Outcome Definition Influences the Relationship between Genetic Polymorphisms of ERCC1, ERCC2, SLC22A2 and Cisplatin Nephrotoxicity in Adult Testicular Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
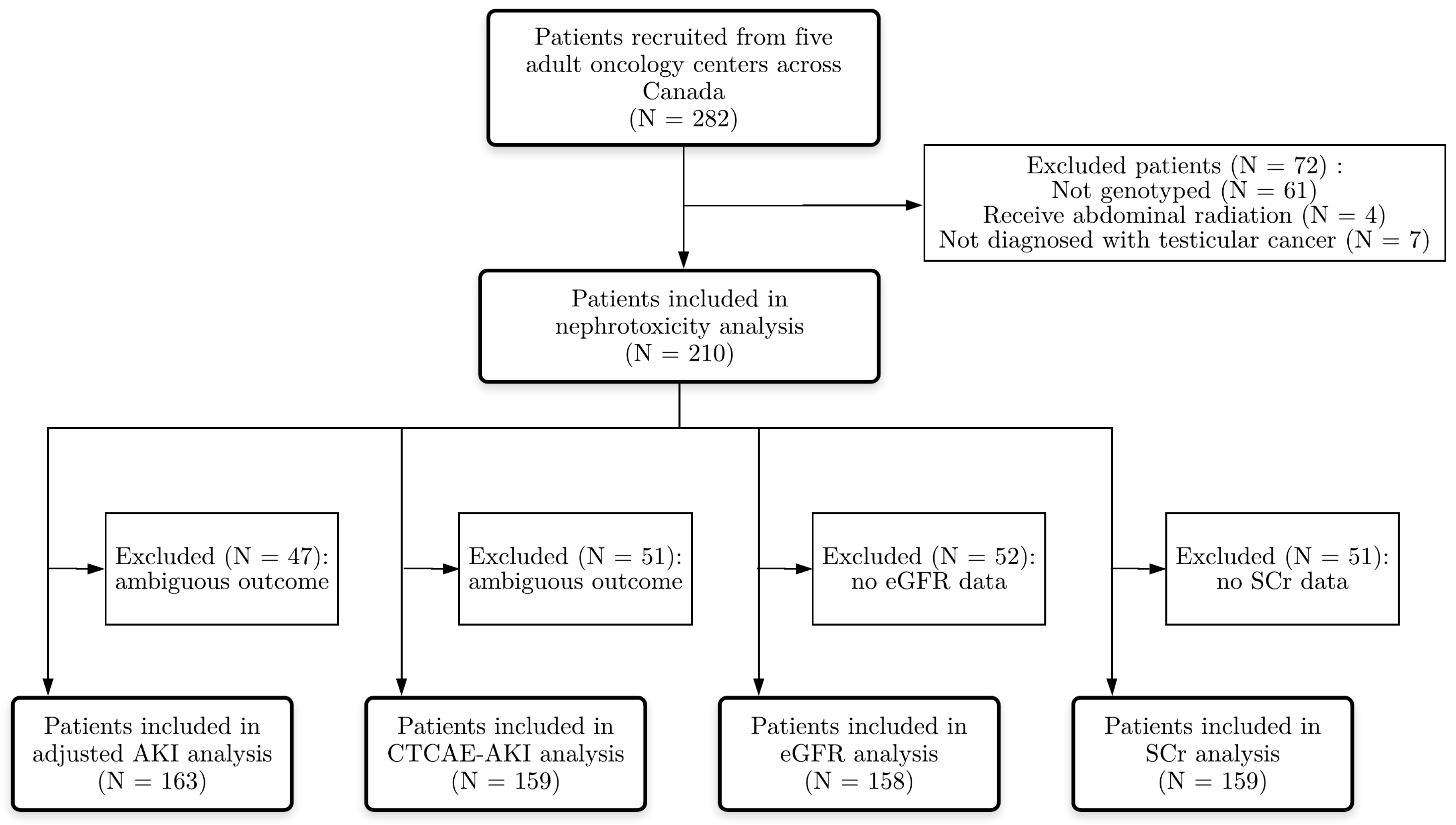
2.1. Study Design and Participants
2.2. Clinical Data Collection
2.3. Outcome Definition
2.3.1. “Adjusted Acute Kidney Injury” (Adjusted-AKI) Outcome Definition
2.3.2. CTCAE-AKI Outcome Definition
2.3.3. “∆SCr and ∆eGFR” Outcome Definition
2.4. Genotype Data
2.4.1. Candidate Genes
2.4.2. Genotyping
2.4.3. Quality Control of Genotype Data
2.5. Statistical Analyses
3. Results
3.1. Study Population
3.2. Genotyping Results
3.3. Adjusted AKI Analysis
3.4. CTCAE-AKI Analysis
3.5. ∆SCr and ∆eGFR Analysis
4. Discussion
4.1. Main Findings
4.2. Gene Expression and Regulation
4.3. Strength and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Rancoule, C.; Guy, J.B.; Vallard, A.; Ben Mrad, M.; Rehailia, A.; Magne, N. 50th anniversary of cisplatin. Bull. Cancer 2017, 104, 167–176. [Google Scholar] [CrossRef]
- Hoffmann, R.; Plug, I.; McKee, M.; Khoshaba, B.; Westerling, R.; Looman, C.; Rey, G.; Jougla, E.; Lang, K.; Parna, K.; et al. Innovations in health care and mortality trends from five cancers in seven European countries between 1970 and 2005. Int. J. Public. Health 2014, 59, 341–350. [Google Scholar] [CrossRef]
- Sakaeda, T.; Kadoyama, K.; Okuno, Y. Adverse event profiles of platinum agents: Data mining of the public version of the FDA adverse event reporting system, AERS and reproducibility of clinical observations. Int. J. Med. Sci. 2011, 8, 487–491. [Google Scholar] [CrossRef]
- Arany, I.; Safirstein, R.L. Cisplatin nephrotoxicity. Semin. Nephrol. 2003, 23, 460–464. [Google Scholar] [CrossRef]
- Sahni, V.; Choudhury, D.; Ahmed, Z. Chemotherapy-associated renal dysfunction. Nat. Rev. Nephrol. 2009, 5, 450–462. [Google Scholar] [CrossRef]
- Chovanec, M.; Abu Zaid, M.; Hanna, N.; El-Kouri, N.; Einhorn, L.H.; Albany, C. Long-term toxicity of cisplatin in germ-cell tumor survivors. Ann. Oncol. 2017, 28, 2670–2679. [Google Scholar] [CrossRef] [PubMed]
- Gietema, J.A.; Meinardi, M.T.; Messerschmidt, J.; Gelevert, T.; Alt, F.; Uges, D.R.A.; Sleijfer, D.T. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet 2000, 355, 1075–1076. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Bosl, G.J.; Alcock, N.W.; Niedzwiecki, D.; Gallina, E.; Shurgot, B. Pharmacokinetics of cis-diamminedichloroplatinum(II) after administration in hypertonic saline. Cancer Res. 1986, 46, 5969–5972. [Google Scholar] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef]
- Khrunin, A.V.; Moisseev, A.; Gorbunova, V.; Limborska, S. Genetic polymorphisms and the efficacy and toxicity of cisplatin-based chemotherapy in ovarian cancer patients. Pharmacogenomics J. 2010, 10, 54–61. [Google Scholar] [CrossRef]
- Tzvetkov, M.V.; Behrens, G.; O’Brien, V.P.; Hohloch, K.; Brockmoller, J.; Benohr, P. Pharmacogenetic analyses of cisplatin-induced nephrotoxicity indicate a renoprotective effect of ERCC1 polymorphisms. Pharmacogenomics 2011, 12, 1417–1427. [Google Scholar] [CrossRef]
- Windsor, R.E.; Strauss, S.J.; Kallis, C.; Wood, N.E.; Whelan, J.S. Germline genetic polymorphisms may influence chemotherapy response and disease outcome in osteosarcoma: A pilot study. Cancer 2012, 118, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Powrozek, T.; Mlak, R.; Krawczyk, P.; Homa, I.; Ciesielka, M.; Koziol, P.; Prendecka, M.; Milanowski, J.; Malecka-Massalska, T. The relationship between polymorphisms of genes regulating DNA repair or cell division and the toxicity of platinum and vinorelbine chemotherapy in advanced NSCLC patients. Clin. Transl. Oncol. 2016, 18, 125–131. [Google Scholar] [CrossRef]
- Iwata, K.; Aizawa, K.; Kamitsu, S.; Jingami, S.; Fukunaga, E.; Yoshida, M.; Yoshimura, M.; Hamada, A.; Saito, H. Effects of genetic variants in SLC22A2 organic cation transporter 2 and SLC47A1 multidrug and toxin extrusion 1 transporter on cisplatin-induced adverse events. Clin. Exp. Nephrol. 2012, 16, 843–851. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, G.; Li, X.; Ren, S.; Li, A.; Xu, J.; Zhang, J.; Zhou, C. Association between single nucleotide polymorphisms (SNPs) and toxicity of advanced non-small-cell lung cancer patients treated with chemotherapy. PLoS ONE 2012, 7, e48350. [Google Scholar] [CrossRef]
- Filipski, K.K.; Mathijssen, R.H.; Mikkelsen, T.S.; Schinkel, A.H.; Sparreboom, A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin. Pharmacol. Ther. 2009, 86, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Zazuli, Z.; Vijverberg, S.; Slob, E.; Liu, G.; Carleton, B.; Veltman, J.; Baas, P.; Masereeuw, R.; Maitland-van der Zee, A.H. Genetic Variations and Cisplatin Nephrotoxicity: A Systematic Review. Front. Pharmacol. 2018, 9, 1111. [Google Scholar] [CrossRef]
- Giachino, D.F.; Ghio, P.; Regazzoni, S.; Mandrile, G.; Novello, S.; Selvaggi, G.; Gregori, D.; DeMarchi, M.; Scagliotti, G.V. Prospective assessment of XPD Lys751Gln and XRCC1 Arg399Gln single nucleotide polymorphisms in lung cancer. Clin. Cancer Res. 2007, 13, 2876–2881. [Google Scholar] [CrossRef]
- Friboulet, L.; Olaussen, K.A.; Pignon, J.P.; Shepherd, F.A.; Tsao, M.S.; Graziano, S.; Kratzke, R.; Douillard, J.Y.; Seymour, L.; Pirker, R.; et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N. Engl. J. Med. 2013, 368, 1101–1110. [Google Scholar] [CrossRef]
- Xiong, Y.; Huang, B.Y.; Yin, J.Y. Pharmacogenomics of platinum-based chemotherapy in non-small cell lung cancer: Focusing on DNA repair systems. Med. Oncol. 2017, 34, 48. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, J.; Sun, M.; Zhang, Z.; Liu, C.; Sun, Y. A Significant Statistical Advancement on the Predictive Values of ERCC1 Polymorphisms for Clinical Outcomes of Platinum-Based Chemotherapy in Non-Small Cell Lung Cancer: An Updated Meta-Analysis. Dis. Markers 2016, 2016, 7643981. [Google Scholar] [CrossRef]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef]
- Wensing, K.U.; Ciarimboli, G. Saving ears and kidneys from cisplatin. Anticancer Res. 2013, 33, 4183–4188. [Google Scholar] [PubMed]
- Little, J.; Higgins, J.P.; Ioannidis, J.P.; Moher, D.; Gagnon, F.; von Elm, E.; Khoury, M.J.; Cohen, B.; Davey-Smith, G.; Grimshaw, J.; et al. Strengthening the reporting of genetic association studies (STREGA): An extension of the STROBE Statement. Hum. Genet. 2009, 125, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Drogemoller, B.I.; Monzon, J.G.; Bhavsar, A.P.; Borrie, A.E.; Brooks, B.; Wright, G.E.B.; Liu, G.; Renouf, D.J.; Kollmannsberger, C.K.; Bedard, P.L.; et al. Association Between SLC16A5 Genetic Variation and Cisplatin-Induced Ototoxic Effects in Adult Patients With Testicular Cancer. JAMA Oncol. 2017, 3, 1558–1562. [Google Scholar] [CrossRef][Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Group, K.D.I.G.O.K.C.W. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Winkelmayer, W.C.; Levin, R.; Setoguchi, S. Associations of kidney function with cardiovascular medication use after myocardial infarction. Clin. J. Am. Soc. Nephrol. 2008, 3, 1415–1422. [Google Scholar] [CrossRef]
- Becquemont, L.; Bauduceau, B.; Benattar-Zibi, L.; Berrut, G.; Bertin, P.; Bucher, S.; Corruble, E.; Danchin, N.; al-Salameh, A.; Derumeaux, G.; et al. Association between Cardiovascular Drugs and Chronic Kidney Disease in Non-Institutionalized Elderly Patients. Basic Clin. Pharmacol. Toxicol. 2015, 117, 137–143. [Google Scholar] [CrossRef]
- Aronow, W.S.; Frishman, W.H.; Cheng-Lai, A. Cardiovascular drug therapy in the elderly. Cardiol. Rev. 2007, 15, 195–215. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Smith, G.L.; Rathore, S.S.; Massie, B.M.; Krumholz, H.M. Renal function, digoxin therapy and heart failure outcomes: Evidence from the digoxin intervention group trial. J. Am. Soc. Nephrol. 2004, 15, 2195–2203. [Google Scholar] [CrossRef]
- Diabetes Canada Clinical Practice Guidelines Expert, C.; Lipscombe, L.; Booth, G.; Butalia, S.; Dasgupta, K.; Eurich, D.T.; Goldenberg, R.; Khan, N.; MacCallum, L.; Shah, B.R.; et al. Pharmacologic Glycemic Management of Type 2 Diabetes in Adults. Can. J. Diabetes 2018, 42, S88–S103. [Google Scholar]
- Awdishu, L.; Mehta, R.L. The 6R’s of drug induced nephrotoxicity. BMC Nephrol. 2017, 18, 124. [Google Scholar] [CrossRef]
- BCCA Genitourinary Chemotherapy Protocols. BC Cancer, Provincial Health Services Authority. Available online: http://www.bccancer.bc.ca/health-professionals/clinical-resources/chemotherapy-protocols/genitourinary#Testicular (accessed on 21 February 2018).
- CCA Genitourinary Cancer Guidelines and Advice. Cancer Care Ontario. Available online: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/genitourinary?f%5B0%5D=field_type_of_cancer%3A656&f%5B1%5D=field_type_of_cancer%3A681 (accessed on 21 February 2018).
- Rantanen, V.; Grenman, S.; Kulmala, J.; Grenman, R. Comparative evaluation of cisplatin and carboplatin sensitivity in endometrial adenocarcinoma cell lines. Br. J. Cancer 1994, 69, 482–486. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. National Cancer Institute. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/About.html (accessed on 15 February 2018).
- Castillo, J.J.; Vincent, M.; Justice, E. Diagnosis and management of hyponatremia in cancer patients. Oncologist 2012, 17, 756–765. [Google Scholar] [CrossRef]
- CPNDS. Welcome to The Canadian Pharmacogenomics Network for Drug Safety (CPNDS). Available online: http://cpnds.ubc.ca/ (accessed on 8 January 2019).
- Hinai, Y.; Motoyama, S.; Niioka, T.; Miura, M. Absence of effect of SLC22A2 genotype on cisplatin-induced nephrotoxicity in oesophageal cancer patients receiving cisplatin and 5-fluorouracil: Report of results discordant with those of earlier studies. J. Clin. Pharm. Ther. 2013, 38, 498–503. [Google Scholar] [CrossRef]
- KimCurran, V.; Zhou, C.; Schmid-Bindert, G.; Shengxiang, R.; Zhou, S.; Zhang, L.; Zhang, J. Lack of correlation between ERCC1 (C8092A) single nucleotide polymorphism and efficacy/toxicity of platinum based chemotherapy in Chinese patients with advanced non-small cell lung cancer. Adv. Med. Sci. 2011, 56, 30–38. [Google Scholar] [CrossRef]
- Goekkurt, E.; Al-Batran, S.E.; Hartmann, J.T.; Mogck, U.; Schuch, G.; Kramer, M.; Jaeger, E.; Bokemeyer, C.; Ehninger, G.; Stoehlmacher, J. Pharmacogenetic analyses of a phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil and leucovorin plus either oxaliplatin or cisplatin: A study of the arbeitsgemeinschaft internistische onkologie. J. Clin. Oncol. 2009, 27, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Erculj, N.; Kovac, V.; Hmeljak, J.; Dolzan, V. The influence of platinum pathway polymorphisms on the outcome in patients with malignant mesothelioma. Ann. Oncol. 2012, 23, 961–967. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, W. Ameliorative effects of SLC22A2 gene polymorphism 808 G/T and cimetidine on cisplatin-induced nephrotoxicity in Chinese cancer patients. Food Chem. Toxicol. 2012, 50, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- Tiao, J.Y.; Semmens, J.B.; Masarei, J.R.; Lawrence-Brown, M.M. The effect of age on serum creatinine levels in an aging population: relevance to vascular surgery. Cardiovasc. Surg. 2002, 10, 445–451. [Google Scholar] [CrossRef]
- McMahon, K.R.; Rod Rassekh, S.; Schultz, K.R.; Pinsk, M.; Blydt-Hansen, T.; Mammen, C.; Tsuyuki, R.T.; Devarajan, P.; Cuvelier, G.D.; Mitchell, L.G.; et al. Design and Methods of the Pan-Canadian Applying Biomarkers to Minimize Long-Term Effects of Childhood/Adolescent Cancer Treatment (ABLE) Nephrotoxicity Study: A Prospective Observational Cohort Study. Can. J. Kidney Health Dis. 2017, 4, 2054358117690338. [Google Scholar] [CrossRef] [PubMed]
- Woelfelschneider, A.; Popanda, O.; Lilla, C.; Linseisen, J.; Mayer, C.; Celebi, O.; Debus, J.; Bartsch, H.; Chang-Claude, J.; Schmezer, P. A distinct ERCC1 haplotype is associated with mRNA expression levels in prostate cancer patients. Carcinogenesis 2008, 29, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.J.; Liu, W.; Zhang, J.; Zhu, J.; Zhang, R.; Tang, J.; Yang, T.; Zou, Y.; He, J.; Xia, H. Functional Polymorphisms at ERCC1/XPF Genes Confer Neuroblastoma Risk in Chinese Children. EBioMedicine 2018, 30, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Consortium, G. The Genotype-Tissue Expression (GTEx), GTEx Consortium, 2017.
- Zolk, O. Disposition of metformin: variability due to polymorphisms of organic cation transporters. Ann. Med. 2012, 44, 119–129. [Google Scholar] [CrossRef] [PubMed]
| Grade | Definition | Characteristic(s) |
|---|---|---|
| 0 | An increase in serum creatinine, up to 1.5 times baseline value AND Electrolyte disorders grade 0 CTCAE: • Hypomagnesemia: ≥LLN–1.2 mg/dL; <LLN–0.5 mmol/L, OR • Hypokalaemia: ≥LLN–3.0 mmol/L, OR • Hypophosphatemia: ≥LLN–2.5 mg/dL; <LLN–0.8 mmol/L, OR • Hyponatremia: ≥LLN–130 mmol/L (>2 months) | Asymptomatic |
| 1 | Between 1.5–1.9 times baseline SCr OR ≥0.3 mg/dL (≥26.5 µmol/L) increase in SCr OR Electrolyte disorders grade 1 CTCAE: • Hypomagnesemia: <LLN–1.2 mg/dL; <LLN–0.5 mmol/L, OR • Hypokalaemia: <LLN–3.0 mmol/L, OR • Hypophosphatemia: <LLN–2.5 mg/dL; <LLN–0.8 mmol/L, OR • Hyponatremia: <LLN–130 mmol/L (>2 months) | Possible Symptomatic |
| 2 | An increase in serum creatinine between 2.0–2.9 times baseline SCr ORElectrolyte disorders grade 2 CTCAE: • Hypomagnesemia: <1.2–0.9 mg/dL; <0.5–0.4 mmol/L, OR • Hypokalaemia: <LLN–3.0 mmol/L, OR • Hypophosphatemia: <2.5–2.0 mg/dL; <0.8–0.6 mmol/L, OR • Hyponatremia: <LLN–130–120 mmol/L (>2 months) | Clinically relevant, required intervention |
| 3 | An increase in serum creatinine at least 3.0 times baseline OR Increase in serum creatinine to ≥4.0 mg/dL (≥353.6 µmol/L) OR Initiation of renal replacement therapy, OR OR Electrolyte disorders ≥grade 3 CTCAE: • Hypomagnesemia: <0.9 mg/dL; <0.4 mmol/L, OR • Hypokalaemia: <3.0 mmol/L; hospitalization indicated, OR • Hypophosphatemia: <2.0 mg/dL; <0.6 mmol/L, OR • Hyponatremia: <LLN–120 mmol/L (>2 months) | Required close monitoring |
| Case | Control | Ambiguous |
|---|---|---|
| Acute nephrotoxicity ≥ grade 1 OR Received electrolyte supplementation | Acute nephrotoxicity < grade 1 AND No supplementation | No lab values available during the time frame (3 months before initiation and 3 months after the last administration of cisplatin) OR Incomplete data e.g., initiation and end date of cisplatin therapy OR Pre-existing renal disease (electrolyte disturbances, not SCr or eGFR) |
| Case | Control | Ambiguous |
|---|---|---|
| Acute kidney injury ≥ grade 1 | Acute kidney injury < grade 1 | No lab values available during the time frame (3 months before initiation and 3 months after the last administration of cisplatin) OR Incomplete data e.g., initiation and end date of cisplatin therapy |
| Characteristics | ||
|---|---|---|
| Age at start treatment, mean ± SD, years | 31.8 ± 10.2 | |
| Ancestry, mean ± SD, proportion | European | 0.72 ± 0.26 |
| East-Asian | 0.09 ± 0.23 | |
| American | 0.05 ± 0.10 | |
| African | 0.03 ± 0.03 | |
| South-Asian | 0.11 ± 0.15 | |
| Cardiovascular disease, no. (%) | 7 (3.3) | |
| Diabetes, no. (%) | 2 (1.0) | |
| Potentially nephrotoxic co-medications, mean ± SD, total number per patient | 2 ± 2 | |
| Potentially nephrotoxic co-medications, no. (%) | ACEIs a | 3 (1.4) |
| Aminoglycosides | 4 (1.9) | |
| ARBs b | 1 (0.5) | |
| Benzodiazepines | 30 (14) | |
| NSAIDs c | 6 (2.9) | |
| Betalactams | 26 (12) | |
| PPIs d | 25 (12) | |
| Quinolones | 29 (14) | |
| Statins | 2 (1.0) | |
| Acetaminophen | 29 (14) | |
| Other | 104 (50) | |
| Baseline [SCr], mean ± SD, umol/L | 84 ± 16 | |
| Baseline [K+], mean ± SD, mmol/L | 4.1 ± 0.4 | |
| Baseline [Mg2+], mean ± SD, mmol/L | 0.85 ± 0.10 | |
| Baseline [Na+], mean ± SD, mmol/L | 138 ± 2.49 | |
| Baseline [PO4-], mean ± SD, mmol/L | 1.09 ± 0.23 | |
| Cumulative platinum dose, mean ± SD, mg/m2 | 380 ± 123 | |
| Duration cisplatin treatment, mean ± SD | Weeks | 8.7 ± 3.3 |
| Cycles | 3.8 ± 1.1 | |
| Chemotherapy protocol, no. (%), BEP | 136 (65) | |
| Chemotherapy hydration, mean ± SD, L/cycle | 10.7 ± 0.5 | |
| Gene–SNP | OR | 95% CI | p-Value | ORadj | 95% CIadj | p-Valueadj |
|---|---|---|---|---|---|---|
| ERCC1 rs11615 | ||||||
| GG | 1 # | 1 # | ||||
| GA | 1.30 | 0.63–2.67 | 0.48 | 1.45 | 0.64–3.27 | 0.38 |
| AA | 1.24 | 0.51–3.02 | 0.63 | 1.47 | 0.50–4.28 | 0.48 |
| ERCC1 rs3212986 | ||||||
| CC | 1 # | 1 # | ||||
| CA | 0.71 | 0.37–1.36 | 0.31 | 0.63 | 0.30–1.34 | 0.23 |
| AA | 1.00 | 0.30–3.37 | 1.00 | 1.44 | 0.32–6.43 | 0.63 |
| ERCC2 rs13181 | ||||||
| AA | 1 # | 1 # | ||||
| CA | 0.84 | 0.42–1.66 | 0.61 | 0.59 | 0.26–1.33 | 0.20 |
| CC | 1.60 | 0.65–3.93 | 0.31 | 1.43 | 0.50–4.07 | 0.51 |
| ERCC2 rs1799793 | ||||||
| AA | 1 # | 1 # | ||||
| CA | 1.00 | 0.49–2.03 | 1.00 | 0.92 | 0.40–2.15 | 0.85 |
| CC | 0.50 | 0.21–1.17 | 0.11 | 0.55 | 0.21–1.43 | 0.22 |
| SLC22A2 rs316019 | ||||||
| CC | 1 # | 1 # | ||||
| AC | 1.15 | 0.51–2.57 | 0.71 | 1.10 | 0.43–2.79 | 0.85 |
| AA | 2.46 | 0.22–27.78 | 0.47 | 1.70 | 0.11–25.57 | 0.70 |
| Gene–SNP | OR | 95% CI | p-Value | ORadj | 95% CIadj | p-Valueadj | Cohcran-Armitage Trend Test p-Value |
|---|---|---|---|---|---|---|---|
| ERCC1 rs11615 GG vs. GA vs. AA | 1.13 | 0.73–1.75 | 0.586 | 1.23 | 0.73–2.05 | 0.436 | 0.586 |
| ERCC1 rs3212986 AA vs. CA vs. CC | 0.86 | 0.54–1.40 | 0.551 | 0.89 | 0.51–1.54 | 0.669 | 0.537 |
| ERCC2 rs13181 CC vs. CA vs. AA | 1.19 | 0.75–1.88 | 0.461 | 1.04 | 0.61–1.78 | 0.875 | 0.497 |
| ERCC2 rs1799793 CC vs. CA vs. AA | 0.70 | 0.45–1.09 | 0.114 | 0.73 | 0.44–1.19 | 0.206 | 0.280 |
| SLC22A2 rs316019 AA vs. CA vs. CC | 1.28 | 0.64–2.59 | 0.488 | 1.17 | 0.53–2.60 | 0.702 | 0.502 |
| Gene–SNP | OR | 95% CI | p-Value | ORadj | 95% CIadj | p-Valueadj |
|---|---|---|---|---|---|---|
| ERCC1 rs11615 | ||||||
| GG | 1 # | 1 # | ||||
| GA | 1.30 | 0.57–2.99 | 0.55 | 1.23 | 0.45–3.39 | 0.68 |
| AA | 0.48 | 0.14–1.65 | 0.24 | 0.53 | 0.12–2.37 | 0.41 |
| ERCC1 rs3212986 | ||||||
| CC | 1 # | 1 # | ||||
| CA | 0.45 | 0.20–1.02 | 0.06 | 0.24 | 0.08–0.70 | 0.009 * |
| AA | 0.48 | 0.10–2.36 | 0.37 | 0.43 | 0.07–2.47 | 0.34 |
| ERCC2 rs13181 | ||||||
| AA | 1 # | 1 # | ||||
| CA | 1.16 | 0.49–2.73 | 0.74 | 0.59 | 0.20–1.76 | 0.37 |
| CC | 3.16 | 1.17–8.58 | 0.02 | 1.72 | 0.53–5.65 | 0.35 |
| ERCC2 rs1799793 | ||||||
| AA | 1 # | 1 # | ||||
| CA | 1.52 | 0.65–3.54 | 0.33 | 2.39 | 0.84–6.77 | 0.10 |
| CC | 0.57 | 0.18–1.79 | 0.33 | 0.66 | 0.16–2.64 | 0.56 |
| SLC22A2 rs316019 | ||||||
| CC | 1 # | 1 # | ||||
| AC | 3.24 | 1.36–7.74 | 0.008 * | 5.06 | 1.69–15.16 | 0.004 * |
| AA | 9.18 | 0.80–105.80 | 0.08 | 38.12 | 1.89–767.51 | 0.02 |
| Gene–SNP | OR | 95% CI | p-Value | ORadj | 95% CIadj | p-Valueadj | Cohcran-Armitage Trend Test p-Value |
|---|---|---|---|---|---|---|---|
| ERCC1 rs11615 GG vs. GA vs. AA | 0.78 | 0.46–1.33 | 0.364 | 0.92 | 0.50–1.68 | 0.777 | 0.368 |
| ERCC1 rs3212986 AA vs. CA vs. CC | 0.57 | 0.30–1.06 | 0.077 | 0.52 | 0.26–1.07 | 0.076 | 0.067 |
| ERCC2 rs13181 CC vs. CA vs. AA | 1.84 | 1.07–3.15 | 0.027 | 1.39 | 0.75–2.58 | 0.293 | 0.039 * |
| ERCC2 rs1799793 CC vs. CA vs. AA | 0.81 | 0.48–1.38 | 0.447 | 0.85 | 0.47–1.53 | 0.578 | 0.473 |
| SLC22A2 rs316019 AA vs. CA vs. CC | 3.29 | 1.60–6.81 | 0.001 ** | 4.41 | 1.96–9.88 | <0.001 ** | 0.001 ** |
| Gene–SNP | ∆SCr a | ∆eGFR b | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | p-Value | R2adj | p-Value adj | R2 | p-Value | R2adj | p-Value adj | |
| ERCC1 rs11615 GG vs. GA vs. AA | 0.01 | 0.218 | 0.055 | 0.17 | 0.006 | 0.347 | 0.042 | 0.20 |
| ERCC1 rs3212986 AA vs. CA vs. CC | 0.008 | 0.268 | 0.058 | 0.16 | 0.013 | 0.167 | 0.052 | 0.12 |
| ERCC2 rs13181 CC vs. CA vs. AA | 0.001 | 0.652 | 0.046 | 0.28 | 0 | 0.796 | 0.035 | 0.29 |
| ERCC2 rs1799793 CC vs. CA vs. AA | 0.001 | 0.77 | 0.046 | 0.27 | 0.001 | 0.668 | 0.036 | 0.29 |
| SLC22A2 rs316019 AA vs. CA vs. CC | 0.002 | 0.599 | 0.047 | 0.27 | 0.006 | 0.343 | 0.039 | 0.25 |
| Adjusted-AKI | CTCAE-AKI | ΔeGFR | ΔSCr | |
|---|---|---|---|---|
| Basis of Determination | SCr + Mg/K/PO4/Na | SCr | CKD-EPI equation (SCr+age+sex+ethnicity) | SCr |
| Data Characteristics | Categorical | Categorical | Continuous | Continuous |
| Advantage | Tailored on cisplatin-induced nephrotoxicity | • Mostly used in clinics and studies in cancer subjects • Easily calculated | • Easily calculated • CKD-EPI is the equation recommended by KDIGO | • Routinely measured in patients |
| Disadvantage | • Not comparable with other studies • Not validated yet | • Is ≥ grade 1 cut-off clinically relevant? • SCr often increase late resulting in failing to detect early stage nephrotoxicity | • Could not correct for cystatin-C due to unavailable data in routine practice • Disregarding the clinical value of baseline eGFR | • Highly influenced by various individual factors (e.g., age, gender, body weight, diet etc.) • SCr often increase late resulting in failing to detect early stage nephrotoxicity • Disregarding the clinical value of baseline SCr |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zazuli, Z.; Otten, L.S.; Drögemöller, B.I.; Medeiros, M.; Monzon, J.G.; Wright, G.E.B.; Kollmannsberger, C.K.; Bedard, P.L.; Chen, Z.; Gelmon, K.A.; et al. Outcome Definition Influences the Relationship between Genetic Polymorphisms of ERCC1, ERCC2, SLC22A2 and Cisplatin Nephrotoxicity in Adult Testicular Cancer Patients. Genes 2019, 10, 364. https://doi.org/10.3390/genes10050364
Zazuli Z, Otten LS, Drögemöller BI, Medeiros M, Monzon JG, Wright GEB, Kollmannsberger CK, Bedard PL, Chen Z, Gelmon KA, et al. Outcome Definition Influences the Relationship between Genetic Polymorphisms of ERCC1, ERCC2, SLC22A2 and Cisplatin Nephrotoxicity in Adult Testicular Cancer Patients. Genes. 2019; 10(5):364. https://doi.org/10.3390/genes10050364
Chicago/Turabian StyleZazuli, Zulfan, Leila S. Otten, Britt I. Drögemöller, Mara Medeiros, Jose G. Monzon, Galen E. B. Wright, Christian K. Kollmannsberger, Philippe L. Bedard, Zhuo Chen, Karen A. Gelmon, and et al. 2019. "Outcome Definition Influences the Relationship between Genetic Polymorphisms of ERCC1, ERCC2, SLC22A2 and Cisplatin Nephrotoxicity in Adult Testicular Cancer Patients" Genes 10, no. 5: 364. https://doi.org/10.3390/genes10050364
APA StyleZazuli, Z., Otten, L. S., Drögemöller, B. I., Medeiros, M., Monzon, J. G., Wright, G. E. B., Kollmannsberger, C. K., Bedard, P. L., Chen, Z., Gelmon, K. A., McGoldrick, N., Kitchlu, A., Vijverberg, S. J. H., Masereeuw, R., Ross, C. J. D., Liu, G., Carleton, B. C., & Maitland-van der Zee, A. H. (2019). Outcome Definition Influences the Relationship between Genetic Polymorphisms of ERCC1, ERCC2, SLC22A2 and Cisplatin Nephrotoxicity in Adult Testicular Cancer Patients. Genes, 10(5), 364. https://doi.org/10.3390/genes10050364







