Characterization of Quantitative Trait Loci for Germination and Coleoptile Length under Low-Temperature Condition Using Introgression Lines Derived from an Interspecific Cross in Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Evaluation of LTG and Coleoptile Length
2.3. DNA Extraction and Marker Analysis
2.4. Statistical Analysis and QTL Analysis
3. Results
3.1. Low-Temperature Germination Rate of Parental Lines and F2 Population
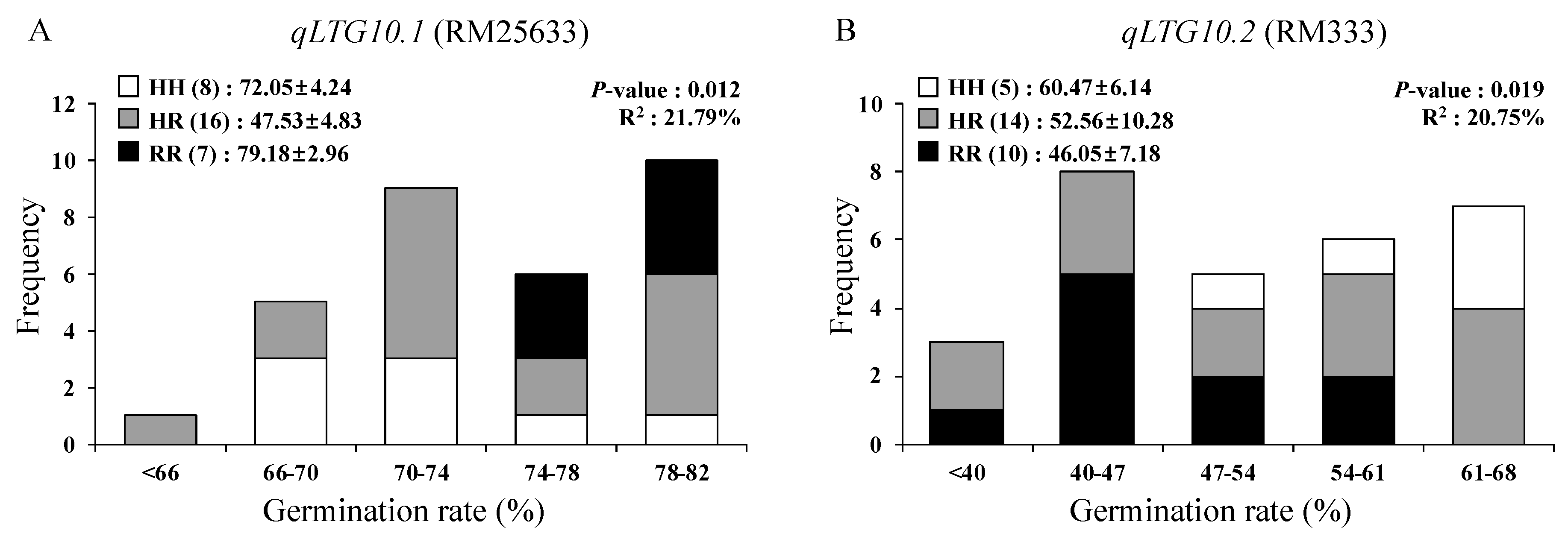
3.2. Mapping QTLs for Low-Temperature Germinability
3.3. Interaction between LTG QTLs
3.4. QTL Pyramiding Line for LTG
3.5. Verification of LTG QTL Using F3 Population
3.6. Coleoptile Length in Parental Lines and F2 Population
3.7. QTLs for Coleoptile Length
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brar, D.S.; Khush, G.S. Wild relatives of rice: A valuable genetic resource for genomics and breeding research. In The Wild Oryza Genomes; Springer: Cham, Switzerland, 2018; pp. 1–25. [Google Scholar]
- Brar, D.; Khush, G. Cytogenetic manipulation and germplasm enhancement of rice (Oryza sativa L.). Genet. Resour. Chromosome Eng. Crop. Improv. 2006, 2, 115–158. [Google Scholar]
- Luo, X.D.; Jun, Z.; Dai, L.F.; Zhang, F.T.; Yi, Z.; Yong, W.; Xie, J.K. Linkage map construction and QTL mapping for cold tolerance in Oryza rufipogon Griff. at early seedling stage. J. Integr. Agric. 2016, 15, 2703–2711. [Google Scholar] [CrossRef]
- McCouch, S.R.; Sweeney, M.; Li, J.; Jiang, H.; Thomson, M.; Septiningsih, E.; Edwards, J.; Moncada, P.; Xiao, J.; Garris, A. Through the genetic bottleneck: O. rufipogon as a source of trait-enhancing alleles for O. sativa. Euphytica 2007, 154, 317–339. [Google Scholar] [CrossRef]
- Wang, Z.F.; Wang, F.H.; Zhou, R.; Wang, J.F.; Zhang, H.S. Identification of quantitative trait loci for cold tolerance during the germination and seedling stages in rice (Oryza sativa L.). Euphytica 2011, 181, 405–413. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, S.W. Analysis of quantitative trait loci associated with seed germination and coleoptile length under low temperature condition. J. Crop Sci. Biotechnol. 2015, 18, 273–278. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, J.H.; Sun, B.Q. Advances on seed vigor physiological and genetic mechanisms. Agric. Sci. China 2007, 6, 1060–1066. [Google Scholar] [CrossRef]
- Teng, S.; Zeng, D.L.; Qian, Q.; Yasufumi, K.; Huang, D.I.; Zhu, L.H. QTL analysis of rice low temperature germinability. Chin. Sci. Bull. 2001, 46, 1800–1804. [Google Scholar] [CrossRef]
- Fujino, K.; Sekiguchi, H.; Sato, T.; Kiuchi, H.; Nonoue, Y.; Takeuchi, Y.; Ando, T.; Lin, S.Y.; Yano, M. Mapping of quantitative trait loci controlling low-temperature germinability in rice (Oryza sativa L.). Appl. Genet. 2004, 108, 794–799. [Google Scholar] [CrossRef]
- Chen, L.; Lou, Q.; Sun, Z.; Xing, Y.; Yu, X.; Luo, L. QTL mapping of low temperature germinability in rice. Zhongguo Shuidao Kexue 2006, 20, 159–164. [Google Scholar]
- Hou, M.Y.; Wang, C.M.; Jiang, L.; Wan, J.M.; Yasui, H.; Yoshimura, A. Inheritance and QTL mapping of low temperature germinability in rice (Oryza sativa L.). Acta Genet. Sin. 2004, 31, 701–706. [Google Scholar]
- Jiang, L.; Liu, S.J.; Hou, M.Y.; Tang, J.Y.; Chen, L.M.; Zhai, H.Q.; Wan, J.M. Analysis of QTLs for seed low temperature germinability and anoxia germinability in rice (Oryza sativa L.). Field Crop. Res. 2006, 98, 68–75. [Google Scholar] [CrossRef]
- Satoh, T.; Tezuka, K.; Kawamoto, T.; Matsumoto, S.; Satoh-Nagasawa, N.; Ueda, K.; Sakurai, K.; Watanabe, A.; Takahashi, H.; Akagi, H. Identification of QTLs controlling low-temperature germination of the East European rice (Oryza sativa L.) variety Maratteli. Euphytica 2016, 207, 245–254. [Google Scholar] [CrossRef]
- Adachi, Y.; Sugiyama, M.; Sakagami, J.I.; Fukuda, A.; Ohe, M.; Watanabe, H. Seed Germination and Coleoptile Growth of New Rice Lines Adapted to Hypoxic Conditions. Plant Prod. Sci. 2015, 18, 471–475. [Google Scholar] [CrossRef]
- Inada, N.; Sakai, A.; Kuroiwa, H.; Kuroiwa, T. Three-dimensional progression of programmed death in the rice coleoptile. Int. Rev. Cytol. 2002, 218, 221–258. [Google Scholar]
- Miura, K.; Lin, S.Y.; Yano, M.; Nagamine, T. Mapping quantitative trait loci controlling low temperature germinability in rice (Oryza sativa L.). Breed. Sci. 2001, 51, 293–299. [Google Scholar] [CrossRef]
- Ji, S.L.; Jiang, L.; Wang, Y.H.; Zhang, W.W.; Liu, X.; Liu, S.J.; Chen, L.M.; Zhai, H.Q.; Wan, J.M. Quantitative trait loci mapping and stability for low temperature germination ability of rice. Plant Breed. 2009, 128, 387–392. [Google Scholar] [CrossRef]
- Fujino, K.; Obara, M.; Shimizu, T.; Koyanagi, K.O.; Ikegaya, T. Genome-wide association mapping focusing on a rice population derived from rice breeding programs in a region. Breed. Sci. 2015, 65, 403–410. [Google Scholar] [CrossRef]
- Ji, S.L.; Ling, J.; Wang, Y.H.; Liu, S.J.; Liu, X.; Zhai, H.Q.; Yoshimura, A.; Wan, J.M. QTL and epistasis for low temperature germinability in rice. Acta Agron. Sin. 2008, 34, 551–556. [Google Scholar]
- Wang, X.; Zou, B.; Shao, Q.; Cui, Y.; Lu, S.; Zhang, Y.; Huang, Q.; Huang, J.; Hua, J. Natural variation reveals that OsSAP16 controls low-temperature germination in rice. J. Exp. Bot. 2018, 69, 413–421. [Google Scholar] [CrossRef]
- Xie, L.X.; Tan, Z.W.; Zhou, Y.; Xu, R.B.; Feng, L.B.; Xing, Y.Z.; Qi, X.Q. Identification and fine mapping of quantitative trait loci for seed vigor in germination and seedling establishment in rice. J. Integr. Plant Biol. 2014, 56, 749–759. [Google Scholar] [CrossRef]
- Li, L.F.; Liu, X.; Xie, K.; Wang, Y.H.; Liu, F.; Lin, Q.Y.; Wang, W.Y.; Yang, C.Y.; Lu, B.Y.; Liu, S.J.; et al. qLTG-9, a stable quantitative trait locus for low-temperature germination in rice (Oryza sativa L.). Appl. Genet. 2013, 126, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.; Kataoka, T.; Shiratsuchi, H.; Fukushima, A.; Yamaguchi, H.; Mochida, H.; Ogiwara, H. QTLs for Seedling Growth of Direct Seeded Rice under Submerged and Low Temperature Conditions. Plant Prod. Sci. 2014, 17, 41–46. [Google Scholar] [CrossRef]
- Shakiba, E.; Edwards, J.D.; Jodari, F.; Duke, S.E.; Baldo, A.M.; Korniliev, P.; McCouch, S.R.; Eizenga, G.C. Genetic architecture of cold tolerance in rice (Oryza sativa) determined through high resolution genome-wide analysis. PLoS ONE 2017, 12, e0172133. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Chen, H.P.; Mao, D.H. Pyramiding of rapid germination loci from Oryza Sativa cultivar ‘Xieqingzao B’ and cold tolerance loci from Dongxiang wild rice to increase climate resilience of cultivated rice. Mol. Breed. 2019, 39, 85. [Google Scholar] [CrossRef]
- Dametto, A.; Sperotto, R.A.; Adamski, J.M.; Blasi, E.A.R.; Cargnelutti, D.; de Oliveira, L.F.V.; Ricachenevsky, F.K.; Fregonezi, J.N.; Mariath, J.E.A.; da Cruz, R.P.; et al. Cold tolerance in rice germinating seeds revealed by deep RNAseq analysis of contrasting indica genotypes. Plant Sci. 2015, 238, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Park, I.K.; Yeo, S.M.; Yun, Y.T.; Ahn, S.N. Mapping quantitative trait loci controlling low-temperature germinability in rice. Korean J. Agric. Sci. 2012, 39, 477–482. [Google Scholar] [CrossRef]
- Shim, K.C.; Kim, S.; Le, A.Q.; Lee, H.S.; Adeva, C.; Jeon, Y.A.; Luong, N.H.; Kim, W.J.; Akhtamov, M.; Ahn, S.N. Fine mapping of a low-temperature Germinability QTL qLTG1 using introgression lines derived from Oryza rufipogon. Plant Breed. Biotechnol. 2019, 7, 141–150. [Google Scholar] [CrossRef]
- Shim, K.C.; Kim, S.H.; Lee, H.S.; Adeva, C.; Jeon, Y.A.; Luong, N.H.; Kim, W.J.; Akhtamov, M.; Park, Y.J.; Ahn, S.N. Characterization of a New qLTG3-1 Allele for Low-temperature Germinability in Rice from the Wild Species Oryza rufipogon. Rice 2020, 13, 10. [Google Scholar] [CrossRef]
- Causse, M.A.; Fulton, T.M.; Cho, Y.G.; Ahn, S.N.; Chunwongse, J.; Wu, K.S.; Xiao, J.H.; Yu, Z.H.; Ronald, P.C.; Harrington, S.E.; et al. Saturated Molecular Map of the Rice Genome Based on an Interspecific Backcross Population. Genetics 1994, 138, 1251–1274. [Google Scholar]
- Panaud, O.; Chen, X.; McCouch, S.R. Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L). Mol. Gen. Genet. 1996, 252, 597–607. [Google Scholar] [CrossRef]
- McCouch, S.R.; Cooperative, R.G. Gene Nomenclature System for Rice. Rice 2008, 1, 72–84. [Google Scholar] [CrossRef]
- Shinada, H.; Iwata, N.; Sato, T.; Fujino, K. QTL pyramiding for improving of cold tolerance at fertilization stage in rice. Breed. Sci. 2014, 63, 483–488. [Google Scholar] [CrossRef][Green Version]
- Najeeb, S.; Ali, J.; Mahender, A.; Pang, Y.L.; Zilhas, J.; Murugaiyan, V.; Vemireddy, L.R.; Li, Z. Identification of main-effect quantitative trait loci (QTLs) for low-temperature stress tolerance germination- and early seedling vigor-related traits in rice (Oryza sativa L.). Mol. Breed. 2020, 40, 10. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Grandillo, S.; Ahn, S.N.; Yuan, L.; Tanksley, S.D.; McCouch, S.R. Identification of trait-improving quantitative trait loci alleles from a wild rice relative, Oryza rufipogon. Genetics 1998, 150, 899–909. [Google Scholar] [PubMed]
- Luo, X.; Ji, S.-D.; Yuan, P.-R.; Lee, H.-S.; Kim, D.-M.; Balkunde, S.; Kang, J.-W.; Ahn, S.-N. QTL mapping reveals a tight linkage between QTLs for grain weight and panicle spikelet number in rice. Rice 2013, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, S.; Saka, N.; Koga, H.; Ono, K.; Shimizu, T.; Ebana, K.; Hayashi, N.; Takahashi, A.; Hirochika, H.; Okuno, K. Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 2009, 325, 998–1001. [Google Scholar] [CrossRef] [PubMed]

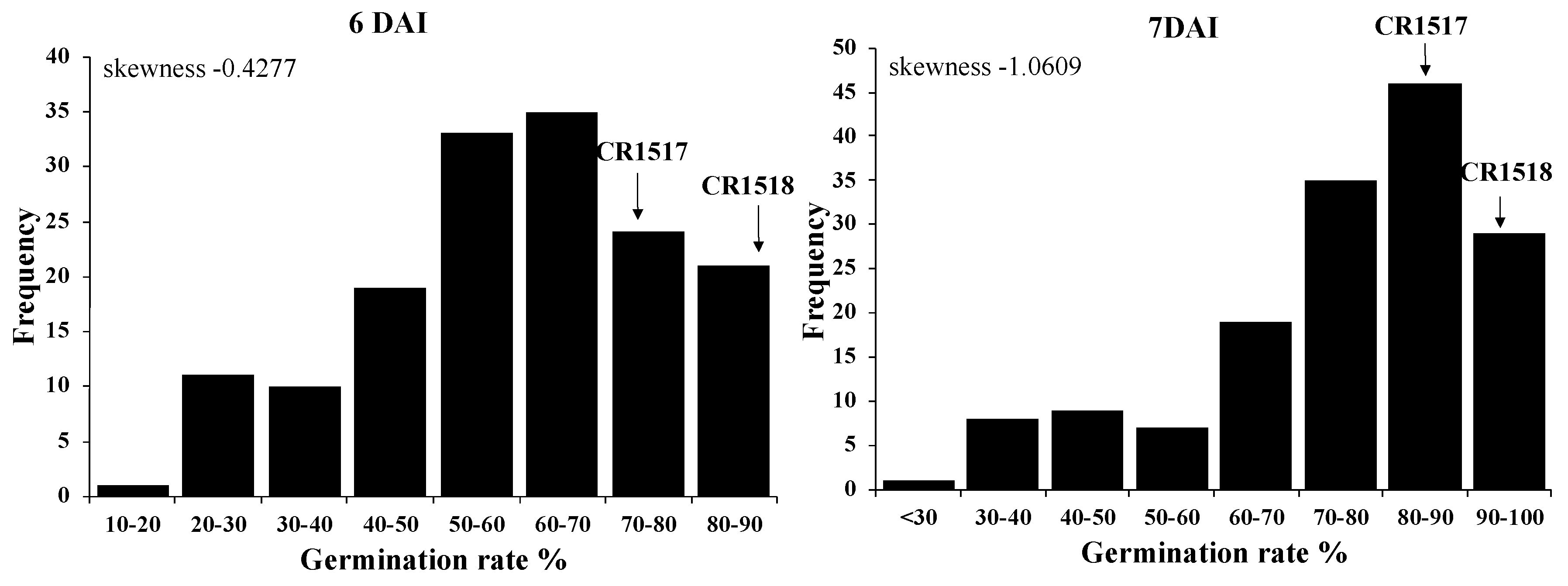

| Germination Rate (%) | ||||||
|---|---|---|---|---|---|---|
| Trait (x) | Lines (n = 5 (z)) | F2 Population | ||||
| O. rufipogon | Hwaseong | CR1517 | CR1518 | Mean | Range | |
| 4 DAI | 60.0 a (y) | 3.3 c | 30.4 b | 34.8 b | 15.6 ± 10.1 | 1.1–48.9 |
| 5 DAI | 91.9 a | 11.1 c | 49.3 b | 56.7 b | 38.3 ± 15.2 | 4.4–72.2 |
| 6 DAI | 97.4 a | 33.9 d | 70.4 c | 78.5 b | 58.6 ± 17.7 | 12.2–88.9 |
| 7 DAI | 100 a | 62.2 d | 84.2 c | 91.9 b | 74.9 ± 16.5 | 25.6–95.6 |
| 8 DAI | 100 a | 80.7 b | 99.3 a | 100 a | 90.7 ± 11.6 | 51.1–100 |
| Population | Trait (x) | QTL | Chr. | Markers | F-Value | p-Value | R2 (%) (y) | Gene Action (z) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| a | d | d/a | ||||||||
| F2 | LTG (6 DAI) | qLTG1 | 1 | RM220-CRM22 | 14.4 | 0.000 | 16.0 | 10.0 | 0.2 | 0.0 |
| qLTG3 | 3 | qLTG3-1_18D | 23.1 | 0.000 | 23.8 | 9.6 | 9.7 | 1.0 | ||
| qLTG10.2 | 10 | RM333-RM591 | 3.3 | 0.038 | 4.2 | −4.2 | 5.1 | −1.2 | ||
| Total | 38.9 | |||||||||
| LTG (7 DAI) | qLTG1 | 1 | RM220-CRM22 | 10.3 | 0.000 | 12.0 | 7.7 | 2.1 | 0.3 | |
| qLTG3 | 3 | qLTG3-1_18D | 21.0 | 0.000 | 22.1 | 8.1 | 9.7 | 1.2 | ||
| qLTG8 | 8 | RM72-RM22705 | 4.4 | 0.014 | 5.4 | 4.9 | 2.4 | 0.5 | ||
| qLTG10.1 | 10 | RM25633 | 4.2 | 0.041 | 4.6 | 2.9 | −1.9 | −0.6 | ||
| qLTG10.2 | 10 | RM333-RM591 | 5.4 | 0.006 | 6.6 | −5.3 | 5.5 | −1.0 | ||
| Total | 42.0 | |||||||||
| Coleoptile Lengths (mm) | ||||||
|---|---|---|---|---|---|---|
| Trait (x) | Lines (n = 5 (z)) | F2 Population | ||||
| O. rufipogon | Hwaseong | CR1517 | CR1518 | Mean | Range | |
| 7 DAI | 2.6 a (y) | 0.7 c | 1.6 b | 1.1 bc | 1.2 ± 0.7 | 0.2–9.2 |
| 8 DAI | 4.5 a | 1.4 d | 2.8 b | 2.0 c | 3.2 ± 0.5 | 0.6–9.5 |
| 9 DAI | 7.0 a | 2.8 c | 5.0 b | 3.5 c | 6.6 ± 1.3 | 2.0–10.0 |
| 10 DAI | 9.0 a | 3.8 d | 7.4 b | 5.6 c | 7.9 ± 1.4 | 5.0–12.0 |
| Population | Trait (x) | QTL | Chr. | Markers | F-Value | p-Value | R2 (%) (y) | Gene Action (z) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| a | d | d/a | ||||||||
| F2 | CCL 9 DAI | qCCL3 | 3 | qLTG3-1_18D | 9.82 | 0.000 | 12.0 | 0.032 | 0.08 | 2.6 |
| qCCL8 | 8 | RM22689-RM22705 | 4.77 | 0.001 | 6.2 | 0.043 | 0.02 | 0.6 | ||
| CCL 10 DAI | qCCL1 | 1 | RM220-CRM22 | 4.97 | 0.008 | 6.4 | 0.037 | 0.02 | 0.1 | |
| qCCL3 | 3 | qLTG3-1_18D | 7.30 | 0.001 | 9.2 | 0.033 | 0.07 | 2.3 | ||
| qCCL8 | 8 | RM22689-RM22705 | 5.27 | 0.006 | 7.1 | 0.046 | 0.03 | 0.7 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtamov, M.; Adeva, C.; Shim, K.-C.; Lee, H.-S.; Kim, S.H.; Jeon, Y.-A.; Luong, N.H.; Kang, J.-W.; Lee, J.-Y.; Ahn, S.-N. Characterization of Quantitative Trait Loci for Germination and Coleoptile Length under Low-Temperature Condition Using Introgression Lines Derived from an Interspecific Cross in Rice. Genes 2020, 11, 1200. https://doi.org/10.3390/genes11101200
Akhtamov M, Adeva C, Shim K-C, Lee H-S, Kim SH, Jeon Y-A, Luong NH, Kang J-W, Lee J-Y, Ahn S-N. Characterization of Quantitative Trait Loci for Germination and Coleoptile Length under Low-Temperature Condition Using Introgression Lines Derived from an Interspecific Cross in Rice. Genes. 2020; 11(10):1200. https://doi.org/10.3390/genes11101200
Chicago/Turabian StyleAkhtamov, Mirjalol, Cheryl Adeva, Kyu-Chan Shim, Hyun-Sook Lee, Sun Ha Kim, Yun-A Jeon, Ngoc Ha Luong, Ju-Won Kang, Ji-Yoon Lee, and Sang-Nag Ahn. 2020. "Characterization of Quantitative Trait Loci for Germination and Coleoptile Length under Low-Temperature Condition Using Introgression Lines Derived from an Interspecific Cross in Rice" Genes 11, no. 10: 1200. https://doi.org/10.3390/genes11101200
APA StyleAkhtamov, M., Adeva, C., Shim, K.-C., Lee, H.-S., Kim, S. H., Jeon, Y.-A., Luong, N. H., Kang, J.-W., Lee, J.-Y., & Ahn, S.-N. (2020). Characterization of Quantitative Trait Loci for Germination and Coleoptile Length under Low-Temperature Condition Using Introgression Lines Derived from an Interspecific Cross in Rice. Genes, 11(10), 1200. https://doi.org/10.3390/genes11101200




