The Role of Hepatitis B Core-Related Antigen
Abstract
:1. Introduction
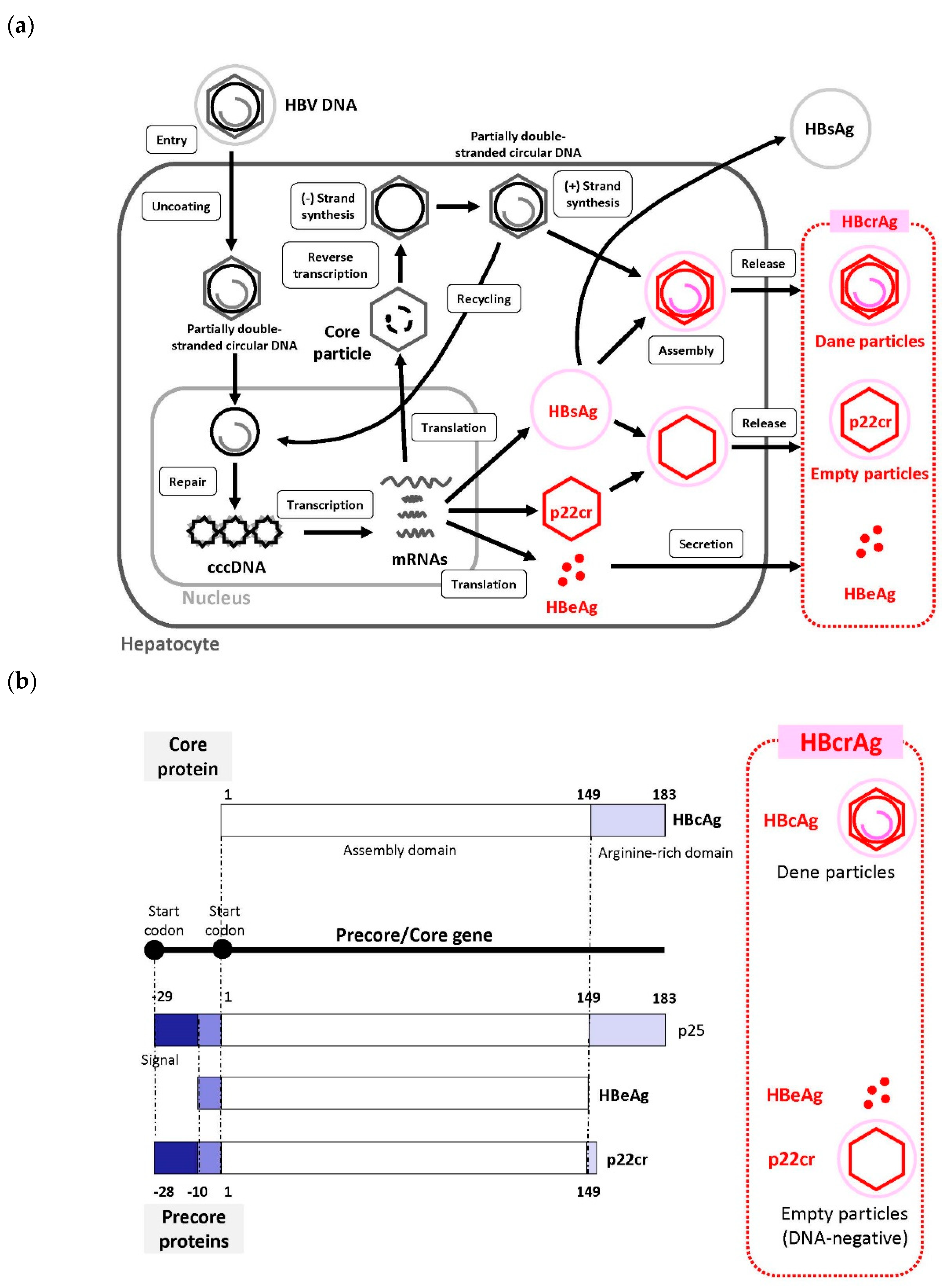
2. HBV Life Cycle
3. HBcrAg and Its Measurement
3.1. What Is HBcrAg?
3.2. HBcrAg Measurement
4. Correlation between HBcrAg and Other HBV Markers
4.1. Serum or Intrahepatic HBV DNA Levels
4.2. Intrahepatic cccDNA Levels
4.3. HBsAg
4.4. HBV RNA
4.5. HBV Gene Deletion
5. CHB Natural History Including HBeAg Seroconversion
5.1. HBcrAg in Different Stages of Chronic Infection
5.2. Prediction of HBeAg Seroconversion
5.3. Prediction of HBsAg Seroconversion
6. Anti-HBV Treatment
6.1. Change in HBcrAg and Other HBV Markers under NA Therapy
6.2. NA Cessation Point
6.3. HBcrAg and HBV RNA Related to NA Treatment
7. HCC Development
7.1. HCC Occurrence
7.2. HCC Recurrence
8. HBV Reactivation
9. HBV Reinfection after Liver Transplantation
10. Upcoming Anti-HBV Strategies
10.1. Capsid Assembly Modifier
10.2. RNA Interference (RNAi)-Based Therapeutic ARC-520
10.3. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-CRISPR Associated Protein 9 (Cas9)
10.4. Other Anti-HBV Strategies
11. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kao, J.H. Hepatitis B vaccination and prevention of hepatocellular carcinoma. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 907–917. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Factsheets for Chronic Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 1 April 2019).
- Lai, C.L.; Yuen, M.F. The natural history and treatment of chronic hepatitis B: A critical evaluation of standard treatment criteria and end points. Ann. Intern. Med. 2007, 147, 58–61. [Google Scholar] [CrossRef]
- Kao, J.H.; Chen, D.S. Global control of hepatitis B virus infection. Lancet Infect. Dis. 2002, 2, 395–403. [Google Scholar] [CrossRef]
- Fattovich, G.; Bortolotti, F.; Donato, F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors. J. Hepatol. 2008, 48, 335–352. [Google Scholar] [CrossRef]
- Inoue, T.; Tanaka, Y. Hepatitis B Virus and Its Sexually Transmitted Infection—An Update. Microb. Cell 2016, 3, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, G.; Allain, J.P.; Brunetto, M.R.; Buendia, M.A.; Chen, D.S.; Colombo, M.; Craxi, A.; Donato, F.; Ferrari, C.; Gaeta, G.B.; et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J. Hepatol. 2008, 49, 652–657. [Google Scholar] [CrossRef]
- Brechot, C.; Thiers, V.; Kremsdorf, D.; Nalpas, B.; Pol, S.; Paterlini-Brechot, P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: Clinically significant or purely “occult”? Hepatology 2001, 34, 194–203. [Google Scholar] [CrossRef]
- Bonilla Guerrero, R.; Roberts, L.R. The role of hepatitis B virus integrations in the pathogenesis of human hepatocellular carcinoma. J. Hepatol. 2005, 42, 760–777. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Bzowej, N.H.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Murad, M.H. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016, 63, 261–283. [Google Scholar] [CrossRef] [PubMed]
- EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [CrossRef] [PubMed]
- Lin, C.L.; Kao, J.H. Perspectives and control of hepatitis B virus infection in Taiwan. J. Formos. Med. Assoc. 2015, 114, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Kao, J.H. Looking into the crystal ball: Biomarkers for outcomes of HBV infection. Hepatol. Int. 2016, 10, 99–101. [Google Scholar] [CrossRef]
- Chen, C.J.; Yang, H.I.; Su, J.; Jen, C.L.; You, S.L.; Lu, S.N.; Huang, G.T.; Iloeje, U.H. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006, 295, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Iloeje, U.H.; Yang, H.I.; Su, J.; Jen, C.L.; You, S.L.; Chen, C.J. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006, 130, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.C.; Liu, C.J.; Yang, H.C.; Su, T.H.; Wang, C.C.; Chen, C.L.; Hsu, C.A.; Kuo, S.F.; Liu, C.H.; Chen, P.J.; et al. Serum hepatitis B surface antigen levels help predict disease progression in patients with low hepatitis B virus loads. Hepatology 2013, 57, 441–450. [Google Scholar] [CrossRef]
- Yuen, M.F.; Wong, D.K.; Fung, J.; Ip, P.; But, D.; Hung, I.; Lau, K.; Yuen, J.C.; Lai, C.L. HBsAg Seroclearance in chronic hepatitis B in Asian patients: Replicative level and risk of hepatocellular carcinoma. Gastroenterology 2008, 135, 1192–1199. [Google Scholar] [CrossRef]
- Chen, J.D.; Yang, H.I.; Iloeje, U.H.; You, S.L.; Lu, S.N.; Wang, L.Y.; Su, J.; Sun, C.A.; Liaw, Y.F.; Chen, C.J. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology 2010, 138, 1747–1754. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.W.; Chen, L.; Hu, P.; Ren, H.; Hu, H.D. Systematic review with meta-analysis: Development of hepatocellular carcinoma in chronic hepatitis B patients with hepatitis B surface antigen seroclearance. Aliment. Pharmacol. Ther. 2016, 43, 1253–1261. [Google Scholar] [CrossRef]
- Kim, G.A.; Lee, H.C.; Kim, M.J.; Ha, Y.; Park, E.J.; An, J.; Lee, D.; Shim, J.H.; Kim, K.M.; Lim, Y.S. Incidence of hepatocellular carcinoma after HBsAg seroclearance in chronic hepatitis B patients: A need for surveillance. J. Hepatol. 2015, 62, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Honer Zu Siederdissen, C.; Maasoumy, B.; Cornberg, M. New viral biomarkers for Hepatitis B: Are we able to change practice? J. Viral Hepat. 2018, 25, 1226–1235. [Google Scholar] [CrossRef]
- Mak, L.Y.; Seto, W.K.; Fung, J.; Yuen, M.F. New Biomarkers of Chronic Hepatitis B. Gut Liver 2019. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, N.; Matsuura, K.; Sugauchi, F.; Watanabe, T.; Murakami, S.; Iio, E.; Ogawa, S.; Nojiri, S.; Joh, T.; Tanaka, Y. Application of a newly developed high-sensitivity HBsAg chemiluminescent enzyme immunoassay for hepatitis B patients with HBsAg seroclearance. J. Clin. Microbiol. 2013, 51, 3484–3491. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, N.; Kusumoto, S.; Murakami, S.; Ogawa, S.; Ri, M.; Matsui, T.; Tamori, A.; Toyoda, H.; Ishida, T.; Iida, S.; et al. Novel monitoring of hepatitis B reactivation based on ultra-high sensitive hepatitis B surface antigen assay. Liver Int. 2017, 37, 1138–1147. [Google Scholar] [CrossRef]
- Seto, W.K.; Wong, D.K.; Fung, J.; Huang, F.Y.; Liu, K.S.; Lai, C.L.; Yuen, M.F. Linearized hepatitis B surface antigen and hepatitis B core-related antigen in the natural history of chronic hepatitis B. Clin. Microbiol. Infect. 2014, 20, 1173–1180. [Google Scholar] [CrossRef]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Correction: Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 2014, 3, e05570. [Google Scholar] [CrossRef]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 2012, 1, e00049. [Google Scholar] [CrossRef]
- Yang, H.C.; Kao, J.H. Persistence of hepatitis B virus covalently closed circular DNA in hepatocytes: Molecular mechanisms and clinical significance. Emerg. Microbes Infect. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Gerlich, W.H. Medical virology of hepatitis B: How it began and where we are now. Virol. J. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Seeger, C.; Mason, W.S. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 2000, 64, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Tuttleman, J.S.; Pourcel, C.; Summers, J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell 1986, 47, 451–460. [Google Scholar] [CrossRef]
- Kimura, T.; Rokuhara, A.; Sakamoto, Y.; Yagi, S.; Tanaka, E.; Kiyosawa, K.; Maki, N. Sensitive enzyme immunoassay for hepatitis B virus core-related antigens and their correlation to virus load. J. Clin. Microbiol. 2002, 40, 439–445. [Google Scholar] [CrossRef]
- Rokuhara, A.; Tanaka, E.; Matsumoto, A.; Kimura, T.; Yamaura, T.; Orii, K.; Sun, X.; Yagi, S.; Maki, N.; Kiyosawa, K. Clinical evaluation of a new enzyme immunoassay for hepatitis B virus core-related antigen; a marker distinct from viral DNA for monitoring lamivudine treatment. J. Viral Hepat. 2003, 10, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, F.; Miyakoshi, H.; Kobayashi, M.; Kumada, H. Correlation between serum hepatitis B virus core-related antigen and intrahepatic covalently closed circular DNA in chronic hepatitis B patients. J. Med. Virol. 2009, 81, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Maasoumy, B.; Wiegand, S.B.; Jaroszewicz, J.; Bremer, B.; Lehmann, P.; Deterding, K.; Taranta, A.; Manns, M.P.; Wedemeyer, H.; Glebe, D.; et al. Hepatitis B core-related antigen (HBcrAg) levels in the natural history of hepatitis B virus infection in a large European cohort predominantly infected with genotypes A and D. Clin. Microbiol. Infect. 2015, 21, 606.e601–606.e610. [Google Scholar] [CrossRef]
- Kimura, T.; Ohno, N.; Terada, N.; Rokuhara, A.; Matsumoto, A.; Yagi, S.; Tanaka, E.; Kiyosawa, K.; Ohno, S.; Maki, N. Hepatitis B virus DNA-negative dane particles lack core protein but contain a 22-kDa precore protein without C-terminal arginine-rich domain. J. Biol. Chem. 2005, 280, 21713–21719. [Google Scholar] [CrossRef]
- Hadziyannis, E.; Laras, A. Viral Biomarkers in Chronic HBeAg Negative HBV Infection. Genes 2018, 9, 469. [Google Scholar] [CrossRef]
- Lin, C.L.; Kao, J.H. New perspectives of biomarkers for the management of chronic hepatitis B. Clin. Mol. Hepatol. 2016, 22, 423–431. [Google Scholar] [CrossRef]
- Mak, L.Y.; Wong, D.K.; Cheung, K.S.; Seto, W.K.; Lai, C.L.; Yuen, M.F. Review article: Hepatitis B core-related antigen (HBcrAg): An emerging marker for chronic hepatitis B virus infection. Aliment. Pharmacol. Ther. 2018, 47, 43–54. [Google Scholar] [CrossRef]
- Wong, D.K.; Tanaka, Y.; Lai, C.L.; Mizokami, M.; Fung, J.; Yuen, M.F. Hepatitis B virus core-related antigens as markers for monitoring chronic hepatitis B infection. J. Clin. Microbiol. 2007, 45, 3942–3947. [Google Scholar] [CrossRef]
- Rokuhara, A.; Sun, X.; Tanaka, E.; Kimura, T.; Matsumoto, A.; Yao, D.; Yin, L.; Wang, N.; Maki, N.; Kiyosawa, K. Hepatitis B virus core and core-related antigen quantitation in Chinese patients with chronic genotype B and C hepatitis B virus infection. J. Gastroenterol. Hepatol. 2005, 20, 1726–1730. [Google Scholar] [CrossRef]
- Wong, D.K.; Seto, W.K.; Cheung, K.S.; Chong, C.K.; Huang, F.Y.; Fung, J.; Lai, C.L.; Yuen, M.F. Hepatitis B virus core-related antigen as a surrogate marker for covalently closed circular DNA. Liver Int. 2017, 37, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.K.; Yuen, M.F.; Yuan, H.; Sum, S.S.; Hui, C.K.; Hall, J.; Lai, C.L. Quantitation of covalently closed circular hepatitis B virus DNA in chronic hepatitis B patients. Hepatology 2004, 40, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Q.; Feng, S.; Wang, M.L.; Liang, L.B.; Zhou, L.Y.; Du, L.Y.; Yan, L.B.; Tao, C.M.; Tang, H. Serum hepatitis B core-related antigen is a satisfactory surrogate marker of intrahepatic covalently closed circular DNA in chronic hepatitis B. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Matsumoto, A.; Suzuki, F.; Kobayashi, M.; Mizokami, M.; Tanaka, Y.; Okanoue, T.; Minami, M.; Chayama, K.; Imamura, M.; et al. Measurement of hepatitis B virus core-related antigen is valuable for identifying patients who are at low risk of lamivudine resistance. Liver Int. 2006, 26, 90–96. [Google Scholar] [CrossRef]
- Wang, L.; Cao, X.; Wang, Z.; Gao, Y.; Deng, J.; Liu, X.; Zhuang, H. Correlation of HBcrAg with Intrahepatic Hepatitis B Virus Total DNA and Covalently Closed Circular DNA in HBeAg-Positive Chronic Hepatitis B Patients. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Hasegawa, K.; Nishikawa, H.; Enomoto, H.; Iwata, Y.; Sakai, Y.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; Yoh, K.; et al. Proposed model for the prediction of intrahepatic covalently closed circular DNA level in patients with chronic hepatitis B. Hepatol. Res. 2018. [Google Scholar] [CrossRef]
- Ganem, D.; Prince, A.M. Hepatitis B virus infection--natural history and clinical consequences. N. Engl. J. Med. 2004, 350, 1118–1129. [Google Scholar] [CrossRef]
- Schadler, S.; Hildt, E. HBV life cycle: Entry and morphogenesis. Viruses 2009, 1, 185–209. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liang, X.S. Progression and status of antiviral monitoring in patients with chronic hepatitis B: From HBsAg to HBV RNA. World J. Hepatol. 2018, 10, 603–611. [Google Scholar] [CrossRef]
- Honer Zu Siederdissen, C.; Maasoumy, B.; Cornberg, M. What is new on HBsAg and other diagnostic markers in HBV infection? Best Pract. Res. Clin. Gastroenterol. 2017, 31, 281–289. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, B.; Valdes, J.D.; Sun, J.; Guo, H. Serum HBV RNA: A New Potential Biomarker for Chronic Hepatitis B Virus Infection. Hepatology 2018. [Google Scholar] [CrossRef]
- Liao, H.; Liu, Y.; Li, X.; Wang, J.; Chen, X.; Zou, J.; Li, Q.; Liu, L.; Wang, J.; Huang, B.; et al. Monitoring of serum HBV RNA, HBcrAg, HBsAg and anti-HBc levels in patients during long-term nucleoside/nucleotide analogue therapy. Antivir. Ther. 2018. [Google Scholar] [CrossRef]
- Huang, H.; Wang, J.; Li, W.; Chen, R.; Chen, X.; Zhang, F.; Xu, D.; Lu, F. Serum HBV DNA plus RNA shows superiority in reflecting the activity of intrahepatic cccDNA in treatment-naive HBV-infected individuals. J. Clin. Virol. 2018, 99–100, 71–78. [Google Scholar] [CrossRef]
- Chen, E.Q.; Wang, M.L.; Tao, Y.C.; Wu, D.B.; Liao, J.; He, M.; Tang, H. Serum HBcrAg is better than HBV RNA and HBsAg in reflecting intrahepatic covalently closed circular DNA. J. Viral Hepat. 2019, 26, 586–595. [Google Scholar] [CrossRef]
- Suzuki, Y.; Maekawa, S.; Komatsu, N.; Sato, M.; Tatsumi, A.; Miura, M.; Matsuda, S.; Muraoka, M.; Nakakuki, N.; Amemiya, F.; et al. HBV preS deletion mapping using deep sequencing demonstrates a unique association with viral markers. PLoS ONE 2019, 14, e0212559. [Google Scholar] [CrossRef]
- Loggi, E.; Vukotic, R.; Conti, F.; Grandini, E.; Gitto, S.; Cursaro, C.; Galli, S.; Furlini, G.; Re, M.C.; Andreone, P. Serum hepatitis B core-related antigen is an effective tool to categorize patients with HBeAg-negative chronic hepatitis B. J. Viral Hepat. 2018, 26, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N.; Matsumoto, A.; Tanaka, E.; Rokuhara, A.; Yoshizawa, K.; Umemura, T.; Maki, N.; Kimura, T.; Kiyosawa, K. Patients with and without loss of hepatitis B virus DNA after hepatitis B e antigen seroconversion have different virological characteristics. J. Med. Virol. 2006, 78, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Testoni, B.; Lebosse, F.; Scholtes, C.; Berby, F.; Miaglia, C.; Subic, M.; Loglio, A.; Facchetti, F.; Lampertico, P.; Levrero, M.; et al. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J. Hepatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Lu, W.; Wang, Y.B.; Weng, Q.C.; Zhang, Z.Y.; Yang, Z.Q.; Feng, Y.L. Measurement of the hepatitis B core-related antigen is valuable for predicting the pathological status of liver tissues in chronic hepatitis B patients. J. Virol. Methods 2016, 235, 92–98. [Google Scholar] [CrossRef]
- Seto, W.K.; Tanaka, Y.; Wong, D.K.; Lai, C.L.; Shinkai, N.; Yuen, J.C.; Tong, T.; Fung, J.; Hung, I.F.; Yuen, M.F. Evidence of serologic activity in chronic hepatitis B after surface antigen (HBsAg) seroclearance documented by conventional HBsAg assay. Hepatol. Int. 2012, 7, 98–105. [Google Scholar] [CrossRef]
- Chuaypen, N.; Posuwan, N.; Payungporn, S.; Tanaka, Y.; Shinkai, N.; Poovorawan, Y.; Tangkijvanich, P. Serum hepatitis B core-related antigen as a treatment predictor of pegylated interferon in patients with HBeAg-positive chronic hepatitis B. Liver Int. 2016, 36, 827–836. [Google Scholar] [CrossRef] [PubMed]
- JSH Guidelines for the Management of Hepatitis B Virus Infection. Hepatol. Res. 2014, 44 (Suppl. S1), 1–58.
- Lai, C.L.; Wong, D.; Ip, P.; Kopaniszen, M.; Seto, W.K.; Fung, J.; Huang, F.Y.; Lee, B.; Cullaro, G.; Chong, C.K.; et al. Reduction of covalently closed circular DNA with long-term nucleos(t)ide analogue treatment in chronic hepatitis B. J. Hepatol. 2017, 66, 275–281. [Google Scholar] [CrossRef]
- Lutgehetmann, M.; Volzt, T.; Quaas, A.; Zankel, M.; Fischer, C.; Dandri, M.; Petersen, J. Sequential combination therapy leads to biochemical and histological improvement despite low ongoing intrahepatic hepatitis B virus replication. Antivir. Ther. 2008, 13, 57–66. [Google Scholar] [PubMed]
- Ma, H.; Yang, R.F.; Li, X.H.; Jin, Q.; Wei, L. HBcrAg Identifies Patients Failing to Achieve HBeAg Seroconversion Treated with Pegylated Interferon Alfa-2b. Chin. Med. J. (Engl.) 2016, 129, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Abate, M.L.; Noviello, D.; Olivero, A.; Rosso, C.; Troshina, G.; Ciancio, A.; Rizzetto, M.; Saracco, G.M.; Smedile, A. Hepatitis B core-related antigen kinetics in chronic hepatitis B virus genotype D-infected patients treated with nucleos(t)ide analogues or pegylated-interferon-alpha. Hepatol. Res. 2017, 47, 747–754. [Google Scholar] [CrossRef]
- Matsumoto, A.; Yatsuhashi, H.; Nagaoka, S.; Suzuki, Y.; Hosaka, T.; Tsuge, M.; Chayama, K.; Kanda, T.; Yokosuka, O.; Nishiguchi, S.; et al. Factors associated with the effect of interferon-alpha sequential therapy in order to discontinue nucleoside/nucleotide analog treatment in patients with chronic hepatitis B. Hepatol. Res. 2015, 45, 1195–1202. [Google Scholar] [CrossRef]
- Martinot-Peignoux, M.; Lapalus, M.; Maylin, S.; Boyer, N.; Castelnau, C.; Giuily, N.; Pouteau, M.; Moucari, R.; Asselah, T.; Marcellin, P. Baseline HBsAg and HBcrAg titres allow peginterferon-based ’precision medicine’ in HBeAg-negative chronic hepatitis B patients. J. Viral Hepat. 2016, 23, 905–911. [Google Scholar] [CrossRef]
- Wang, B.; Carey, I.; Bruce, M.; Montague, S.; Dusheiko, G.; Agarwal, K. HBsAg and HBcrAg as predictors of HBeAg seroconversion in HBeAg-positive patients treated with nucleos(t)ide analogues. J. Viral Hepat. 2018, 25, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Deng, R.; Chen, E.Q.; Tao, C.M.; Liao, J.; Zhou, T.Y.; Wang, J.; Tang, H. Performance of serum HBcrAg in chronic hepatitis B patients with 8-year nucleos(t)ide analogs therapy. Clin. Res. Hepatol. Gastroenterol. 2018. [Google Scholar] [CrossRef]
- Jung, K.S.; Park, J.Y.; Chon, Y.E.; Kim, H.S.; Kang, W.; Kim, B.K.; Kim, S.U.; Kim do, Y.; Han, K.H.; Ahn, S.H. Clinical outcomes and predictors for relapse after cessation of oral antiviral treatment in chronic hepatitis B patients. J. Gastroenterol. 2016, 51, 830–839. [Google Scholar] [CrossRef]
- Matsumoto, A.; Tanaka, E.; Minami, M.; Okanoue, T.; Yatsuhashi, H.; Nagaoka, S.; Suzuki, F.; Kobayashi, M.; Chayama, K.; Imamura, M.; et al. Low serum level of hepatitis B core-related antigen indicates unlikely reactivation of hepatitis after cessation of lamivudine therapy. Hepatol. Res. 2007, 37, 661–666. [Google Scholar] [CrossRef]
- Shinkai, N.; Tanaka, Y.; Orito, E.; Ito, K.; Ohno, T.; Hirashima, N.; Hasegawa, I.; Sugauchi, F.; Ueda, R.; Mizokami, M. Measurement of hepatitis B virus core-related antigen as predicting factor for relapse after cessation of lamivudine therapy for chronic hepatitis B virus infection. Hepatol. Res. 2006, 36, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Nguyen, M.H.; Mo, L.R.; Wu, M.S.; Yang, T.H.; Chen, C.C.; Tseng, C.H.; Tai, C.M.; Wu, C.Y.; Lin, J.T.; et al. Combining hepatitis B core-related and surface antigens at end of nucleos(t)ide analogue treatment to predict off-therapy relapse risk. Aliment. Pharmacol. Ther. 2019, 49, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Suzuki, F.; Kobayashi, M.; Fujiyama, S.; Kawamura, Y.; Sezaki, H.; Akuta, N.; Suzuki, Y.; Saitoh, S.; Arase, Y.; et al. Impact of hepatitis B core-related antigen on the incidence of hepatocellular carcinoma in patients treated with nucleos(t)ide analogues. Aliment. Pharmacol. Ther. 2019, 49, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Kumada, T.; Toyoda, H.; Tada, T.; Kiriyama, S.; Tanikawa, M.; Hisanaga, Y.; Kanamori, A.; Niinomi, T.; Yasuda, S.; Andou, Y.; et al. Effect of nucleos(t)ide analogue therapy on hepatocarcinogenesis in chronic hepatitis B patients: A propensity score analysis. J. Hepatol. 2013, 58, 427–433. [Google Scholar] [CrossRef]
- Honda, M.; Shirasaki, T.; Terashima, T.; Kawaguchi, K.; Nakamura, M.; Oishi, N.; Wang, X.; Shimakami, T.; Okada, H.; Arai, K.; et al. Hepatitis B Virus (HBV) Core-Related Antigen During Nucleos(t)ide Analog Therapy Is Related to Intra-hepatic HBV Replication and Development of Hepatocellular Carcinoma. J. Infect. Dis. 2016, 213, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Toyoda, H.; Kiriyama, S.; Tanikawa, M.; Hisanaga, Y.; Kanamori, A.; Kitabatake, S.; Yama, T.; Tanaka, J. HBcrAg predicts hepatocellular carcinoma development: An analysis using time-dependent receiver operating characteristics. J. Hepatol. 2016, 65, 48–56. [Google Scholar] [CrossRef]
- Chen, S.; Jia, J.; Gao, Y.; Li, H.; Fang, M.; Feng, H.; Guan, W.; Ji, J.; Gao, Z.; Gao, C. Clinical evaluation of hepatitis B core-related antigen in chronic hepatitis B and hepatocellular carcinoma patients. Clin. Chim. Acta 2018, 486, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.L.; Wong, V.W. Risk prediction of hepatitis B virus-related hepatocellular carcinoma in the era of antiviral therapy. World J. Gastroenterol. 2013, 19, 6515–6522. [Google Scholar] [CrossRef]
- Cheung, K.S.; Seto, W.K.; Wong, D.K.; Lai, C.L.; Yuen, M.F. Relationship between HBsAg, HBcrAg and hepatocellular carcinoma in patients with undetectable HBV DNA under nucleos(t)ide therapy. J. Viral Hepat. 2017, 24, 654–661. [Google Scholar] [CrossRef]
- Suzuki, Y.; Maekawa, S.; Komatsu, N.; Sato, M.; Tatsumi, A.; Miura, M.; Matsuda, S.; Muraoka, M.; Nakakuki, N.; Shindo, H.; et al. Hepatitis B virus (HBV)-infected patients with low hepatitis B surface antigen and high hepatitis B core-related antigen titers have a high risk of HBV-related hepatocellular carcinoma. Hepatol. Res. 2019, 49, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Lin, J.T.; Zeng, Y.S.; Chen, Y.J.; Wu, M.S.; Wu, C.Y. Association between nucleos(t)ide analog and tumor recurrence in hepatitis B virus-related hepatocellular carcinoma after radiofrequency ablation. Hepatology 2016, 63, 1517–1527. [Google Scholar] [CrossRef]
- Huang, G.; Lau, W.Y.; Zhou, W.P.; Shen, F.; Pan, Z.Y.; Yuan, S.X.; Wu, M.C. Prediction of hepatocellular carcinoma recurrence in patients with low hepatitis B Virus DNA levels and high preoperative hepatitis B surface antigen levels. JAMA Surgery 2014, 149, 519–527. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Luo, Y.; Chen, W.D.; Gong, G.Z. Hepatitis B virus mutation may play a role in hepatocellular carcinoma recurrence: A systematic review and meta-regression analysis. J. Gastroenterol. Hepatol. 2015, 30, 977–983. [Google Scholar] [CrossRef]
- Urabe, A.; Imamura, M.; Tsuge, M.; Kan, H.; Fujino, H.; Fukuhara, T.; Masaki, K.; Kobayashi, T.; Ono, A.; Nakahara, T.; et al. The relationship between HBcrAg and HBV reinfection in HBV related post-liver transplantation patients. J. Gastroenterol. 2017, 52, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Seto, W.K.; Wong, D.K.; Chan, T.S.; Hwang, Y.Y.; Fung, J.; Liu, K.S.; Gill, H.; Lam, Y.F.; Cheung, K.S.; Lie, A.K.; et al. Association of Hepatitis B Core-Related Antigen with Hepatitis B Virus Reactivation in Occult Viral Carriers Undergoing High-Risk Immunosuppressive Therapy. Am. J. Gastroenterol. 2016, 111, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Sevastianos, V.; Burroughs, A.K. Prevention of and treatment for hepatitis B virus infection after liver transplantation in the nucleoside analogues era. Am. J. Transplant. 2003, 3, 250–258. [Google Scholar] [CrossRef]
- Ferretti, G.; Merli, M.; Ginanni Corradini, S.; Callejon, V.; Tanzilli, P.; Masini, A.; Ferretti, S.; Iappelli, M.; Rossi, M.; Rivanera, D.; et al. Low-dose intramuscular hepatitis B immune globulin and lamivudine for long-term prophylaxis of hepatitis B recurrence after liver transplantation. Transplant. Proc. 2004, 36, 535–538. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Tatsuki, I.; Otani, M.; Akiyama, M.; Ozawa, E.; Miuma, S.; Miyaaki, H.; Taura, N.; Hayashi, T.; Okudaira, S.; et al. Significance of hepatitis B virus core-related antigen and covalently closed circular DNA levels as markers of hepatitis B virus re-infection after liver transplantation. J. Gastroenterol. Hepatol. 2013, 28, 1217–1222. [Google Scholar] [CrossRef]
- Fujimoto, M.; Ichikawa, T.; Nakao, K.; Miyaaki, H.; Shibata, H.; Eguchi, S.; Takatsuki, M.; Nagaoka, S.; Yatsuhashi, H.; Kanematsu, T.; et al. The significance of enzyme immunoassay for the assessment of hepatitis B virus core-related antigen following liver transplantation. Intern. Med. 2009, 48, 1577–1583. [Google Scholar] [CrossRef]
- Yasunaka, T.; Takaki, A.; Yagi, T.; Iwasaki, Y.; Sadamori, H.; Koike, K.; Hirohata, S.; Tatsukawa, M.; Kawai, D.; Shiraha, H.; et al. Serum hepatitis B virus DNA before liver transplantation correlates with HBV reinfection rate even under successful low-dose hepatitis B immunoglobulin prophylaxis. Hepatol. Int. 2011, 5, 918–926. [Google Scholar] [CrossRef]
- Liu, S.H.; Seto, W.K.; Lai, C.L.; Yuen, M.F. Hepatitis B: Treatment choice and monitoring for response and resistance. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 697–707. [Google Scholar] [CrossRef]
- Corcuera, A.; Stolle, K.; Hillmer, S.; Seitz, S.; Lee, J.Y.; Bartenschlager, R.; Birkmann, A.; Urban, A. Novel non-heteroarylpyrimidine (HAP) capsid assembly modifiers have a different mode of action from HAPs in vitro. Antivir. Res. 2018, 158, 135–142. [Google Scholar] [CrossRef]
- Berke, J.M.; Tan, Y.; Verbinnen, T.; Dehertogh, P.; Vergauwen, K.; Vos, A.; Lenz, O.; Pauwels, F. Antiviral profiling of the capsid assembly modulator BAY41-4109 on full-length HBV genotype A-H clinical isolates and core site-directed mutants in vitro. Antivir. Res. 2017, 144, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Wooddell, C.I.; Rozema, D.B.; Hossbach, M.; John, M.; Hamilton, H.L.; Chu, Q.; Hegge, J.O.; Klein, J.J.; Wakefield, D.H.; Oropeza, C.E.; et al. Hepatocyte-targeted RNAi therapeutics for the treatment of chronic hepatitis B virus infection. Mol. Ther. 2013, 21, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Klein, J.J.; Hamilton, H.L.; Chu, Q.; Frey, C.L.; Trubetskoy, V.S.; Hegge, J.; Wakefield, D.; Rozema, D.B.; Lewis, D.L. Co-injection of a targeted, reversibly masked endosomolytic polymer dramatically improves the efficacy of cholesterol-conjugated small interfering RNAs in vivo. Nucl. Acid Ther. 2012, 22, 380–390. [Google Scholar] [CrossRef]
- Gish, R.G.; Yuen, M.F.; Chan, H.L.; Given, B.D.; Lai, C.L.; Locarnini, S.A.; Lau, J.Y.; Wooddell, C.I.; Schluep, T.; Lewis, D.L. Synthetic RNAi triggers and their use in chronic hepatitis B therapies with curative intent. Antivir. Res. 2015, 121, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Trubetskoy, V.S.; Griffin, J.B.; Nicholas, A.L.; Nord, E.M.; Xu, Z.; Peterson, R.M.; Wooddell, C.I.; Rozema, D.B.; Wakefield, D.H.; Lewis, D.L.; et al. Phosphorylation-specific status of RNAi triggers in pharmacokinetic and biodistribution analyses. Nucl. Acids Res. 2017, 45, 1469–1478. [Google Scholar] [CrossRef]
- Wooddell, C.I.; Yuen, M.F.; Chan, H.L.; Gish, R.G.; Locarnini, S.A.; Chavez, D.; Ferrari, C.; Given, B.D.; Hamilton, J.; Kanner, S.B.; et al. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Sakuma, T.; Masaki, K.; Abe-Chayama, H.; Mochida, K.; Yamamoto, T.; Chayama, K. Highly multiplexed CRISPR-Cas9-nuclease and Cas9-nickase vectors for inactivation of hepatitis B virus. Genes Cells 2016, 21, 1253–1262. [Google Scholar] [CrossRef]
- Lin, S.R.; Yang, H.C.; Kuo, Y.T.; Liu, C.J.; Yang, T.Y.; Sung, K.C.; Lin, Y.Y.; Wang, H.Y.; Wang, C.C.; Shen, Y.C.; et al. The CRISPR/Cas9 System Facilitates Clearance of the Intrahepatic HBV Templates In Vivo. Mol. Ther. Nucl. Acids 2014, 3, e186. [Google Scholar] [CrossRef]
- Dong, C.; Qu, L.; Wang, H.; Wei, L.; Dong, Y.; Xiong, S. Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antivir. Res. 2015, 118, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hensel, K.O.; Rendon, J.C.; Navas, M.C.; Rots, M.G.; Postberg, J. Virus-host interplay in hepatitis B virus infection and epigenetic treatment strategies. FEBS J. 2017, 284, 3550–3572. [Google Scholar] [CrossRef]
- Straubeta, A.; Lahaye, T. Zinc fingers, TAL effectors, or Cas9-based DNA binding proteins: what’s best for targeting desired genome loci? Mol. Plant 2013, 6, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.C.; Holmes, M.C.; Wang, J.; Guschin, D.Y.; Lee, Y.L.; Rupniewski, I.; Beausejour, C.M.; Waite, A.J.; Wang, N.S.; Kim, K.A.; et al. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat. Biotechnol. 2007, 25, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Doyon, Y.; Vo, T.D.; Mendel, M.C.; Greenberg, S.G.; Wang, J.; Xia, D.F.; Miller, J.C.; Urnov, F.D.; Gregory, P.D.; Holmes, M.C. Enhancing zinc-finger-nuclease activity with improved obligate heterodimeric architectures. Nat. Methods 2011, 8, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Xirong, L.; Rui, L.; Xiaoli, Y.; Qiuyan, H.; Bikui, T.; Sibo, Z.; Naishuo, Z. Hepatitis B virus can be inhibited by DNA methyltransferase 3a via specific zinc-finger-induced methylation of the X promoter. Biochemistry 2014, 79, 111–123. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Z.; Guo, J.; Huang, P.; Zhu, X.; Zhou, X.; Yang, Z.; Zhao, L.; Xu, L.; Xu, J.; et al. Creation of a six-fingered artificial transcription factor that represses the hepatitis B virus HBx gene integrated into a human hepatocellular carcinoma cell line. J. Biomol. Screening 2013, 18, 378–387. [Google Scholar] [CrossRef]
| No. of Patients | (a) Serum HBV DNA | (b) Intrahepatic Total HBV DNA | (c) Intrahepatic cccDNA | Year | Country Where the Patients Were Enrolled | References | |||
|---|---|---|---|---|---|---|---|---|---|
| Correlation Coefficient | p Value | Correlation Coefficient | p Value | Correlation Coefficient | p Value | ||||
| 82 | Total: 0.807 | <0.001 | 2003 | Japan | [33] | ||||
| HBeAg-positive: 0.847 | <0.001 | ||||||||
| HBeAg-negative:0.632 | <0.001 | ||||||||
| 190 | 0.79 (HBV genotype B) | <0.001 | 2005 | Japan | [41] | ||||
| 0.87 (HBV genotype C) | <0.001 | ||||||||
| 93 | 0.82 | <0.001 | 0.7 | <0.001 | 0.664 | <0.001 | 2007 | Japan | [40] |
| 31 | 0.482 | <0.006 | 2009 | Japan | [34] | ||||
| 138 | Total: 0.69 | <0.0001 | 0.7 | <0.0001 | 2017 | Hong Kong | [42] | ||
| HBeAg-positive: 0.66 | <0.0001 | ||||||||
| HBeAg-negative:0.59 | <0.0001 | ||||||||
| 305 | 0.67 | <0.001 | 2017 | Hong Kong | [42] | ||||
| 79 of 82* | 0.323 | 0.004 | 2019 | China | [46] | ||||
| Disease Phase | HBeAg | No. of Patients | Serum HBV DNA | Serum HBsAg | Serum HBsAg-HQ | Year | Country Where the Patients Were Enrolled | References | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation Coefficient | p Value | Correlation Coefficient | p Value | Correlation Coefficient | p Value | ||||||
| Immune tolerance | Positive | 30 | 0.45 | 0.013 | 0.47 | 0.0095 | 2015 | Germany | [35] | ||
| 52 | 0.369 | 0.007 | 0.286 | 0.04 | 0.401 | 0.003 | 2014 | Hong Kong | [25] | ||
| Immune clearance | Positive | 60 | 0.66 | <0.0001 | 0.53 | <0.0001 | 2015 | Germany | [35] | ||
| 105 | 0.484 | <0.001 | 0.406 | 0.017 | 0.596 | 0.401 | 2014 | Hong Kong | [25] | ||
| Hepatitis | Negative | 50 | 0.74 | <0.0001 | 0.4 | 0.0045 | 2015 | Germany | [35] | ||
| 97 | 0.537 | <0.001 | 0.245 | <0.001 | 0.401 | <0.001 | 2014 | Hong Kong | [25] | ||
| Inactive/quiescent carrier | Negative | 109 | 0.18 | 0.054 | 0.47 | <0.0001 | 2015 | Germany | [35] | ||
| 95 | 0.472 | <0.001 | 0.388 | <0.001 | 0.605 | <0.001 | 2014 | Hong Kong | [25] | ||
| Category | Findings | HBcrAg Level (log U/mL) and Point | References |
|---|---|---|---|
| Natural history | HBeAg seroconversion | <4.92 log U/mL during the clinical course | [25] |
| HBsAg seroclearance | Undetectable (79%), 2.7 log U/mL (median of 21%) during the clinical course | [25,61] | |
| Anti-HBV treatment | HBeAg seroconversion by PEG-IFN at 12 weeks | >8 log U/mL (no response) at the beginning of the therapy | [62] |
| HBeAg seroconversion by PEG-IFN plus NA for 4 weeks followed by PEG-IFN for 20 weeks | >4.5 log U/mL (no response) at the beginning of the therapy | [68] | |
| No LAM resistance | <4.6 log U/mL at 6 months of treatment | [45] | |
| Virological relapse within 1 year of NA cessation | >3.7 log U/mL at NA cessation | [72] | |
| Virological relapse regardless of undetectable HBV DNA for at least 6 months | 3.2–3.7 log U/mL at NA (LAM or ETV) cessation | [73,74] | |
| Virological relapse regardless of undetectable HBV DNA for at least 6 months | <3.4 log U/mL at LAM cessation | [74] | |
| HCC occurrence | Incidence of HCC for treatment-naïve patients | >2.9 log U/mL during the follow-up period | [79] |
| Incidence of HCC for treatment-experienced patients | >4.67 log U/mL at pre-treatment, >3.89 log U/mL at post-treatment | [82] | |
| HCC development during NA treatment | Detectable HBcrAg during NA treatment | [78] | |
| HCC recurrence within 2 years | >4.8 log U/mL at the time of HCC diagnosis | [76] | |
| HBV reactivation | HBV reactivation by high-risk immunosuppressive therapy within 2 years | Detectable HBcrAg at baseline | [88] |
| HBV reinfection | High levels of post-liver transplantation cccDNA | >4 log U/mL before liver transplantation | [93] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, T.; Tanaka, Y. The Role of Hepatitis B Core-Related Antigen. Genes 2019, 10, 357. https://doi.org/10.3390/genes10050357
Inoue T, Tanaka Y. The Role of Hepatitis B Core-Related Antigen. Genes. 2019; 10(5):357. https://doi.org/10.3390/genes10050357
Chicago/Turabian StyleInoue, Takako, and Yasuhito Tanaka. 2019. "The Role of Hepatitis B Core-Related Antigen" Genes 10, no. 5: 357. https://doi.org/10.3390/genes10050357
APA StyleInoue, T., & Tanaka, Y. (2019). The Role of Hepatitis B Core-Related Antigen. Genes, 10(5), 357. https://doi.org/10.3390/genes10050357




