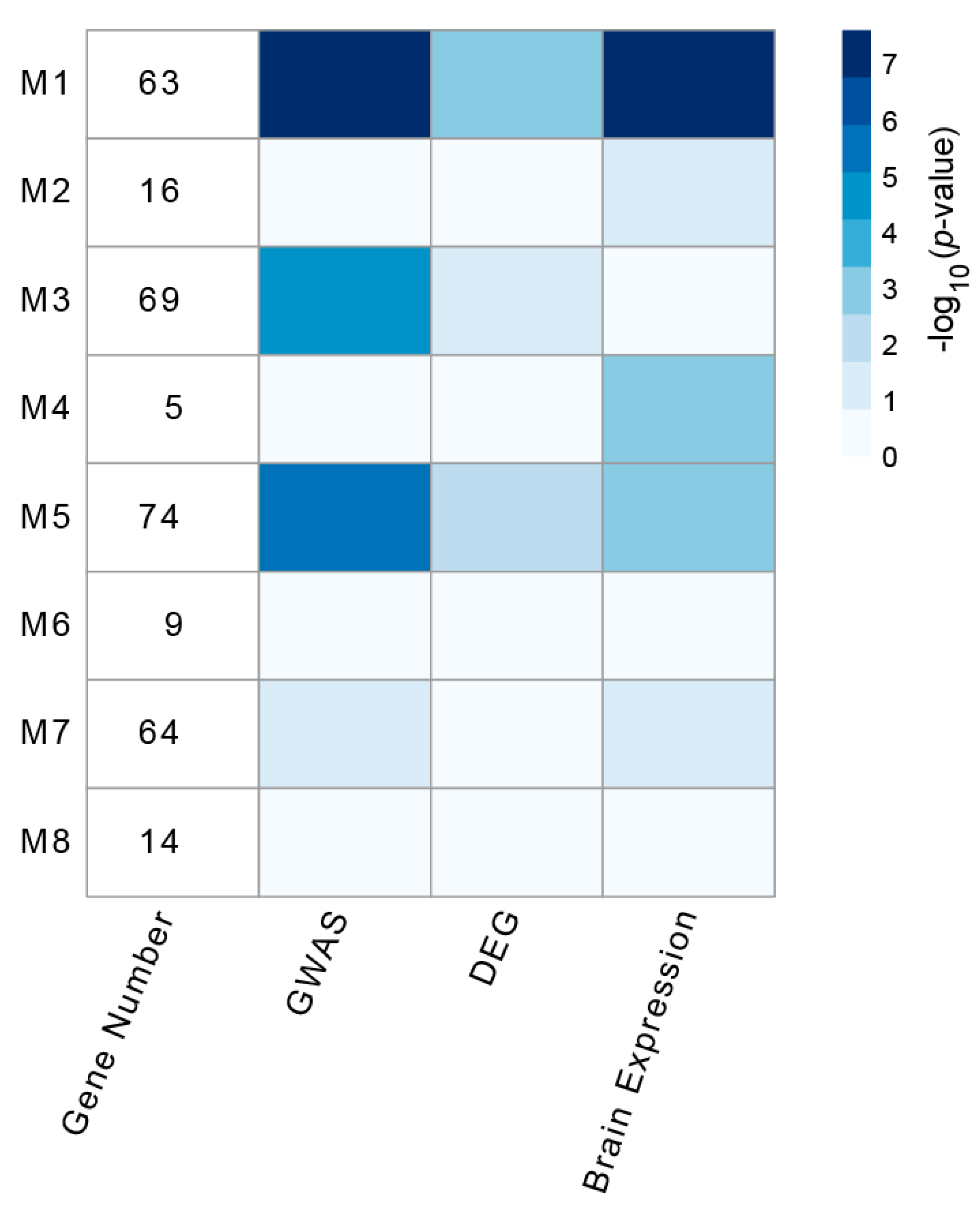
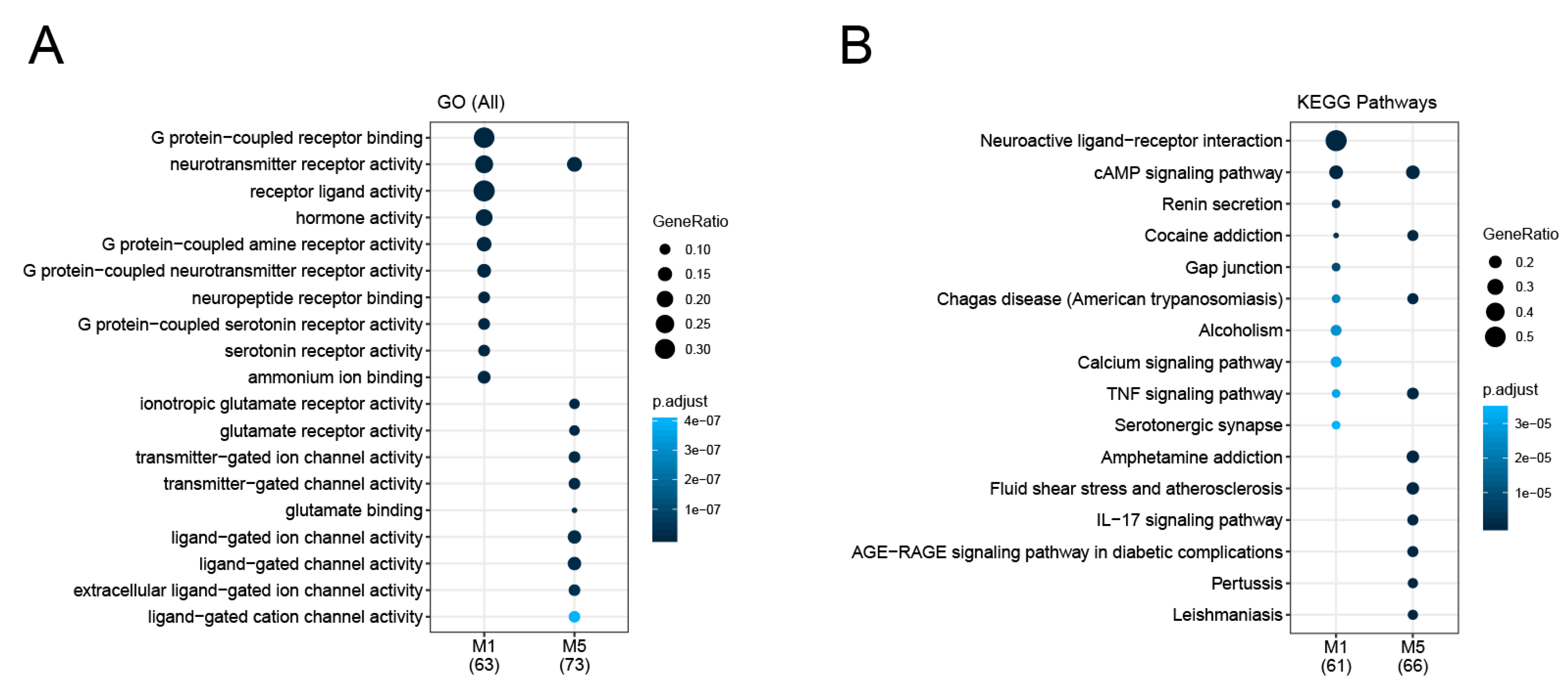
3.2. Identification and Analysis of Densely Connected Modules in the Delirium PPI Network
3.3. Psychiatric Disease Association of Delirium-Related Modules
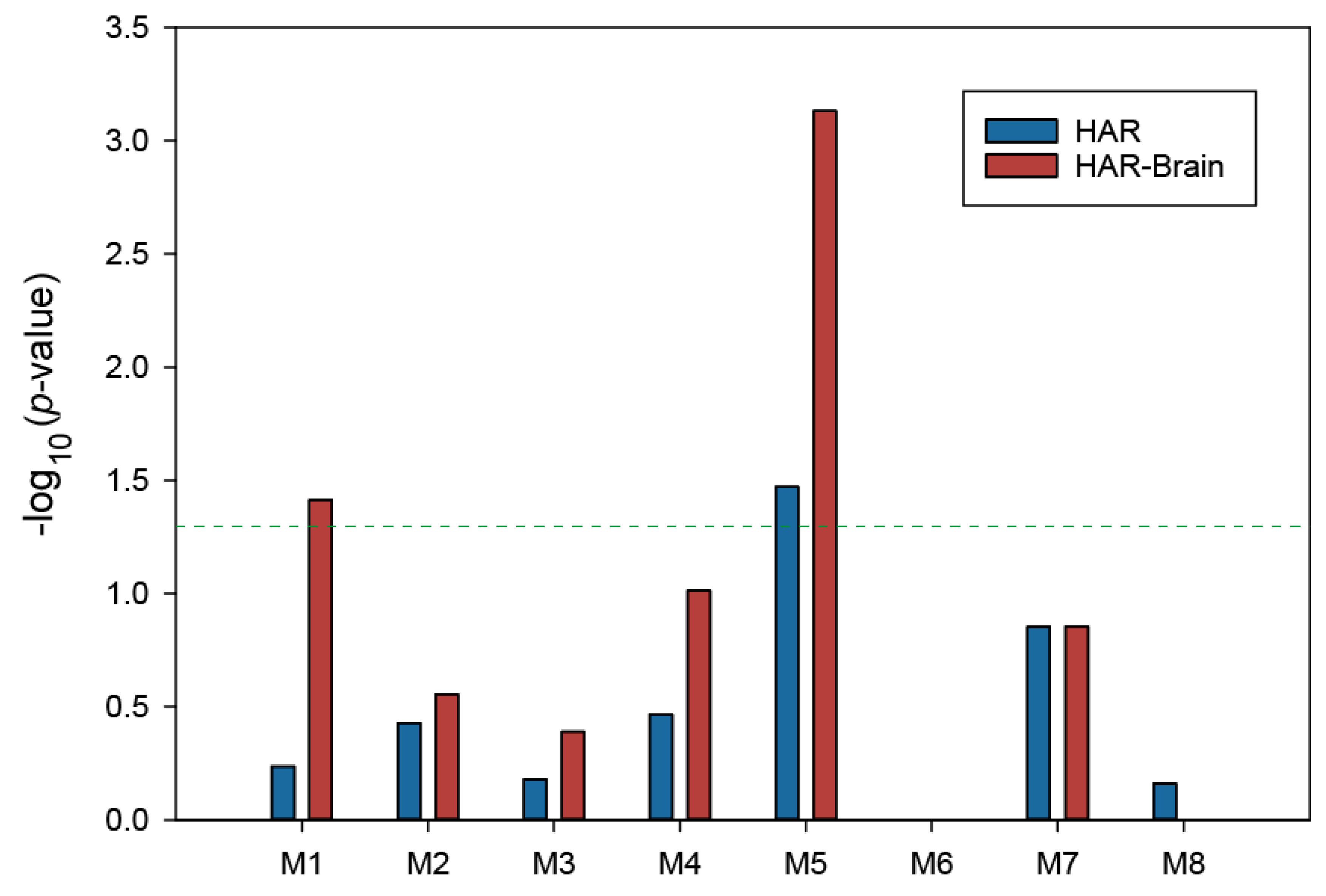
3.4. Enrichment of HAR-Brain Genes in Delirium-Related Modules
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Han, J.H.; Suyama, J. Delirium and Dementia. Clin. Geriatr. Med. 2018, 34, 327–354. [Google Scholar] [CrossRef]
- Inouye, S.K. Delirium in older persons. N. Engl. J. Med. 2006, 354, 1157–1165. [Google Scholar] [CrossRef]
- Kim, M.S.; Rhim, H.C.; Park, A.; Kim, H.; Han, K.M.; Patkar, A.A.; Pae, C.U.; Han, C. Comparative efficacy and acceptability of pharmacological interventions for the treatment and prevention of delirium: A systematic review and network meta-analysis. J. Psychiatr. Res. 2020, 125, 164–176. [Google Scholar] [CrossRef]
- Hshieh, T.T.; Inouye, S.K.; Oh, E.S. Delirium in the Elderly. Psychiatr. Clin. N. Am. 2018, 41, 1–17. [Google Scholar] [CrossRef]
- Maldonado, J.R. Neuropathogenesis of delirium: Review of current etiologic theories and common pathways. Am. J. Geriatr. Psychiatry 2013, 21, 1190–1222. [Google Scholar] [CrossRef] [PubMed]
- McCoy, T.H., Jr.; Hart, K.; Pellegrini, A.; Perlis, R.H. Genome-wide association identifies a novel locus for delirium risk. Neurobiol. Aging 2018, 68, 160.e9–160.e14. [Google Scholar] [CrossRef]
- Maclullich, A.M.; Ferguson, K.J.; Miller, T.; de Rooij, S.E.; Cunningham, C. Unravelling the pathophysiology of delirium: A focus on the role of aberrant stress responses. J. Psychosom. Res. 2008, 65, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Vasunilashorn, S.M.; Ngo, L.H.; Inouye, S.K.; Fong, T.G.; Jones, R.N.; Dillon, S.T.; Libermann, T.A.; O’Connor, M.; Arnold, S.E.; Xie, Z.; et al. Apolipoprotein E genotype and the association between C-reactive protein and postoperative delirium: Importance of gene-protein interactions. Alzheimer’s Dement. 2020, 16, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Vasunilashorn, S.M.; Ngo, L.H.; Jones, R.N.; Inouye, S.K.; Hall, K.T.; Gallagher, J.; Dillon, S.T.; Xie, Z.; Libermann, T.A.; Marcantonio, E.R. The Association Between C-Reactive Protein and Postoperative Delirium Differs by Catechol-O-Methyltransferase Genotype. Am. J. Geriatr. Psychiatry 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Van Munster, B.C.; de Rooij, S.; Yazdanpanah, M.; Tienari, P.J.; Pitkala, K.H.; Osse, R.J.; Adamis, D.; Smit, O.; van der Steen, M.S.; van Houten, M.; et al. The association of the dopamine transporter gene and the dopamine receptor 2 gene with delirium, a meta-analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010, 153B, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Alagiakrishnan, K.; Wiens, C.A. An approach to drug induced delirium in the elderly. Postgrad. Med. J. 2004, 80, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, J.D.; Narasimhan, M. Delirium and antipsychotics: A systematic review of epidemiology and somatic treatment options. Psychiatry 2008, 5, 29–36. [Google Scholar] [PubMed]
- Davis, A.P.; Grondin, C.J.; Johnson, R.J.; Sciaky, D.; McMorran, R.; Wiegers, J.; Wiegers, T.C.; Mattingly, C.J. The Comparative Toxicogenomics Database: Update 2019. Nucleic Acids Res. 2019, 47, D948–D954. [Google Scholar] [CrossRef] [PubMed]
- Conte, F.; Fiscon, G.; Licursi, V.; Bizzarri, D.; D’Anto, T.; Farina, L.; Paci, P. A paradigm shift in medicine: A comprehensive review of network-based approaches. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194416. [Google Scholar] [CrossRef]
- Menche, J.; Sharma, A.; Kitsak, M.; Ghiassian, S.D.; Vidal, M.; Loscalzo, J.; Barabasi, A.L. Disease networks. Uncovering disease-disease relationships through the incomplete interactome. Science 2015, 347, 1257601. [Google Scholar] [CrossRef]
- Ramly, B.; Afiqah-Aleng, N.; Mohamed-Hussein, Z.A. Protein-Protein Interaction Network Analysis Reveals Several Diseases Highly Associated with Polycystic Ovarian Syndrome. Int. J. Mol. Sci. 2019, 20, 2959. [Google Scholar] [CrossRef] [PubMed]
- Razick, S.; Magklaras, G.; Donaldson, I.M. iRefIndex: A consolidated protein interaction database with provenance. BMC Bioinform. 2008, 9, 405. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Bruckner, R.J.; Paulo, J.A.; Cannon, J.R.; Ting, L.; Baltier, K.; Colby, G.; Gebreab, F.; Gygi, M.P.; Parzen, H.; et al. Architecture of the human interactome defines protein communities and disease networks. Nature 2017, 545, 505–509. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]
- Xiang, X.; Yu, Y.; Tang, X.; Chen, M.; Zheng, Y.; Zhu, S. Transcriptome Profile in Hippocampus during Acute Inflammatory Response to Surgery: Toward Early Stage of PND. Front. Immunol. 2019, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, J.; Bowler, E.; Cerezo, M.; Gil, L.; Hall, P.; Hastings, E.; Junkins, H.; McMahon, A.; Milano, A.; Morales, J.; et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 2017, 45, D896–D901. [Google Scholar] [CrossRef] [PubMed]
- Pinero, J.; Bravo, A.; Queralt-Rosinach, N.; Gutierrez-Sacristan, A.; Deu-Pons, J.; Centeno, E.; Garcia-Garcia, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef] [PubMed]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Z.; Sun, M.; Liu, Q.; Wei, M.; Gao, H. Identification of cancer/testis antigen 2 gene as a potential hepatocellular carcinoma therapeutic target by hub gene screening with topological analysis. Oncol. Lett. 2019, 18, 4778–4788. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Gutierrez-Sacristan, A.; Grosdidier, S.; Valverde, O.; Torrens, M.; Bravo, A.; Pinero, J.; Sanz, F.; Furlong, L.I. PsyGeNET: A knowledge platform on psychiatric disorders and their genes. Bioinformatics 2015, 31, 3075–3077. [Google Scholar] [CrossRef]
- Gutierrez-Sacristan, A.; Hernandez-Ferrer, C.; Gonzalez, J.R.; Furlong, L.I. psygenet2r: A R/Bioconductor package for the analysis of psychiatric disease genes. Bioinformatics 2017, 33, 4004–4006. [Google Scholar] [CrossRef]
- Doan, R.N.; Bae, B.I.; Cubelos, B.; Chang, C.; Hossain, A.A.; Al-Saad, S.; Mukaddes, N.M.; Oner, O.; Al-Saffar, M.; Balkhy, S.; et al. Mutations in Human Accelerated Regions Disrupt Cognition and Social Behavior. Cell 2016, 167, 341–354.e312. [Google Scholar] [CrossRef]
- Wei, Y.; de Lange, S.C.; Scholtens, L.H.; Watanabe, K.; Ardesch, D.J.; Jansen, P.R.; Savage, J.E.; Li, L.; Preuss, T.M.; Rilling, J.K.; et al. Genetic mapping and evolutionary analysis of human-expanded cognitive networks. Nat. Commun. 2019, 10, 4839. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Menche, J.; Huang, C.C.; Ort, T.; Zhou, X.; Kitsak, M.; Sahni, N.; Thibault, D.; Voung, L.; Guo, F.; et al. A disease module in the interactome explains disease heterogeneity, drug response and captures novel pathways and genes in asthma. Hum. Mol. Genet. 2015, 24, 3005–3020. [Google Scholar] [CrossRef] [PubMed]
- Salzer, I.; Ray, S.; Schicker, K.; Boehm, S. Nociceptor Signalling through ion Channel Regulation via GPCRs. Int. J. Mol. Sci. 2019, 20, 2488. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.S.; Witkin, J.M. Protein complexes as psychiatric and neurological drug targets. Biochem. Pharmacol. 2018, 151, 263–281. [Google Scholar] [CrossRef]
- Rifkin, R.A.; Moss, S.J.; Slesinger, P.A. G Protein-Gated Potassium Channels: A Link to Drug Addiction. Trends Pharmacol. Sci. 2017, 38, 378–392. [Google Scholar] [CrossRef]
- Fromer, M.; Pocklington, A.J.; Kavanagh, D.H.; Williams, H.J.; Dwyer, S.; Gormley, P.; Georgieva, L.; Rees, E.; Palta, P.; Ruderfer, D.M.; et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 2014, 506, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.Y.; Quan, Y.; Zhang, H.Y. Human accelerated genome regions with value in medical genetics and drug discovery. Drug Discov. Today 2020, 25, 821–827. [Google Scholar] [CrossRef]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef]
- Volpicelli, F.; Perrone-Capano, C.; Bellenchi, G.C.; Colucci-D’Amato, L.; di Porzio, U. Molecular Regulation in Dopaminergic Neuron Development. Cues to Unveil Molecular Pathogenesis and Pharmacological Targets of Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 3995. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.R.; Orlandi, C. Orphan G Protein Coupled Receptors in Affective Disorders. Genes 2020, 11, 694. [Google Scholar] [CrossRef]
- Van den Heuvel, M.P.; Scholtens, L.H.; de Lange, S.C.; Pijnenburg, R.; Cahn, W.; van Haren, N.E.M.; Sommer, I.E.; Bozzali, M.; Koch, K.; Boks, M.P.; et al. Evolutionary modifications in human brain connectivity associated with schizophrenia. Brain 2019, 142, 3991–4002. [Google Scholar] [CrossRef] [PubMed]
- Coronel, R.; Bernabeu-Zornoza, A.; Palmer, C.; Muniz-Moreno, M.; Zambrano, A.; Cano, E.; Liste, I. Role of Amyloid Precursor Protein (APP) and Its Derivatives in the Biology and Cell Fate Specification of Neural Stem Cells. Mol. Neurobiol. 2018, 55, 7107–7117. [Google Scholar] [CrossRef] [PubMed]
- Triaca, V.; Calissano, P. Impairment of the nerve growth factor pathway driving amyloid accumulation in cholinergic neurons: The incipit of the Alzheimer’s disease story? Neural Regen. Res. 2016, 11, 1553–1556. [Google Scholar] [CrossRef]
- Feher, A.; Juhasz, A.; Laszlo, A.; Pakaski, M.; Kalman, J.; Janka, Z. Serotonin transporter and serotonin receptor 2A gene polymorphisms in Alzheimer’s disease. Neurosci. Lett. 2013, 534, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, X.; Liu, S.; Zhao, Y.; Zhu, J.; Liu, K. Roles of Neuropeptide Y in Neurodegenerative and Neuroimmune Diseases. Front. Neurosci. 2019, 13, 869. [Google Scholar] [CrossRef]
- Yang, Y.R.; Kang, D.S.; Lee, C.; Seok, H.; Follo, M.Y.; Cocco, L.; Suh, P.G. Primary phospholipase C and brain disorders. Adv. Biol. Regul. 2016, 61, 80–85. [Google Scholar] [CrossRef]
- Wang, P.; Velagapudi, R.; Kong, C.; Rodriguiz, R.M.; Wetsel, W.C.; Yang, T.; Berger, M.; Gelbard, H.A.; Colton, C.A.; Terrando, N. Neurovascular and immune mechanisms that regulate postoperative delirium superimposed on dementia. Alzheimer’s Dement. 2020, 16, 734–749. [Google Scholar] [CrossRef]

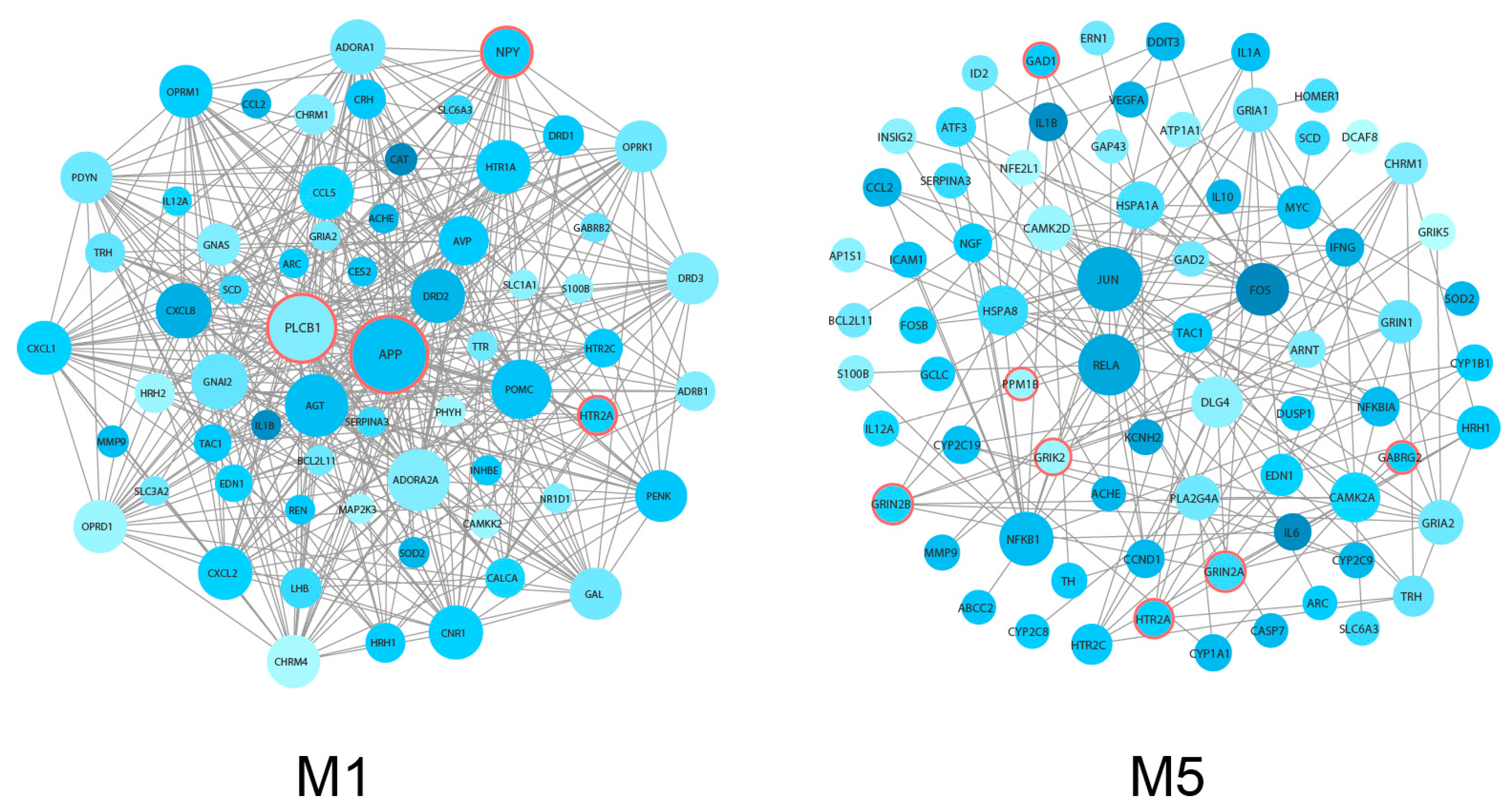
| Symbol | Gene Full Name | Module | Degree | Betweenness | MCC |
|---|---|---|---|---|---|
| APP | amyloid beta precursor protein | M1 | 45 | 1862.02222 | 9.22 × 1013 |
| PLCB1 | phospholipase C beta 1 | M1 | 37 | 353.11032 | 9.22 × 1013 |
| NPY | neuropeptide Y | M1 | 21 | 0.58095 | 9.22 × 1013 |
| HTR2A | 5-hydroxytryptamine receptor 2A | M1, M5 | 10 | 12.21667 | 15,144 |
| GRIN2A | glutamate ionotropic receptor NMDA type subunit 2A | M5 | 7 | 0 | 5040 |
| GRIN2B | glutamate ionotropic receptor NMDA type subunit 2B | M5 | 7 | 0 | 5040 |
| GRIK2 | glutamate ionotropic receptor kainate type subunit 2 | M5 | 4 | 1.33333 | 12 |
| GAD1 | glutamate decarboxylase | M5 | 3 | 36.12047 | 4 |
| PPM1B | protein phosphatase, Mg2+/Mn2+ dependent 1B | M5 | 1 | 0 | 1 |
| GABRG2 | gamma-aminobutyric acid type A receptor subunit gamma2 | M5 | 1 | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, Y.; Terada, T.; Muto, Y. Systems Level Analysis and Identification of Pathways and Key Genes Associated with Delirium. Genes 2020, 11, 1225. https://doi.org/10.3390/genes11101225
Takahashi Y, Terada T, Muto Y. Systems Level Analysis and Identification of Pathways and Key Genes Associated with Delirium. Genes. 2020; 11(10):1225. https://doi.org/10.3390/genes11101225
Chicago/Turabian StyleTakahashi, Yukiko, Tomoyoshi Terada, and Yoshinori Muto. 2020. "Systems Level Analysis and Identification of Pathways and Key Genes Associated with Delirium" Genes 11, no. 10: 1225. https://doi.org/10.3390/genes11101225
APA StyleTakahashi, Y., Terada, T., & Muto, Y. (2020). Systems Level Analysis and Identification of Pathways and Key Genes Associated with Delirium. Genes, 11(10), 1225. https://doi.org/10.3390/genes11101225





