The Variant rs1784042 of the SIDT2 Gene is Associated with Metabolic Syndrome through Low HDL-c Levels in a Mexican Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Demographic, Anthropometric and Clinical Measurements
2.3. Sample Genotyping and Selection of SNPs for Validation
2.3.1. Discovery Phase
2.3.2. Replication Phase
2.4. Statistical Power of the Study
2.5. Conditional and Haplotype Association Analysis
2.6. Statistical Analysis
3. Results
3.1. Demographic Data of the Study Population
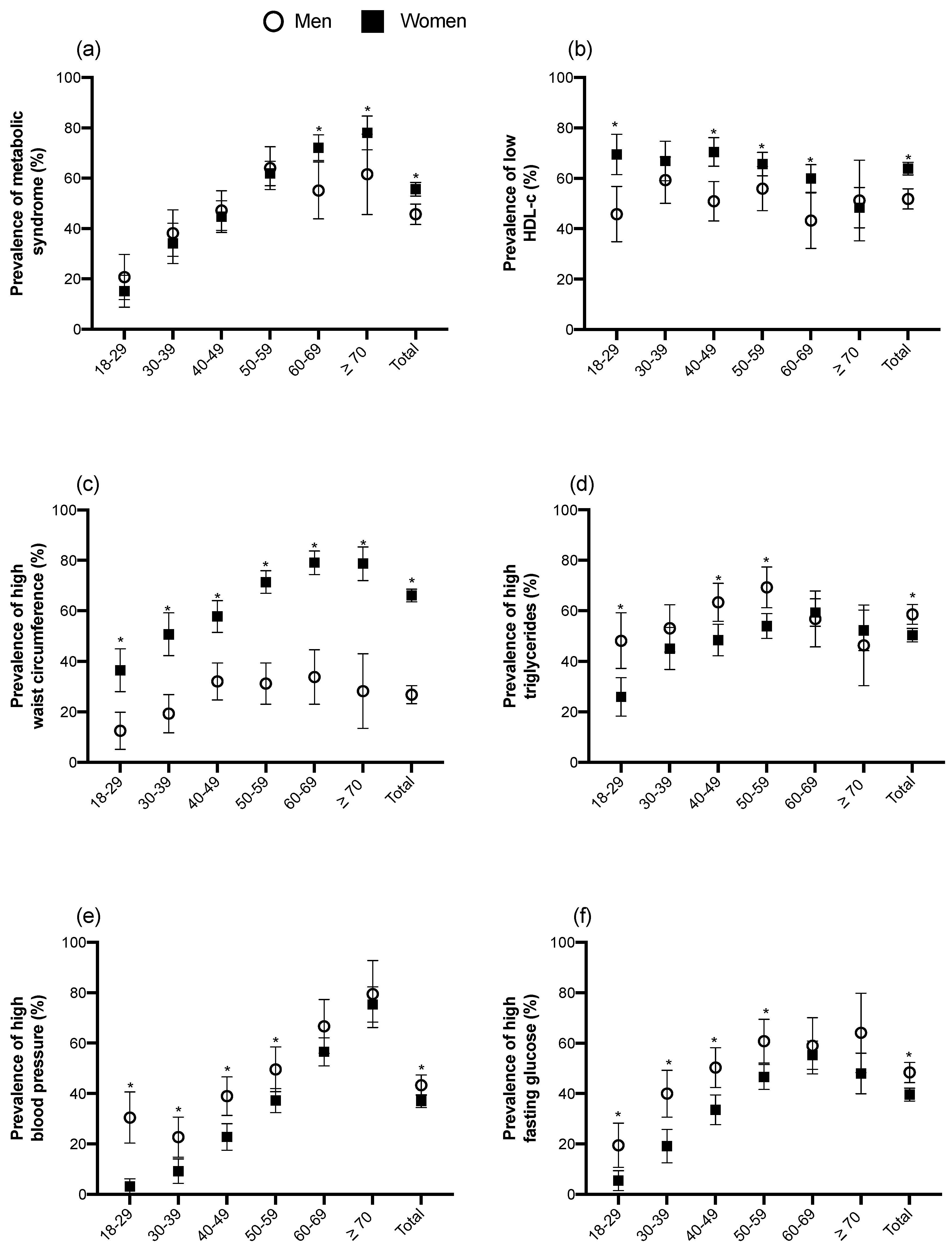
3.2. Prevalence of MetS and Its Components by Gender and Age Groups
3.3. Association Analyses of Genetic Variants rs17120425 and rs1784042 of SIDT2 with MetS
3.4. Conditional Analysis of SIDT2 Locus
3.5. Haplotype Association Analysis
3.6. In Silico Functional Analysis of Genetic Variants rs17120425 and rs1784042 in SIDT2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Publisher’s Note
References
- McNeill, A.M.; Rosamond, W.D.; Girman, C.J.; Golden, S.H.; Schmidt, M.I.; East, H.E.; Ballantyne, C.M.; Heiss, G. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care 2005, 28, 385–390. [Google Scholar] [CrossRef]
- Punthakee, Z.; Goldenberg, R.; Katz, P. 2018 Clinical Practice Guidelines Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome Diabetes Canada Clinical Practice Guidelines Expert Committee. Can. J. Diabetes 2018, 42 (Suppl. 1), S10–S15. [Google Scholar] [CrossRef]
- Rojas, R.; Aguilar-Salinas, C.A.; Jiménez-Corona, A.; Shamah-Levy, T.; Rauda, J.; Ávila-Burgos, L.; Villalpando, S.; Lazcano Ponce, E. Metabolic syndrome in Mexican adults: Results from the National Health and Nutrition Survey 2006. Salud Publica Mex. 2010, 52 (Suppl. 1), S11–S18. [Google Scholar] [CrossRef]
- Terán-García, M.; Bouchard, C. Genetics of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 89–114. [Google Scholar] [CrossRef]
- Watanabe, R.M.; Valle, T.; Hauser, E.R.; Ghosh, S.; Eriksson, J.; Kohtamäki, K.; Ehnholm, C.; Tuomilehto, J.; Collins, F.S.; Bergman, R.N.; et al. Familiality of quantitative metabolic traits in Finnish families with non-insulin-dependent diabetes mellitus. Finland-United States Investigation of NIDDM Genetics (FUSION) Study investigators. Hum. Hered. 1999, 49, 159–168. [Google Scholar] [CrossRef]
- Ziki, M.D.A.; Mani, A. Metabolic syndrome: Genetic insights into disease pathogenesis. Curr. Opin. Lipidol. 2016, 27, 162–171. [Google Scholar] [CrossRef]
- Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; Buchkovich, M.L.; Mora, S.; et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 2013, 45, 1274–1283. [Google Scholar] [CrossRef]
- Bandesh, K.; Prasad, G.; Giri, A.K.; Kauser, Y.; Upadhyay, M.; Basu, A.; Tandon, N.; Bharadwaj, D. Genome-wide association study of blood lipids in Indians confirms universality of established variants. J. Hum. Genet. 2019, 64, 573–587. [Google Scholar] [CrossRef]
- Dupuis, J.; Langenberg, C.; Prokopenko, I.; Saxena, R.; Soranzo, N.; Jackson, A.U.; Wheeler, E.; Glazer, N.L.; Bouatia-Naji, N.; Gloyn, A.L.; et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 2010, 42, 105–116. [Google Scholar] [CrossRef]
- Stančáková, A.; Laakso, M. Genetics of metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 243–252. [Google Scholar] [CrossRef]
- Kristiansson, K.; Perola, M.; Tikkanen, E.; Kettunen, J.; Surakka, I.; Havulinna, A.S.; Stančáková, A.; Barnes, C.; Widen, E.; Kajantie, E.; et al. Genome-wide screen for metabolic syndrome susceptibility loci reveals strong lipid gene contribution but no evidence for common genetic basis for clustering of metabolic syndrome traits. Circ. Cardiovasc. Genet. 2012, 5, 242–249. [Google Scholar] [CrossRef]
- Zafar, U.; Khaliq, S.; Ahmad, H.U.; Manzoor, S.; Lone, K.P. Metabolic syndrome: An update on diagnostic criteria, pathogenesis, and genetic links. Hormones 2018, 17, 299–313. [Google Scholar] [CrossRef]
- Brown, B.C.; Ye, C.J.; Price, A.L.; Zaitlen, N. Transethnic Genetic-Correlation Estimates from Summary Statistics. Am. J. Hum. Genet. 2016, 99, 76–88. [Google Scholar] [CrossRef]
- Gombojav, B.; Lee, S.J.; Kho, M.; Song, Y.M.; Lee, K.; Sung, J. Multiple susceptibility loci at chromosome 11q23.3 are associated with plasma triglyceride in East Asians. J. Lipid Res. 2016, 57, 318–324. [Google Scholar] [CrossRef]
- Andaleon, A.; Mogil, L.S.; Wheeler, H.E. Gene-based association study for lipid traits in diverse cohorts implicates BACE1 and SIDT2 regulation in triglyceride levels. PeerJ 2018, 6, e4314. [Google Scholar] [CrossRef]
- Moon, S.; Lee, Y.; Won, S.; Lee, J. Multiple genotype-phenotype association study reveals intronic variant pair on SIDT2 associated with metabolic syndrome in a Korean population. Hum. Genomics 2018, 12, 48. [Google Scholar] [CrossRef]
- Kulminski, A.M.; Loika, Y.; Huang, J.; Arbeev, K.G.; Bagley, O.; Ukraintseva, S.; Yashin, A.I.; Culminskaya, I. Pleiotropic meta-analysis of age-related phenotypes addressing evolutionary uncertainty in their molecular mechanisms. Front. Genet. 2019, 10, 433. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Y.; Yu, C.; Tan, F.; Wang, L. Spontaneous nonalcoholic fatty liver disease and ER stress in Sidt2 deficiency mice. Biochem. Biophys. Res. Commun. 2016, 476, 326–332. [Google Scholar] [CrossRef]
- Chen, X.; Gu, X.; Zhang, H. Sidt2 regulates hepatocellular lipid metabolism through autophagy. J. Lipid Res. 2018, 59, 404–415. [Google Scholar] [CrossRef]
- Jialin, G.; Xuefan, G.; Huiwen, Z. SID1 transmembrane family, member 2 (Sidt2): A novel lysosomal membrane protein. Biochem. Biophys. Res. Commun. 2010, 402, 588–594. [Google Scholar] [CrossRef]
- Méndez-Acevedo, K.M.; Valdes, V.J.; Asanov, A.; Vaca, L. A novel family of mammalian transmembrane proteins involved in cholesterol transport. Sci. Rep. 2017, 7, 7450. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gu, X.; Mahuran, D.J.; Wang, Z.; Zhang, H. Impaired Glucose Tolerance in a Mouse Model of Sidt2 Deficiency. PLoS ONE 2013, 8, e66139. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yu, C.; Xiong, Q.; Zhang, Y.; Wang, L. Lysosomal integral membrane protein Sidt2 plays a vital role in insulin secretion. Int. J. Clin. Exp. Pathol. 2015, 8, 15622–15631. [Google Scholar] [PubMed]
- Moreno-Estrada, A.; Gignoux, C.R.; Fernández-López, J.C.; Zakharia, F.; Sikora, M.; Contreras, A.V.; Acuña-Alonzo, V.; Sandoval, K.; Eng, C.; Romero-Hidalgo, S.; et al. The genetics of Mexico recapitulates Native American substructure and affects biomedical traits. Science 2014, 344, 1280–1285. [Google Scholar] [CrossRef]
- Pedroza-Tobias, A.; Trejo-Valdivia, B.; Sanchez-Romero, L.M.; Barquera, S. Classification of metabolic syndrome according to lipid alterations: Analysis from the Mexican National Health and Nutrition Survey 2006. BMC Public Health 2014, 14, 1056. [Google Scholar] [CrossRef]
- Aguilar-Salinas, C.A.; Olaiz, G.; Valles, V.; Torres, J.M.R.; Gómez Pérez, F.J.; Rull, J.A.; Rojas, R.; Franco, A.; Sepulveda, J. High prevalence of low HDL cholesterol concentrations and mixed hyperlipidemia in a Mexican nationwide survey. J. Lipid Res. 2001, 42, 1298–1307. [Google Scholar]
- Acuña-Alonzo, V.; Flores-Dorantes, T.; Kruit, J.K.; Villarreal-Molina, T.; Arellano-Campos, O.; Hünemeier, T.; Moreno-Estrada, A.; Ortiz-López, M.G.; Villamil-Ramírez, H.; León-Mimila, P.; et al. A functional ABCA1 gene variant is associated with low HDL-cholesterol levels and shows evidence of positive selection in Native Americans. Hum. Mol. Genet. 2010, 19, 2877–2885. [Google Scholar] [CrossRef]
- The SIGMA Type 2 Diabetes Consortium; Williams, A.L.; Jacobs, S.B.R.; Moreno-Macías, H.; Huerta-Chagoya, A.; Churchhouse, C.; Márquez-Luna, C.; García-Ortíz, H.; Gómez-Vázquez, M.J. Sequence variants in SLC16A11 are a common risk factor for type 2 diabetes in Mexico. Nature 2014, 506, 97–101. [Google Scholar] [CrossRef]
- Villalobos-Comparán, M.; Jiménez-Ortega, R.F.; Estrada, K.; Parra-Torres, A.Y.; González-Mercado, A.; Patiño, N.; Castillejos-López, M.; Quiterio, M.; Fernandez-López, J.C.; Ibarra, B.; et al. A pilot genome-wide association study in postmenopausal Mexican-Mestizo women implicates the RMND1/CCDC170 locus is associated with bone mineral density. Int. J. Genomics 2017, 2017, 5831020. [Google Scholar] [CrossRef]
- Rivera-Paredez, B.; Macías-Kauffer, L.; Fernandez-Lopez, J.C.; Villalobos-Comparán, M.; Martinez-Aguilar, M.M.; De la Cruz-Montoya, A.; Ramírez-Salazar, E.G.; Villamil-Ramírez, H.; Quiterio, M.; Ramírez-Palacios, P.; et al. Influence of genetic and non-genetic risk factors for serum uric acid levels and hyperuricemia in mexicans. Nutrients 2019, 11, 1336. [Google Scholar] [CrossRef]
- Denova-Gutierrez, E.; Flores, Y.N.; Gallegos-Carrillo, K.; Ramirez-Palacios, P.; Rivera-Paredez, B.; Munoz-Aguirre, P.; Velazquez-Cruz, R.; Torres-Ibarra, L.; Meneses-Leon, J.; Mendez-Hernandez, P.; et al. Health workers cohort study: Methods and study design. Salud Publica Mex. 2016, 58, 708–716. [Google Scholar] [CrossRef]
- Loh, P.R.; Danecek, P.; Palamara, P.F.; Fuchsberger, C.; Reshef, Y.A.; Finucane, H.K.; Schoenherr, S.; Forer, L.; McCarthy, S.; Abecasis, G.R.; et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat. Genet. 2016, 48, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, J.; Bowler, E.; Cerezo, M.; Gil, L.; Hall, P.; Hastings, E.; Junkins, H.; McMahon, A.; Milano, A.; Morales, J.; et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 2017, 45, D896–D901. [Google Scholar] [CrossRef] [PubMed]
- Gauderman, W.J. Sample size requirements for association studies of gene-gene interaction. Am. J. Epidemiol. 2002, 155, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Leon-Mimila, P.; Villamil-Ramirez, H.; Macias-Kauffer, L.R.; Jacobo-Albavera, L.; Lopez-Contreras, B.E.; Posadas-Sanchez, R.; Posadas-Romero, C.; Romero-Hidalgo, S.; Moran-Ramos, S.; Dominguez-Perez, M.; et al. A functional variant of the SIDT2 gene involved in cholesterol transport is associated with HDL-C levels and premature coronary artery disease. medRxiv 2020. [Google Scholar] [CrossRef]
- Aguilar Salinas, C.; Cruz-Bautista, I.; Mehta, R.; Villarreal-Molina, M.; Perez, F.; Tusie-Luna, M.; Canizales-Quinteros, S. The ATP-Binding Cassette Transporter Subfamily A Member 1 (ABC-A1) and Type 2 Diabetes: An Association Beyond HDL Cholesterol. Curr. Diabetes Rev. 2007, 3, 264–267. [Google Scholar] [CrossRef]
- Aguilar-Salinas, C.A.; Canizales-Quinteros, S.; Rojas-Martfnez, R.; Mehta, R.; Ma, T.V.M.; Arellano-Campos, O.; Riba, L.; Gómez-Pérez, F.J.; Tusié-Luna, M.T. Hypoalphalipoproteinemia in populations of Native American ancestry: An opportunity to assess the interaction of genes and the environment. Curr. Opin. Lipidol. 2009, 20, 92–97. [Google Scholar] [CrossRef]
- Khramtsova, E.A.; Davis, L.K.; Stranger, B.E. The role of sex in the genomics of human complex traits. Nat. Rev. Genet. 2019, 20, 173–190. [Google Scholar] [CrossRef]
- Kong, S.; Cho, Y.S. Identification of female-specific genetic variants for metabolic syndrome and its component traits to improve the prediction of metabolic syndrome in females. BMC Med. Genet. 2019, 20, 99. [Google Scholar] [CrossRef]
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 2017, 120, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Corpeleijn, E.; Gansevoort, R.T.; Gans, R.O.B.; Hillege, H.L.; Stolk, R.P.; Navis, G.; Bakker, S.J.L.; Dullaart, R.P.F. Role of HDL cholesterol and estimates of HDL particle composition in future development of type 2 diabetes in the general population: The PREVEND study. J. Clin. Endocrinol. Metab. 2013, 98, E1352–E1359. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Nakanishi, S.; Kubota, M.; Maeda, S.; Yoneda, M.; Yamane, K.; Kira, S.; Sasaki, H.; Kohno, N. Low high-density lipoprotein cholesterol level is a significant risk factor for development of type 2 diabetes: Data from the Hawaii-Los Angeles-Hiroshima study. J. Diabetes Investig. 2014, 5, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Brunham, L.R.; Kruit, J.K.; Pape, T.D.; Timmins, J.M.; Reuwer, A.Q.; Vasanji, Z.; Marsh, B.J.; Rodrigues, B.; Johnson, J.D.; Parks, J.S.; et al. β-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 2007, 13, 340–347. [Google Scholar] [CrossRef]
- Brunham, L.R.; Kruit, J.K.; Verchere, C.B.; Hayden, M.R. Cholesterol in islet dysfunction and type 2 diabetes. J. Clin. Investig. 2008, 118, 403–408. [Google Scholar] [CrossRef]
- Vergeer, M.; Brunham, L.R.; Koetsveld, J.; Kruit, J.K.; Verchere, C.B.; Kastelein, J.J.P.; Hayden, M.R.; Stroes, E.S.G. Carriers of loss-of-function mutations in ABCA1 display pancreatic β-cell dysfunction. Diabetes Care 2010, 33, 869–874. [Google Scholar] [CrossRef][Green Version]
- Fryirs, M.A.; Barter, P.J.; Appavoo, M.; Tuch, B.E.; Tabet, F.; Heather, A.K.; Rye, K.A. Effects of high-density lipoproteins on pancreatic β-cell insulin secretion. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1642–1648. [Google Scholar] [CrossRef]
- Cochran, B.J.; Bisoendial, R.J.; Hou, L.; Glaros, E.N.; Rossy, J.; Thomas, S.R.; Barter, P.J.; Rye, K.A. Apolipoprotein A-I increases insulin secretion and production from pancreatic β-cells via a G-protein-cAMPPKA-FoxO1-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2261–2267. [Google Scholar] [CrossRef]
- Von Eckardstein, A.; Widmann, C. High-density lipoprotein, β cells, and diabeteś. Cardiovasc. Res. 2014, 103, 384–394. [Google Scholar] [CrossRef]
- Mendoza-Caamal, E.C.; Barajas-Olmos, F.; Garciá-Ortiz, H.; Cicerón-Arellano, I.; Martínez-Hernández, A.; Córdova, E.J.; Esparza-Aguilar, M.; Contreras-Cubas, C.; Centeno-Cruz, F.; Cid-Soto, M.; et al. Metabolic syndrome in indigenous communities in Mexico: A descriptive and cross-sectional study. BMC Public Health 2020, 20, 339. [Google Scholar] [CrossRef]
- Aguilar-Salinas, C.A.; Canizales-Quinteros, S.; Rojas-Martínez, R.; Mehta, R.; Rodriguez-Guillén, R.; Ordoñez-Sanchez, M.L.; Riba, L.; Tusié-Luna, M.T. The non-synonymous Arg230Cys variant (R230C) of the ATP-binding cassette transporter A1 is associated with low HDL cholesterol concentrations in Mexican adults: A population based nation wide study. Atherosclerosis 2011, 216, 146–150. [Google Scholar] [CrossRef]
- Veenstra, J.; Kalsbeek, A.; Koster, K.; Ryder, N.; Bos, A.; Huisman, J.; Vanderberg, L.; Vanderwoude, J.; Tintle, N.L. Epigenome wide association study of SNP-CpG interactions on changes in triglyceride levels after pharmaceutical intervention: A GAW20 analysis 06 Biological Sciences 0604 Genetics. BMC Proc. 2018, 12 (Suppl. 9), 58. [Google Scholar] [CrossRef]
- Povel, C.M.; Boer, J.M.A.; Reiling, E.; Feskens, E.J.M. Genetic variants and the metabolic syndrome: A systematic review. Obes. Rev. 2011, 12, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Orozco-González, N.; Cortés-Sanabria, L.; Viera-Franco, J.J.; Ramírez-Márquez, J.J.; Cueto-Manzano, A.M. Prevalence of cardiovascular risk factors in a population of health-care workers. Rev. Med. Inst. Mex. Seguro Soc. 2016, 54, 594–601. [Google Scholar]
- Padierna-Luna, J.L.; Ochoa-Rosas, F.S.; Jaramillo-Villalobos, B. Prevalence of metabolic syndrome in health employees. Rev. Med. Inst. Mex. Seguro Soc. 2007, 45, 593–599. [Google Scholar] [PubMed]
- Palacios-Rodríguez, R.G.; Paulín-Villalpando, P.; López-Carmona, J.M.; Valerio-Acosta, M.M.L.; Cabrera-Gaytán, D.A. Metabolic syndrome in health care personnel from a primary care unit. Rev. Med. Inst. Mex. Seguro Soc. 2010, 48, 297–302. [Google Scholar]
- Mathiew-Quirós, Á.; Salinas-Martínez, A.M.; Hernández-Herrera, R.J.; Gallardo-Vela, J.A. Metabolic syndrome in workers of a second level hospital. Rev. Med. Inst. Mex. Seguro Soc. 2014, 52, 580–587. [Google Scholar]
- Cruz-Dominguez, M.P.; González-Márquez, F.; Ayala-López, E.A.; Vera-Lastra, L.O.; Vargas- Rendón, G.H.; Zarate-Amador, A.; Jara-Quezada, L.J. Sobrepeso, obesidad, síndrome metabólico e índice cintura/talla en el personal de salud [Overweight, obesity, metabolic syndrome and waist/height index in health staff]. Rev. Med. Inst. Mex. Seguro Soc. 2015, 53, S36–S41. [Google Scholar]
- Lavalle, F.J.; Villarreal, J.Z.; Montes, J.; Mancillas, L.G.; Rodríguez, S.E.; González, P.; Lara, R. Change in the prevalence of metabolic syndrome in a population of medical students: 6-year follow-up. J. Diabetes Metab. Disord. 2015, 14, 85. [Google Scholar] [CrossRef][Green Version]
- Vizmanos, B.; Betancourt-Nuñez, A.; Márquez-Sandoval, F.; González-Zapata, L.I.; Monsalve-Álvarez, J.; Bressan, J.; De Carvalho Vidigal, F.; Figueredo, R.; López, L.B.; Babio, N.; et al. Metabolic Syndrome among Young Health Professionals in the Multicenter Latin America Metabolic Syndrome Study. Metab. Syndr. Relat. Disord. 2020, 18, 86–95. [Google Scholar] [CrossRef]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic syndrome prevalence by race/ethnicity and sex in the united states, national health and nutrition examination survey, 1988–2012. Prev. Chronic Dis. 2017, 14, E24. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Sandoval, F.; MacEdo-Ojeda, G.; Viramontes-Hörner, D.; Fernández Ballart, J.D.; Salas Salvadó, J.; Vizmanos, B. The prevalence of metabolic syndrome in Latin America: A systematic review. Public Health Nutr. 2011, 14, 1702–1713. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, J.; Schargrodsky, H.; Champagne, B.; Silva, H.; Boissonnet, C.P.; Vinueza, R.; Torres, M.; Hernandez, R.; Wilson, E. Prevalence of the Metabolic Syndrome in Latin America and its association with sub-clinical carotid atherosclerosis: The CARMELA cross sectional study. Cardiovasc. Diabetol. 2009, 8, 52. [Google Scholar] [CrossRef]
- Méndez-Hernández, P.; Flores, Y.; Siani, C.; Lamure, M.; Dosamantes-Carrasco, L.D.; Halley-Castillo, E.; Huitrn, G.; Talavera, J.O.; Gallegos-Carrillo, K.; Salmerón, J. Physical activity and risk of Metabolic Syndrome in an urban Mexican cohort. BMC Public Health 2009, 9, 276. [Google Scholar] [CrossRef]
- Ervin, R.B. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl. Health Stat. Rep. 2009, 13, 1–7. [Google Scholar]
- Cuevas, A.; Alvarez, V.; Carrasco, F. Epidemic of metabolic syndrome in Latin America. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 134–138. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Parameter | Total Sample | Men | Women | p Value |
|---|---|---|---|---|
| n = 1963 | n = 593 | n = 1370 | ||
| Age(years) 1 | 52.0 (40.0–62.0) | 46.0 (36.0–57.0) | 54.0 (43.0–63.0) | <0.001 |
| Body mass index (kg/m2) 1 | 26.7 (24.0–29.7) | 26.5 (24.1–29.0) | 26.8 (24.0–30.1) | 0.133 |
| Overweight 2 | 42.9 (40.7–45.0) | 48.8 (44.9–52.8) | 40.2 (37.7–42.8) | <0.001 |
| Obesity 2 | 23.9 (22.0–25.8) | 19.6 (16.5–22.8) | 25.7 (23.4–28.0) | 0.003 |
| Waist circumference (cm) 1 | 94.0 (86.0–101.0) | 96.0 (90.0–102.0) | 92.0 (85–100.0) | <0.001 |
| Body fat proportion 1 | 41.9 (34.0–47.2) | 31.5 (27.7–34.7) | 45.1 (40.7–49.1) | <0.001 |
| Leisure time physical activity (hour/week) 1 | 1.5 (0.3–3.5) | 1.7 (0.4–5.0) | 1.1 (0.2–3.5) | <0.001 |
| Active (>150 min/week) 2 | 34.9 (32.8–37.0) | 36.6 (32.8–40.4) | 28.2 (25.8–30.6) | <0.001 |
| Smoking current 2 | 12.3 (10.9–13.7) | 21.3 (18.0–26.6) | 9.0 (7.5–10.6) | <0.001 |
| Smoking past 2 | 27.5 (25.5–29.4) | 39.7 (35.7–43.6) | 23.8 (21.5–26.1) | <0.001 |
| Systolic blood pressure (mmHg) 1 | 118.0 (108.0–129.5) | 122.0 (113.0–131.0) | 116.0 (106.0–129.0) | <0.001 |
| Diastolic blood pressure (mmHg) 1 | 74.0 (68.0–81.0) | 77.0 (70–84) | 73.0 (66.0–79.0) | <0.001 |
| Fasting plasma glucose (mg/dL) 1 | 97.0 (90.0–106.0) | 98.0 (92–107) | 96.0 (90.0–104.0) | <0.001 |
| Total cholesterol (mg/dL) 1 | 140.6 (85.4–213.6) | 167.0 (105.0–266.0) | 128.0 (83.0–193.0) | <0.001 |
| Low density lipoprotein-c (mg/dL) 1,3 | 120.0 (98.0–145.4) | 116.0 (97–143.0) | 121.0 (99.0–147.0) | 0.009 |
| High density lipoprotein-c (mg/dL) 1,4 | 44.0 (37.0–52.0) | 39.0 (34.0–46.0) | 46.0 (39.0–54.0) | <0.001 |
| Metabolic Syndrome 2,5 | 52.6 (50.4–54.8) | 45.7 (41.6–49.7) | 55.6 (52.9–58.3) | <0.001 |
| Triglycerides (mg/dL) 1 | 156.0 (112.0–209.0) | 168.0 (118.0–247.0) | 151.0 (109.0–199.0) | <0.001 |
| Total | Men | Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Genotype | Control, n (%) | Case, n (%) | OR 1,7 (95% CI) | p Value | Control, n (%) | Case, n (%) | OR 2 (95% CI) | p Value | Control, n (%) | Case, n (%) | OR 2 (95% CI) | p Value |
| MetS 3 | GG | 742 (80.2) | 858 (83.3) | Ref. | 256 (80.3) | 232 (86.3) | Ref. | 486 (80.2) | 626 (82.4) | Ref. | |||
| GA | 173 (18.8) | 160(15.5) | 0.79 (0.61–1.01) | 0.065 | 58 (18.2) | 36 (13.4) | 0.66 (0.41–1.05) | 0.078 | 115 (19.0) | 124 (16.3) | 0.86 (0.63–1.15) | 0.307 | |
| AA | 10 (1.0) | 11 (1.1) | 0.83 (0.33–2.10) | 0.700 | 5 (1.6) | 1 (0.4) | 0.15 (0.02–1.39) | 0.095 | 5 (0.8) | 10 (1.3) | 1.59 (0.48–5.35) | 0.450 | |
| Additive model | 0.82 (0.65–1.02) | 0.074 | 0.60 (0.39–0.92) | 0.018 | 0.93 (0.71–1.21) | 0.575 | |||||||
| Low HDL- cholesterol 4 | GG | 514 (76.4) | 1086 (84.8) | Ref. | 203 (79.9) | 285 (85.3) | Ref. | 311 (74.2) | 801 (84.6) | Ref. | |||
| GA | 151 (22.4) | 182 (14.2) | 0.54 (0.42–0.69) | 7.5 × 10−7 | 48 (18.9) | 46 (13.8) | 0.65 (0.42–1.02) | 0.059 | 103 (24.6) | 136 (14.4) | 0.50 (0.37–0.66) | 2.7 × 10−6 | |
| AA | 8 (1.2) | 13 (1.0) | 0.73 (0.30–1.79) | 0.492 | 3 (1.2) | 3 (0.9) | 0.66 (0.13–3.32) | 0.613 | 5 (1.2) | 10 (1.1) | 0.78 (0.26–2.31) | 0.652 | |
| Additive model | 0.60 (0.48–0.74) | 4.3 × 10−6 | 0.68 (0.46–1.02) | 0.062 | 0.57 (0.44–0.74) | 2 × 10−5 | |||||||
| Impaired glucose tolerance 5 | GG | 934 (82.6) | 446 (82.8) | Ref. | 251 (82.0) | 165 (84.6) | Ref. | 683 (82.8) | 281 (81.7) | Ref. | |||
| GA | 184 (16.3) | 89 (16.5) | 1.03 (0.77–1.38) | 0.825 | 50 (16.3) | 30 (15.4) | 0.84 (0.51–1.40) | 0.51 | 134 (16.2) | 59 (17.2) | 1.12 (0.79–1.59) | 0.52 | |
| AA | 13 (1.2) | 4 (0.7) | 0.54 (0.17–1.70) | 0.292 | 5 (1.6) | - | 8 (1.0) | 4 (1.2) | 1.01 (0.30–3.47) | 0.983 | |||
| Additive model | 0.96 (0.74–1.25) | 0.773 | 0.70 (0.44–1.12) | 0.135 | 1.10 (0.80–1.50) | 0.56 | |||||||
| Type 2 Diabetes 6 | GG | 934 (82.6) | 220 (77.6) | Ref. | 251 (82.0) | 72 (82.8) | Ref. | 683 (82.8) | 148 (75.1) | Ref. | |||
| GA | 184 (16.3) | 60 (21.1) | 1.56 (1.11–2.21) | 0.011 | 50 (16.3) | 14 (16.1) | 0.98 (0.49–1.97) | 0.954 | 134 (16.2) | 46 (23.4) | 1.82 (1.22–2.71) | 0.003 | |
| AA | 13 (1.2) | 4 (1.4) | 0.97 (0.29–3.20) | 0.959 | 5 (1.6) | 1 (1.2) | 0.32 (0.03–3.61) | 0.355 | 8 (1.0) | 3 (1.5) | 1.39 (0.35–5.54) | 0.644 | |
| Additive model | 1.39 (1.03–1.88) | 0.033 | 0.86 (0.46–1.59) | 0.634 | 1.62 (1.14–2.30) | 0.007 | |||||||
| Total | Men | Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Genotype | Control, n (%) | Case, n (%) | OR 1,7 (95% CI) | p Value | Control, n (%) | Case, n (%) | OR 2 (95% CI) | p Value | Control, n (%) | Case, n (%) | OR 2 (95% CI) | p Value |
| MetS 3 | GG | 445 (49.0) | 534 (52.0) | Ref. | 161 (50.2) | 138 (51.3) | Ref. | 294 (48.4) | 396 (52.2) | Ref. | |||
| GA | 391 (42.1) | 417 (40.6) | 0.84 (0.69–1.03) | 0.097 | 135 (42.1) | 117 (43.5) | 0.98 (0.67–1.34) | 0.780 | 256 (42.2) | 300 (39.5) | 0.80 (0.62–1.02) | 0.066 | |
| AA | 82 (8.8) | 77 (7.5) | 0.65 (0.45–0.93) | 0.018 | 25 (7.8) | 14 (5.2) | 0.59 (0.29–1.19) | 0.138 | 63 (8.3) | 63 (8.3) | 0.67 (0.44–1.02) | 0.061 | |
| Additive model | 0.82 (0.71–0.95) | 0.010 | 0.85 (0.65–1.12) | 0.258 | 0.81 (0.68–0.97) | 0.020 | |||||||
| Low HDL-cholesterol 4 | GG | 308 (45.5) | 681 (53.2) | Ref. | 124 (48.4) | 175 (52.4) | Ref. | 184 (43.7) | 506 (53.5) | Ref. | |||
| GA | 304 (44.9) | 504 (39.4) | 0.75 (0.61, 0.91) | 0.004 | 112 (43.8) | 140 (41.9) | 0.90 (0.64–1.27) | 0.546 | 192 (45.6) | 364 (38.5) | 0.67 (0.53–0.86) | 0.002 | |
| AA | 65 (9.6) | 94 (7.4) | 0.63 (0.45, 0.90) | 0.011 | 20 (7.8) | 19 (5.7) | 0.66 (0.34–1.30) | 0.23 | 45 (10.7) | 75 (7.9) | 0.61 (0.40–0.93) | 0.020 | |
| Additive model | 0.77 (0.67, 0.90) | 0.001 | 0.85 (0.65–1.12) | 0.249 | 0.74 (0.62–0.88) | 0.001 | |||||||
| Impaired glucose tolerance 5 | GG | 567 (50.3) | 280 (51.7) | Ref. | 158 (52.0) | 102 (51.5) | Ref. | 409 (49.6) | 178 (51.7) | Ref. | |||
| GA | 468 (41.5) | 222 (41.0) | 0.93 (0.75, 1.16) | 0.542 | 126 (41.5) | 87 (43.9) | 1.04 (0.71–1.52) | 0.831 | 342 (41.5) | 135 (39.2) | 0.89 (0.69–1.17) | 0.418 | |
| AA | 93 (8.2) | 40 (7.4) | 0.83 (0.55, 1.26) | 0.388 | 20 (6.6) | 9 (4.6) | 0.65 (0.28–1.50) | 0.309 | 73 (8.9) | 31 (9.0) | 0.92 (0.57–1.47) | 0.729 | |
| Additive model | 0.92 (0.78, 1.09) | 0.344 | 0.93 (0.69–1.26) | 0.625 | 0.93 (0.76–1.14) | 0.492 | |||||||
| Type 2 Diabetes 6 | GG | 567 (50.3) | 142 (49.7) | Ref. | 158 (52.0) | 39 (44.3) | Ref. | 409 (49.6) | 103 (52.0) | Ref. | |||
| GA | 468 (41.5) | 118 (41.3) | 0.96 (0.72, 1.29) | 0.800 | 126 (41.5) | 39 (44.3) | 1.25 (0.72–2.16) | 0.43 | 342 (41.5) | 79 (39.9) | 0.87 (0.62–1.23) | 0.432 | |
| AA | 93 (8.2) | 26 (9.1) | 0.99 (0.60, 1.63) | 0.967 | 20 (6.6) | 10 (11.4) | 1.76 (0.70–4.41) | 0.23 | 73 (8.9) | 16 (8.1) | 0.78 (0.42–1.43) | 0.419 | |
| Additive model | 0.98 (0.79, 1.22) | 0.87 | 1.31 (0.88–1.97) | 0.187 | 0.88 (0.68–1.13) | 0.314 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Reyes, G.; Rivera-Paredez, B.; López, J.C.F.; Ramírez-Salazar, E.G.; Aquino-Gálvez, A.; Gallegos-Carrillo, K.; Denova-Gutiérrez, E.; Salmerón, J.; Velázquez-Cruz, R. The Variant rs1784042 of the SIDT2 Gene is Associated with Metabolic Syndrome through Low HDL-c Levels in a Mexican Population. Genes 2020, 11, 1192. https://doi.org/10.3390/genes11101192
León-Reyes G, Rivera-Paredez B, López JCF, Ramírez-Salazar EG, Aquino-Gálvez A, Gallegos-Carrillo K, Denova-Gutiérrez E, Salmerón J, Velázquez-Cruz R. The Variant rs1784042 of the SIDT2 Gene is Associated with Metabolic Syndrome through Low HDL-c Levels in a Mexican Population. Genes. 2020; 11(10):1192. https://doi.org/10.3390/genes11101192
Chicago/Turabian StyleLeón-Reyes, Guadalupe, Berenice Rivera-Paredez, Juan Carlos Fernandez López, Eric G. Ramírez-Salazar, Arnoldo Aquino-Gálvez, Katia Gallegos-Carrillo, Edgar Denova-Gutiérrez, Jorge Salmerón, and Rafael Velázquez-Cruz. 2020. "The Variant rs1784042 of the SIDT2 Gene is Associated with Metabolic Syndrome through Low HDL-c Levels in a Mexican Population" Genes 11, no. 10: 1192. https://doi.org/10.3390/genes11101192
APA StyleLeón-Reyes, G., Rivera-Paredez, B., López, J. C. F., Ramírez-Salazar, E. G., Aquino-Gálvez, A., Gallegos-Carrillo, K., Denova-Gutiérrez, E., Salmerón, J., & Velázquez-Cruz, R. (2020). The Variant rs1784042 of the SIDT2 Gene is Associated with Metabolic Syndrome through Low HDL-c Levels in a Mexican Population. Genes, 11(10), 1192. https://doi.org/10.3390/genes11101192






