The Singularity of Cetacea Behavior Parallels the Complete Inactivation of Melatonin Gene Modules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synteny Maps
2.2. Sequence Retrieval
2.3. Gene Annotation and Mutational Validation
2.4. Phylogenetic Analysis
3. Results
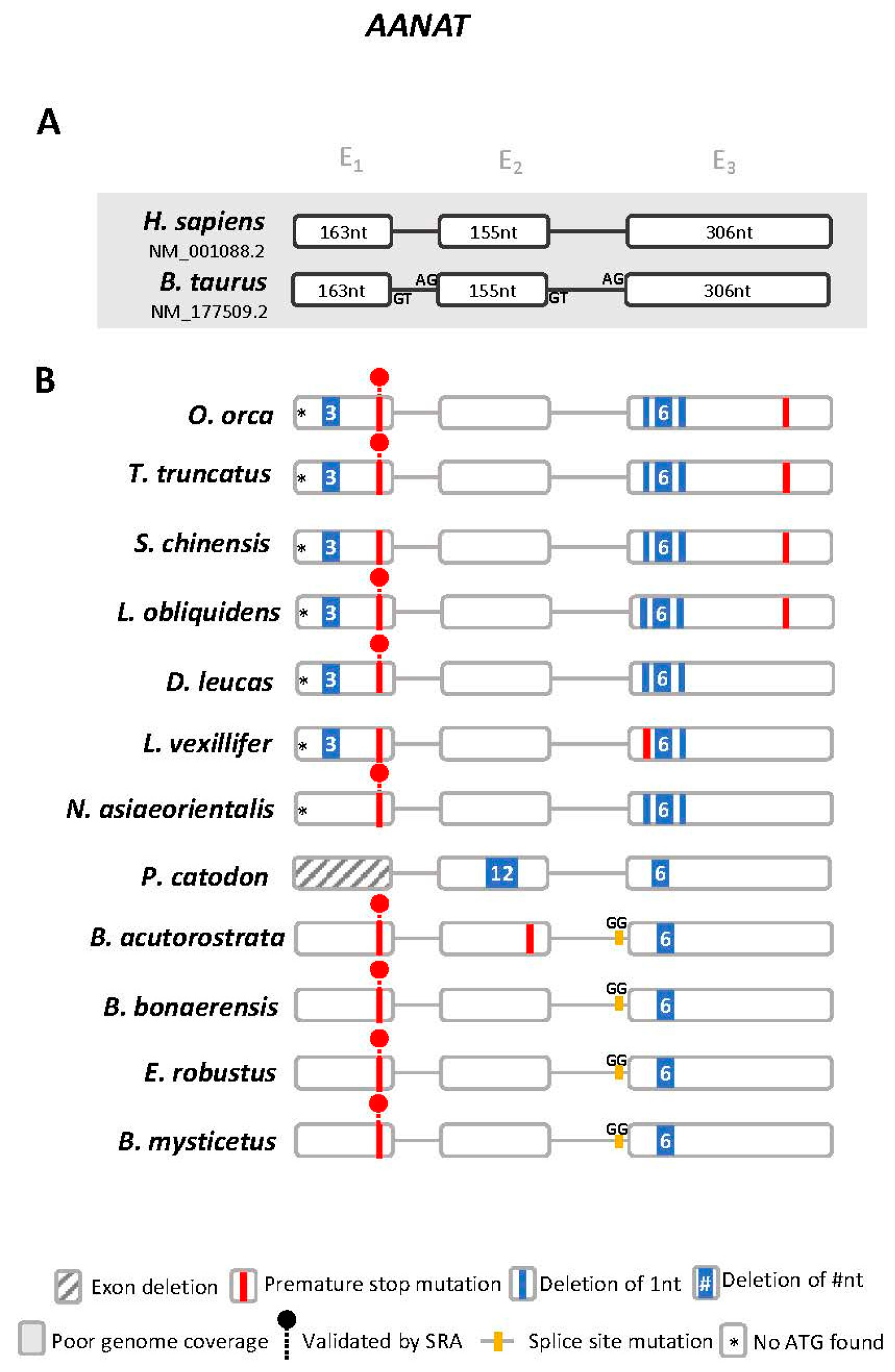
3.1. Aanat Exhibits Conserved Disruptive Mutations in Cetacea
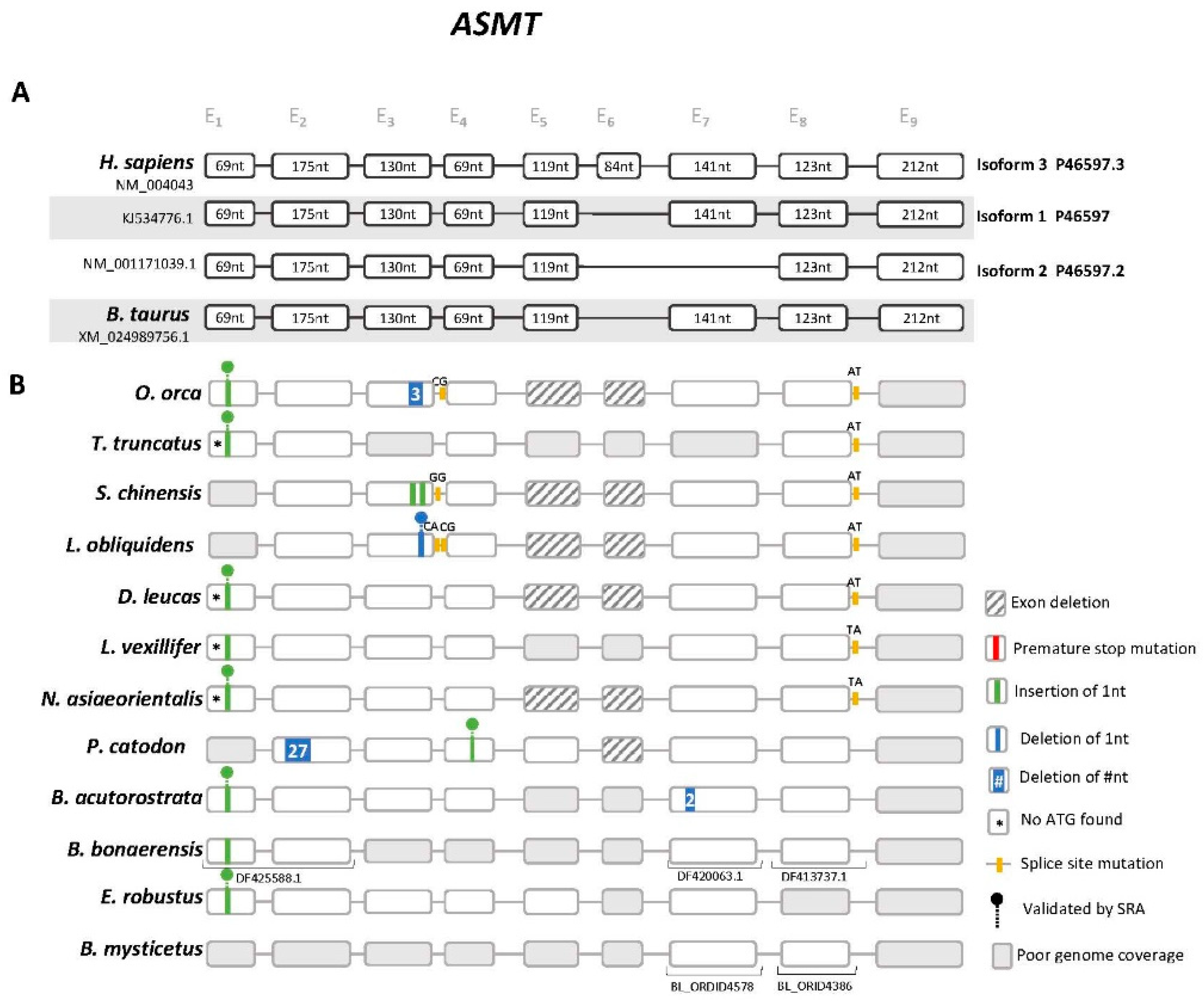
3.2. Cetacea Asmt Reveals Conserved Frameshift Mutation in Exon 1
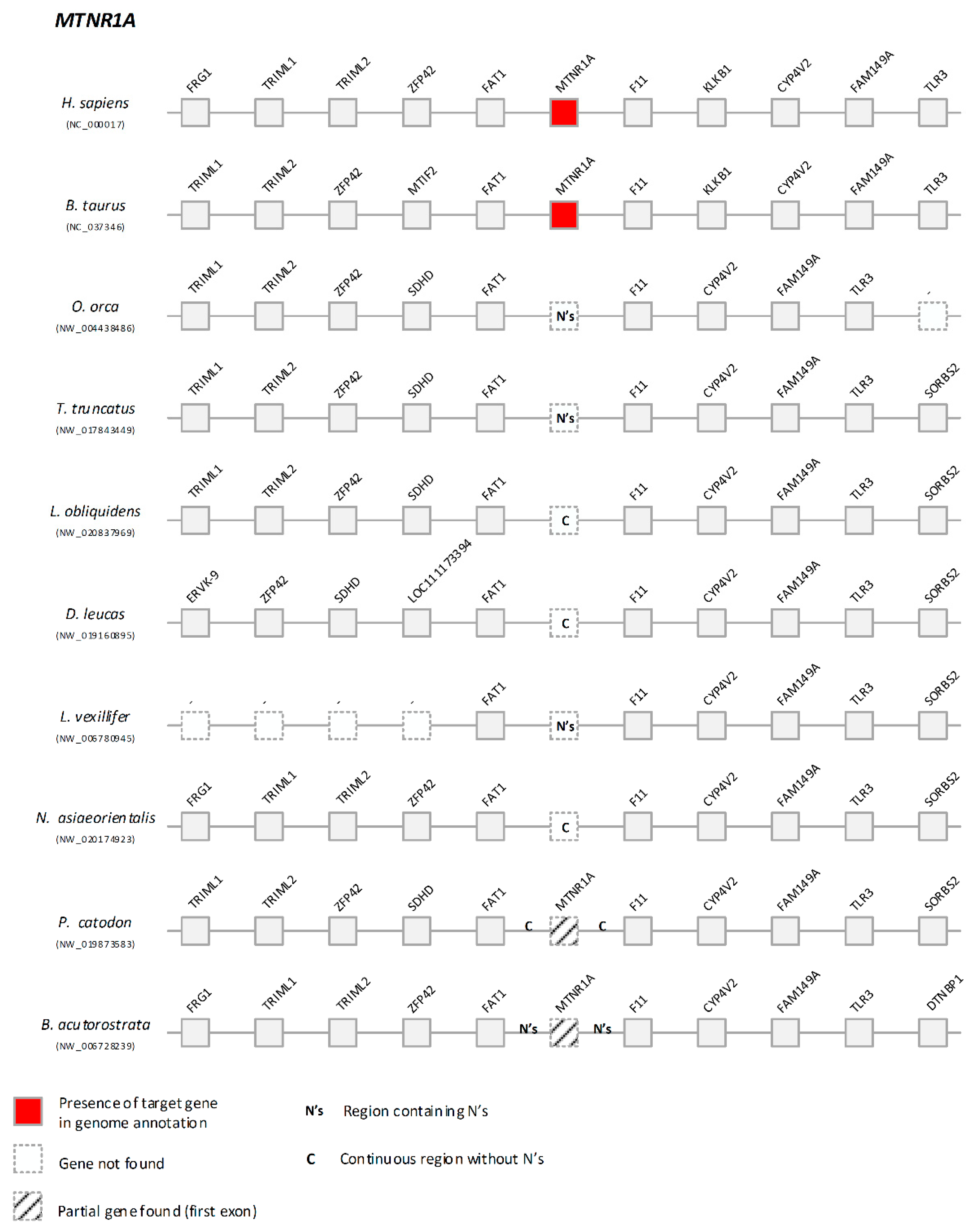
3.3. Signalling Gene Modules are Absent or Mutated in Cetacea
3.4. Hippopotamuses Have Coding Melatonin Synthesis and Signalling Orthologues
3.5. Convergent Disruption of Melatonin Metabolism and Signalling Modules in the Fully Aquatic Manatee
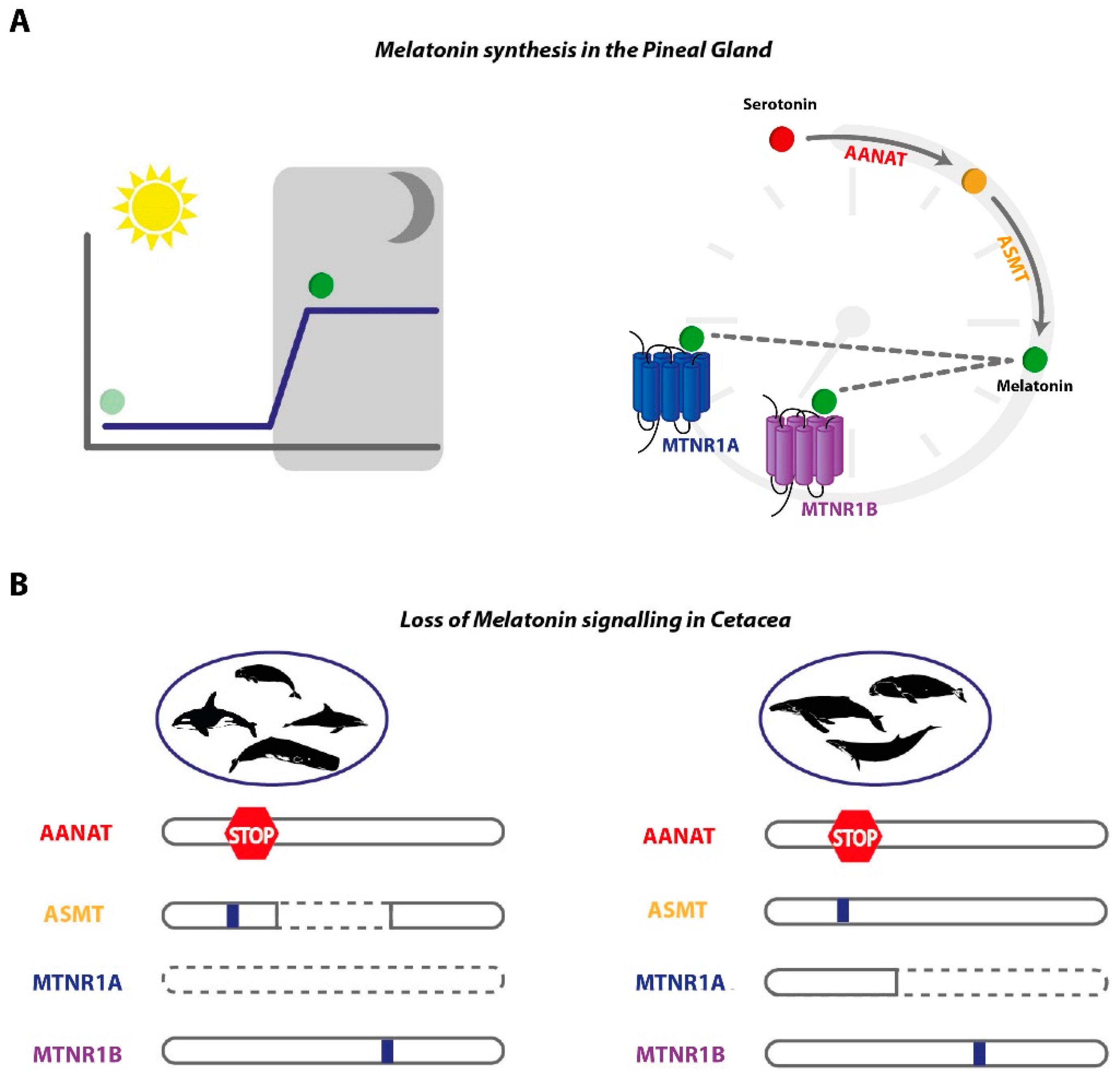
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pevet, P.; Challet, E. Melatonin: Both master clock output and internal time-giver in the circadian clocks network. J. Physiol.-Paris 2011, 105, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y. Suprachiasmatic nucleus in sleep-wake regulation. Sleep Med. 2007, 8 (Suppl. 3), 27–33. [Google Scholar] [CrossRef] [PubMed]
- Pévet, P. Melatonin. Dialogues Clin. Neurosci. 2002, 4, 57–72. [Google Scholar] [PubMed]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.M.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.J. The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc. 2010, 85, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J. Melatonin. Clin. Endocrinol. 1988, 29, 205–229. [Google Scholar] [CrossRef]
- Tosches, M.A.; Bucher, D.; Vopalensky, P.; Arendt, D. Melatonin signaling controls circadian swimming behavior in marine zooplankton. Cell 2014, 159, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the Pineal Gland factor that lightens melanocytes1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Klein, D.; Coon, S.; Roseboom, P.; Weller, J.L.; Bernard, M.; Gastel, J.A.; Zatz, M.; Iuvone, P.; Rodriguez, I.; Bégay, V.; et al. The melatonin rhythm-generating enzyme: Molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog. Horm. Res. 1997, 52, 307–357. [Google Scholar]
- Simonneaux, V.; Ribelayga, C. Generation of the melatonin endocrine message in mammals: A review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol. Rev. 2003, 55, 325. [Google Scholar] [CrossRef]
- Reppart, S.M.; Weaver, D.R.; Godson, C. Melatonin receptors step into the light: Cloning and classification of subtypes. Trends Pharmacol. Sci. 1996, 17, 100–102. [Google Scholar] [CrossRef]
- Comai, S.; Ochoa-Sanchez, R.; Gobbi, G. Sleep–wake characterization of double MT1/MT2 receptor knockout mice and comparison with MT1 and MT2 receptor knockout mice. Behav. Brain Res. 2013, 243, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Falcón, J.; Coon, S.L.; Besseau, L.; Cazaméa-Catalan, D.; Fuentès, M.; Magnanou, E.; Paulin, C.-H.; Boeuf, G.; Sauzet, S.; Jørgensen, E.H.; et al. Drastic neofunctionalization associated with evolution of the timezyme AANAT 500 Mya. Proc. Natl. Acad. Sci. USA 2014, 111, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.C. Evolution of the vertebrate pineal gland: The aanat hypothesis. Chronobiol. Int. 2006, 23, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.C. Arylalkylamine N-acetyltransferase: “The Timezyme”. J. Biol. Chem. 2007, 282, 4233–4237. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.B.; Fang, X.; Fushan, A.A.; Huang, Z.; Lobanov, A.V.; Han, L.; Marino, S.M.; Sun, X.; Turanov, A.A.; Yang, P.; et al. Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature 2011, 479, 223. [Google Scholar] [CrossRef] [PubMed]
- Panin, M.; Gabai, G.; Ballarin, C.; Peruffo, A.; Cozzi, B. Evidence of melatonin secretion in cetaceans: Plasma concentration and extrapineal HIOMT-like presence in the bottlenose dolphin Tursiops truncatus. Gen. Comp. Endocrinol. 2012, 177, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Ralph, C.L. The pineal gland and geographical distribution of animals. Int. J. Biometeorol. 1975, 19, 289–303. [Google Scholar] [CrossRef]
- Ralph, C.L.; Young, S.; Gettinger, R.; O’Shea, T.J. Does the manatee have a pineal body? Acta Zool. 1985, 66, 55–60. [Google Scholar] [CrossRef]
- Fang, X.; Seim, I.; Huang, Z.; Gerashchenko, M.V.; Xiong, Z.; Turanov, A.A.; Zhu, Y.; Lobanov, A.V.; Fan, D.; Yim, S.H.; et al. Adaptations to a subterranean environment and longevity revealed by the analysis of mole rat genomes. Cell Rep. 2014, 8, 1354–1364. [Google Scholar] [CrossRef]
- Quay, W.B. Pineal atrophy and other neuroendocrine and circumventricular features of the naked mole-rat, heterocephalusglaber (Rüppell), a fossorial, equatorial rodent. J. Neural Transm. 1981, 52, 107–115. [Google Scholar] [CrossRef]
- Cajochen, C.; Pache, M.; Flammer, J.; Wirz-Justice, A. Thermoregulatory effects of melatonin in relation to sleepiness AU - Kräuchi, Kurt. Chronobiol. Int. 2006, 23, 475–484. [Google Scholar] [CrossRef]
- Dauchy, R.T.; Wren-Dail, M.A.; Hoffman, A.E.; Hanifin, J.P.; Warfield, B.; Brainard, G.C.; Hill, S.M.; Belancio, V.P.; Dauchy, E.M.; Blask, D.E. Effects of daytime exposure to light from blue-enriched light-emitting diodes on the nighttime melatonin amplitude and circadian regulation of rodent metabolism and physiology. Comp. Med. 2016, 66, 373–383. [Google Scholar] [PubMed]
- Fasick, J.I.; Robinson, P.R. Adaptations of cetacean retinal pigments to aquatic environments. Front. Ecol. Evol. 2016, 4. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Stanke, M.; Morgenstern, B. AUGUSTUS: A web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 2005, 33, W465–W467. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2017. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Lefort, V.; Longueville, J.-E.; Gascuel, O. SMS: Smart model selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef]
- Huson, D.H.; Scornavacca, C. Dendroscope 3: An interactive tool for rooted phylogenetic trees and networks. Syst. Biol. 2012, 61, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Foote, A.D.; Liu, Y.; Thomas, G.W.C.; Vinař, T.; Alföldi, J.; Deng, J.; Dugan, S.; van Elk, C.E.; Hunter, M.E.; Joshi, V.; et al. Convergent evolution of the genomes of marine mammals. Nat. Genet. 2015, 47, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Zimin, A.V.; Delcher, A.L.; Florea, L.; Kelley, D.R.; Schatz, M.C.; Puiu, D.; Hanrahan, F.; Pertea, G.; Van Tassell, C.P.; Sonstegard, T.S.; et al. A whole-genome assembly of the domestic cow, Bos taurus. Genome Biol. 2009, 10, R42. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.J.M.; Taylor, G.A.; Chan, S.; Warren, R.L.; Hammond, S.A.; Bilobram, S.; Mordecai, G.; Suttle, C.A.; Miller, K.M.; Schulze, A.; et al. The genome of the beluga whale (Delphinapterus leucas). Genes 2017, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sun, F.; Xu, S.; Fan, G.; Zhu, K.; Liu, X.; Chen, Y.; Shi, C.; Yang, Y.; Huang, Z.; et al. Baiji genomes reveal low genetic variability and new insights into secondary aquatic adaptations. Nat. Commun. 2013, 4, 2708. [Google Scholar] [CrossRef] [PubMed]
- DeWoody, J.A.; Fernandez, N.B.; Bruniche-Olsen, A.; Antonides, J.D.; Doyle, J.M.; San Miguel, P.; Westerman, R.; Vertyankin, V.V.; Godard-Codding, C.A.J.; Bickham, J.W. Characterization of the gray whale Eschrichtius robustus genome and a genotyping array based on single-nucleotide polymorphisms in candidate genes. Biol. Bull. 2017, 232, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; An, Y.-R.; Kanda, N.; An, C.-M.; An, H.S.; Kang, J.-H.; Kim, E.M.; An, D.-H.; Jung, H.; Joung, M.; et al. Cetaceans evolution: Insights from the genome sequences of common Minke whales. BMC Genom. 2015, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.; Jian, J.; Yu, F.; Yu, X.; Wang, J.; Liu, W. Molecular footprints of inshore aquatic adaptation in Indo-Pacific humpback dolphin (Sousa chinensis). Genomics 2018. [Google Scholar] [CrossRef]
- Arnason, U.; Lammers, F.; Kumar, V.; Nilsson, M.A.; Janke, A. Whole-genome sequencing of the blue whale and other rorquals finds signatures for introgressive gene flow. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef]
- Yim, H.-S.; Cho, Y.S.; Guang, X.; Kang, S.G.; Jeong, J.-Y.; Cha, S.-S.; Oh, H.-M.; Lee, J.-H.; Yang, E.C.; Kwon, K.K.; et al. Minke whale genome and aquatic adaptation in cetaceans. Nat. Genet. 2014, 46, 88–92. [Google Scholar] [CrossRef]
- Jebb, D.; Hiller, M. Recurrent loss of HMGCS2 shows that ketogenesis is not essential for the evolution of large mammalian brains. Elife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Marques, M.; Machado, A.M.; Barbosa, S.; Fonseca, M.M.; Ruivo, R.; Castro, L.F.C. Cetacea are natural knockouts for IL20. Immunogenetics 2018. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Hecker, N.; Roscito, J.G.; Foerster, L.; Langer, B.E.; Hiller, M. A genomics approach reveals insights into the importance of gene losses for mammalian adaptations. Nat. Commun. 2018, 9, 1215. [Google Scholar] [CrossRef]
- Springer, M.S.; Gatesy, J. Evolution of the MC5R gene in placental mammals with evidence for its inactivation in multiple lineages that lack sebaceous glands. Mol. Phylogenet. Evol. 2018, 120, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Botros, H.G.; Legrand, P.; Pagan, C.; Bondet, V.; Weber, P.; Ben-Abdallah, M.; Lemière, N.; Huguet, G.; Bellalou, J.; Maronde, E.; et al. Crystal structure and functional mapping of human ASMT, the last enzyme of the melatonin synthesis pathway. J. Pineal Res. 2012, 54, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Marti-Solans, J.; Belyaeva, O.V.; Torres-Aguila, N.P.; Kedishvili, N.Y.; Albalat, R.; Canestro, C. Coelimination and survival in gene network evolution: Dismantling the RA-signaling in a chordate. Mol. Biol. Evol. 2016, 33, 2401–2416. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef]
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Wortsman, J. Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur. J. Biochem. 2003, 270, 3335–3344. [Google Scholar] [CrossRef]
- Ried, K.; Rao, E.; Schiebel, K.; Rappold, G.A. Gene duplications as a recurrent theme in the evolution of the human pseudoautosomal region 1: isolation of the gene ASMTL. Hum. Mol. Genet. 1998, 7, 1771–1778. [Google Scholar] [CrossRef]
- Zhang, K.; Ruan, Z.; Li, J.; Bian, C.; You, X.; Coon, L.S.; Shi, Q. A comparative genomic and transcriptomic survey provides novel insights into N-acetylserotonin methyltransferase (ASMT) in Fish. Molecules 2017, 22, 1653. [Google Scholar] [CrossRef]
- Pfeffer, M.; Korf, H.-W.; Wicht, H. Synchronizing effects of melatonin on diurnal and circadian rhythms. Gen. Comp. Endocrinol. 2017. [Google Scholar] [CrossRef]
- Lyamin, O.I.; Manger, P.R.; Ridgway, S.H.; Mukhametov, L.M.; Siegel, J.M. Cetacean sleep: An unusual form of mammalian sleep. Neurosci. Biobehav. Rev. 2008, 32, 1451–1484. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, Y.; Arai, K.; Kohshima, S. Sleep in continuously active dolphins. Nature 2006, 441, E9. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef]
- Albalat, R.; Canestro, C. Evolution by gene loss. Nat. Rev. Genet. 2016, 17, 379–391. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes-Marques, M.; Ruivo, R.; Alves, L.Q.; Sousa, N.; Machado, A.M.; Castro, L.F.C. The Singularity of Cetacea Behavior Parallels the Complete Inactivation of Melatonin Gene Modules. Genes 2019, 10, 121. https://doi.org/10.3390/genes10020121
Lopes-Marques M, Ruivo R, Alves LQ, Sousa N, Machado AM, Castro LFC. The Singularity of Cetacea Behavior Parallels the Complete Inactivation of Melatonin Gene Modules. Genes. 2019; 10(2):121. https://doi.org/10.3390/genes10020121
Chicago/Turabian StyleLopes-Marques, Mónica, Raquel Ruivo, Luís Q. Alves, Nelson Sousa, André M. Machado, and L. Filipe C. Castro. 2019. "The Singularity of Cetacea Behavior Parallels the Complete Inactivation of Melatonin Gene Modules" Genes 10, no. 2: 121. https://doi.org/10.3390/genes10020121
APA StyleLopes-Marques, M., Ruivo, R., Alves, L. Q., Sousa, N., Machado, A. M., & Castro, L. F. C. (2019). The Singularity of Cetacea Behavior Parallels the Complete Inactivation of Melatonin Gene Modules. Genes, 10(2), 121. https://doi.org/10.3390/genes10020121




