NF-YA Overexpression in Lung Cancer: LUSC
Abstract
1. Introduction
2. Materials and Methods
2.1. RNA-seq Datasets
2.2. Classification of All TCGA LUSC Tumors
2.3. Global Gene Expression Analysis
2.4. Gene Ontology and Pathway Enrichment Analysis
2.5. Transcription Factor Binding Site (TFBS) and De Novo Motif Discovery
2.6. Analysis of Clinical Data
2.7. Statistical Analysis
3. Results
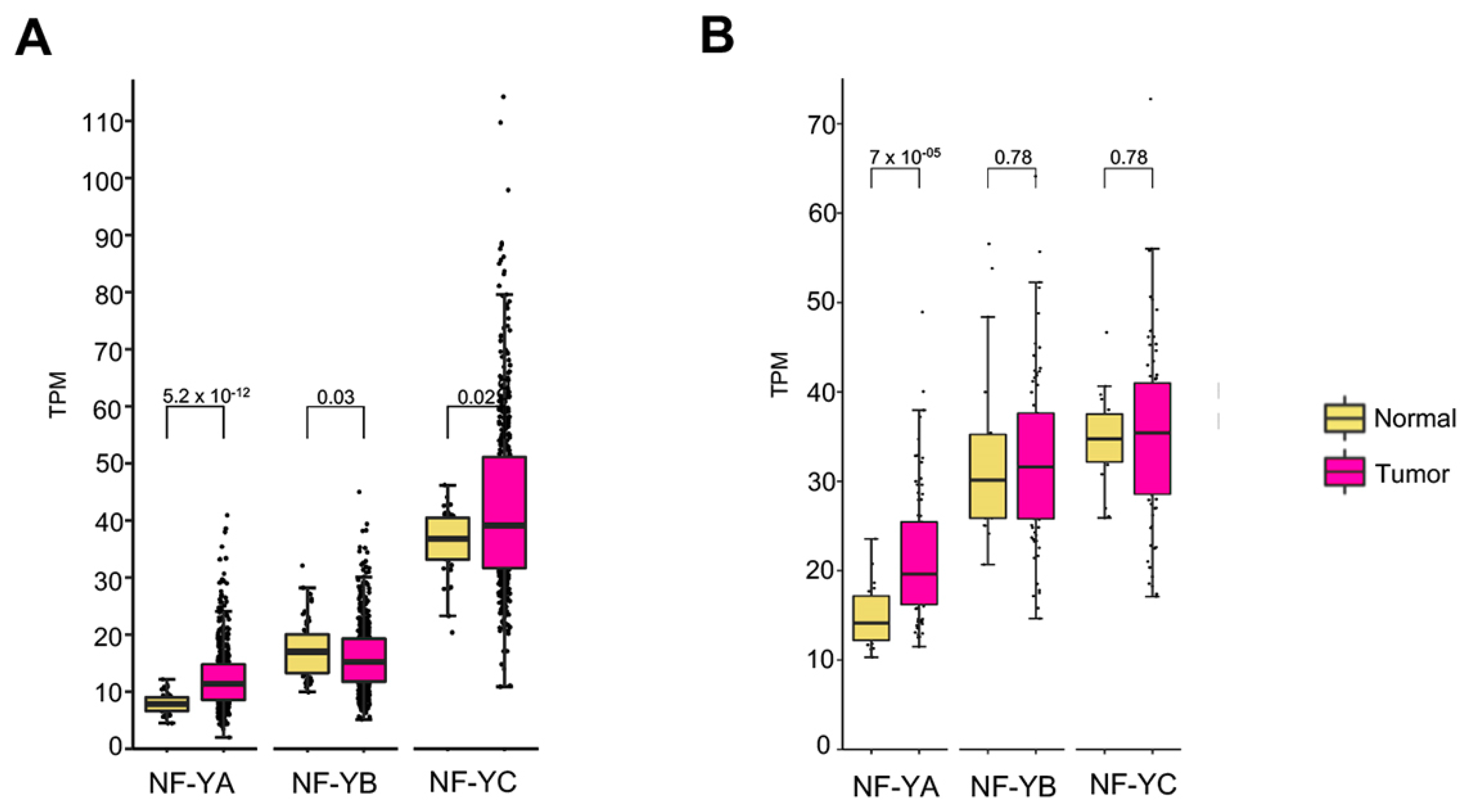
3.1. NF-YA is Overexpressed in Lung Tumors
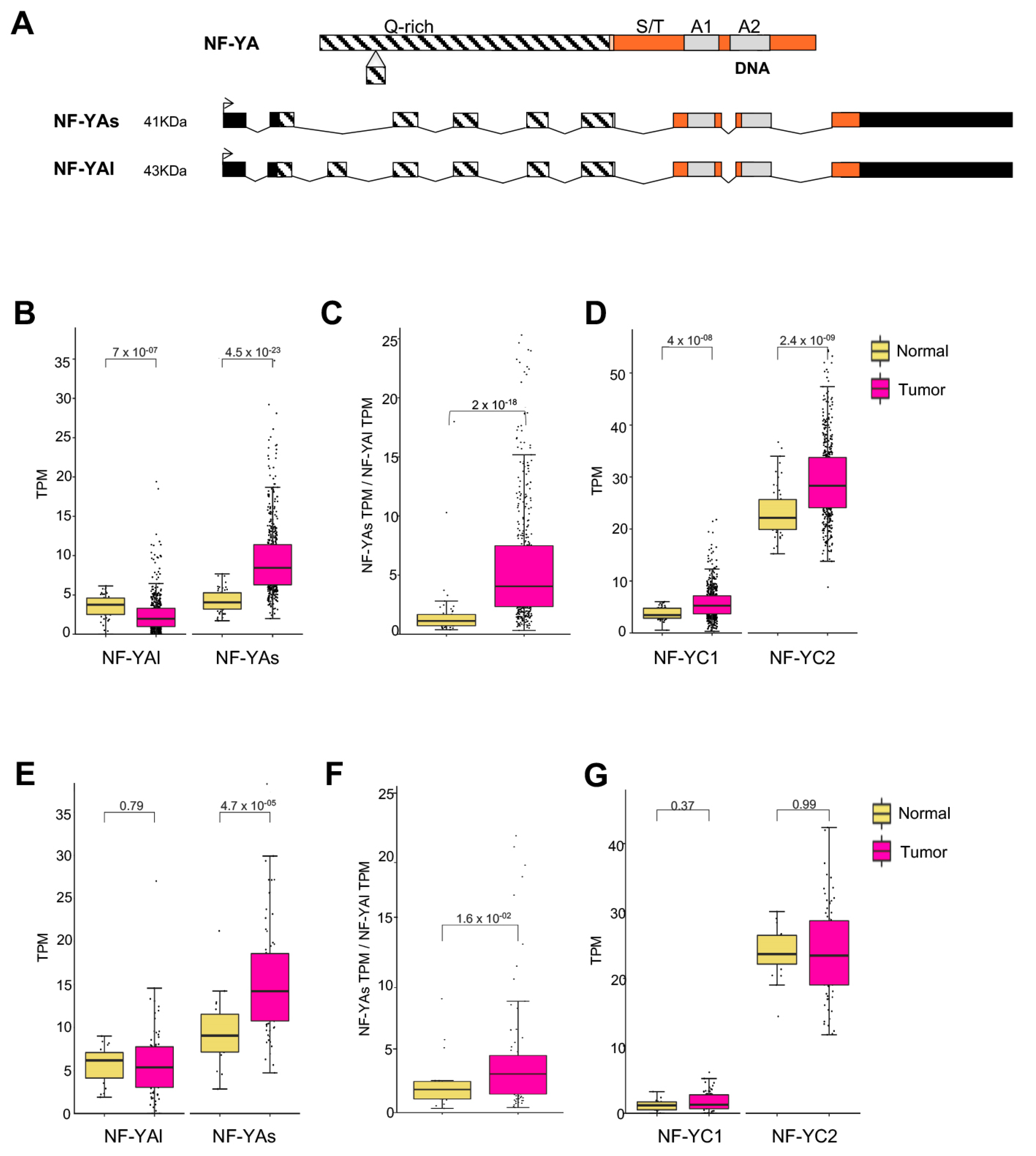
3.2. Splicing Isoforms of NF-YA Are Differentially Regulated in LUSC
3.3. NF-YA Levels Correlate with Proliferative Markers
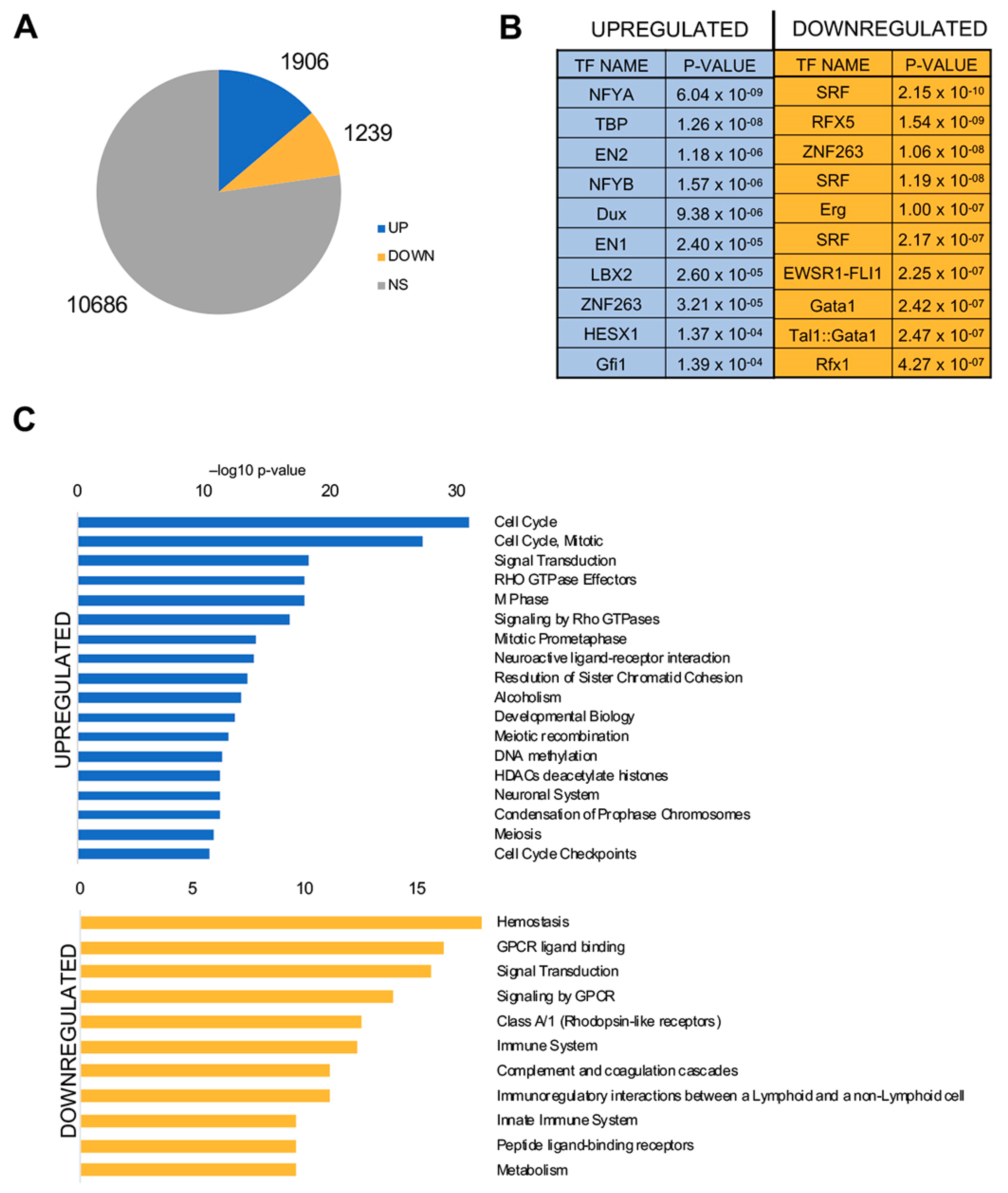
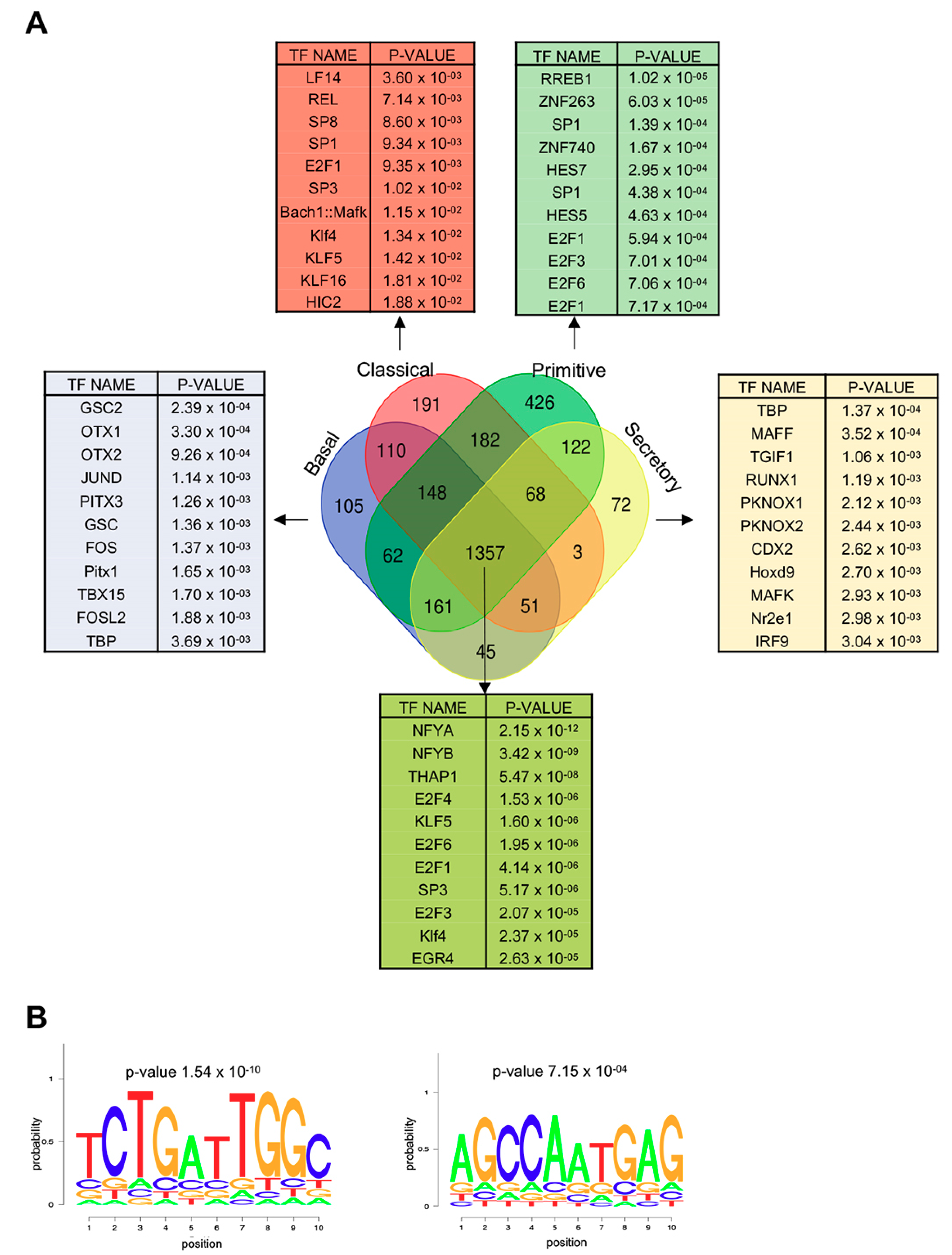
3.4. NF-Y Sites are Enriched in Promoters of Genes Overexpressed in LUSC
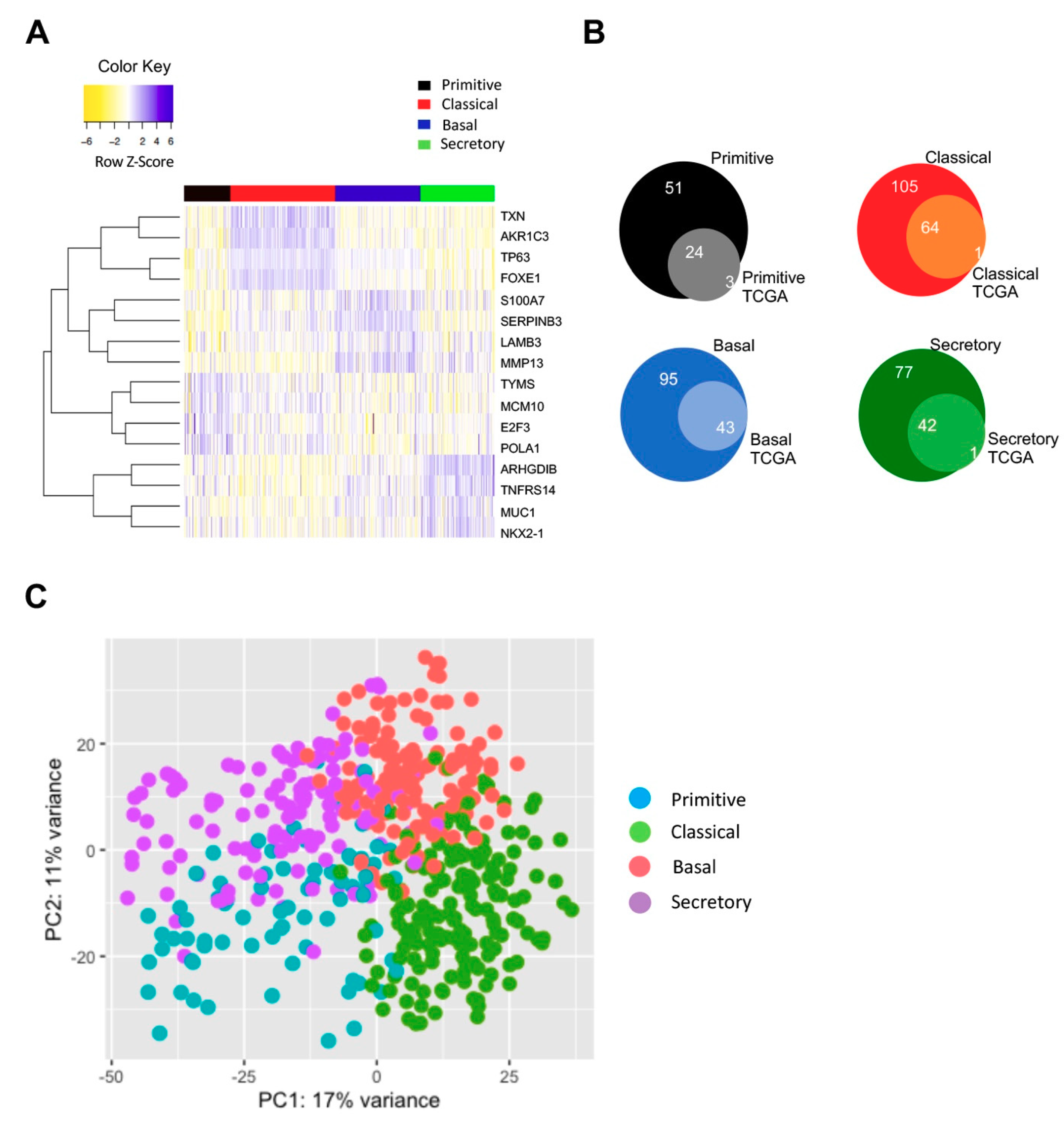
3.5. Classification of All TCGA LUSC Tumors in Subtypes
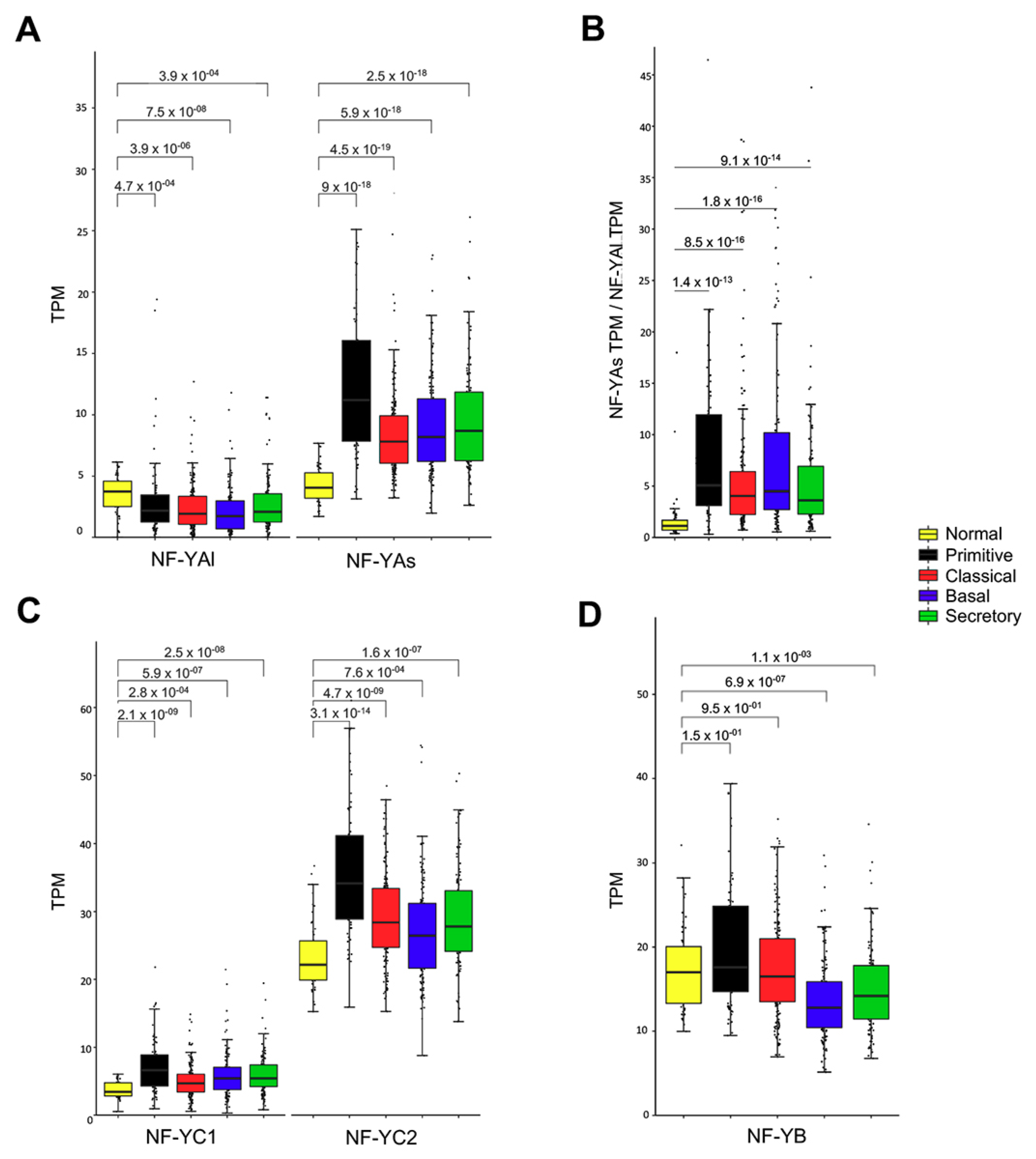
3.6. Expression of NF-YA Isoforms in LUSC Subtypes
3.7. Genes Overexpressed in All LUSC Subtypes Have CCAAT in Promoters
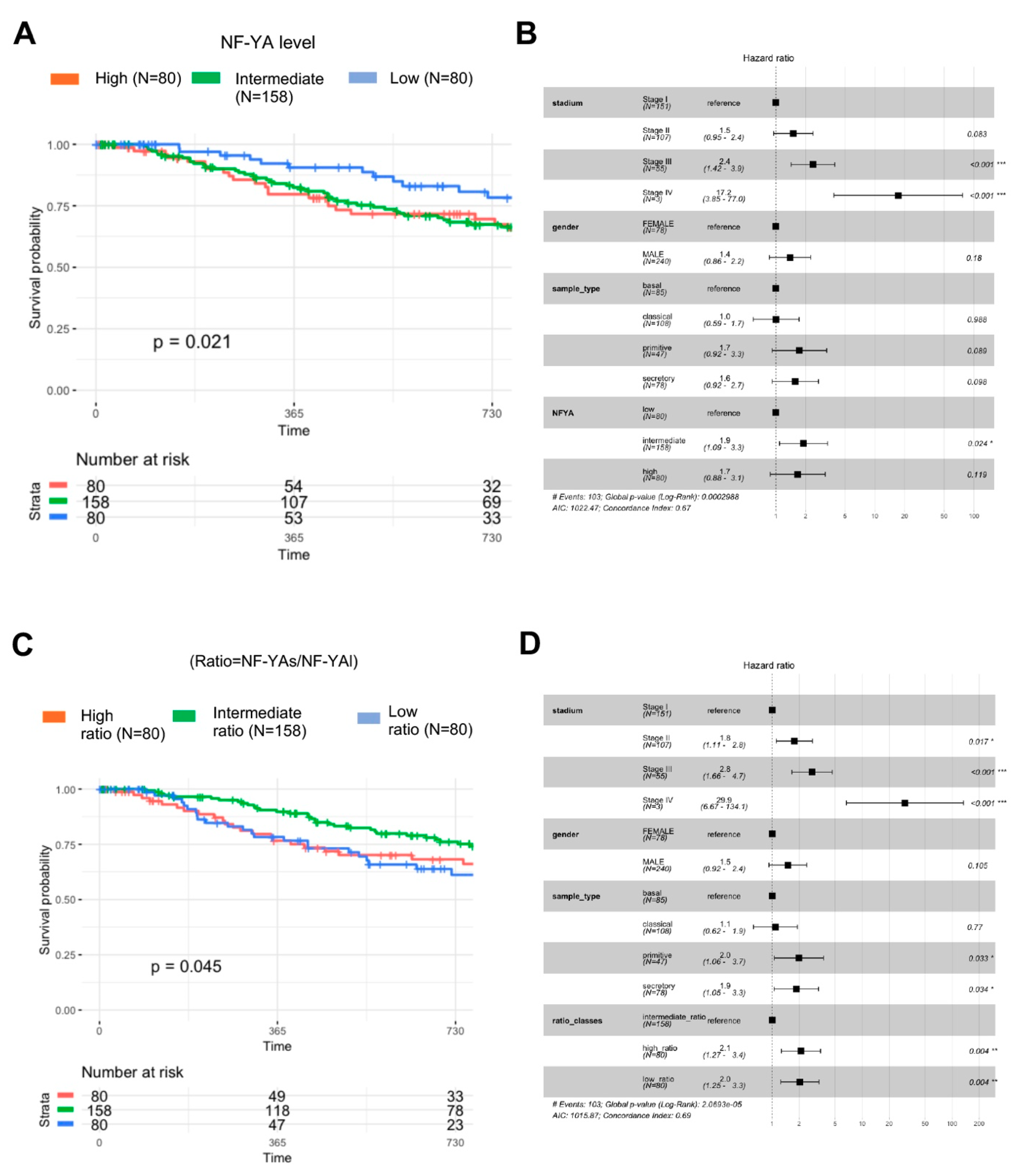
3.8. Clinical Outcomes of NF-YA Isoforms Overexpression
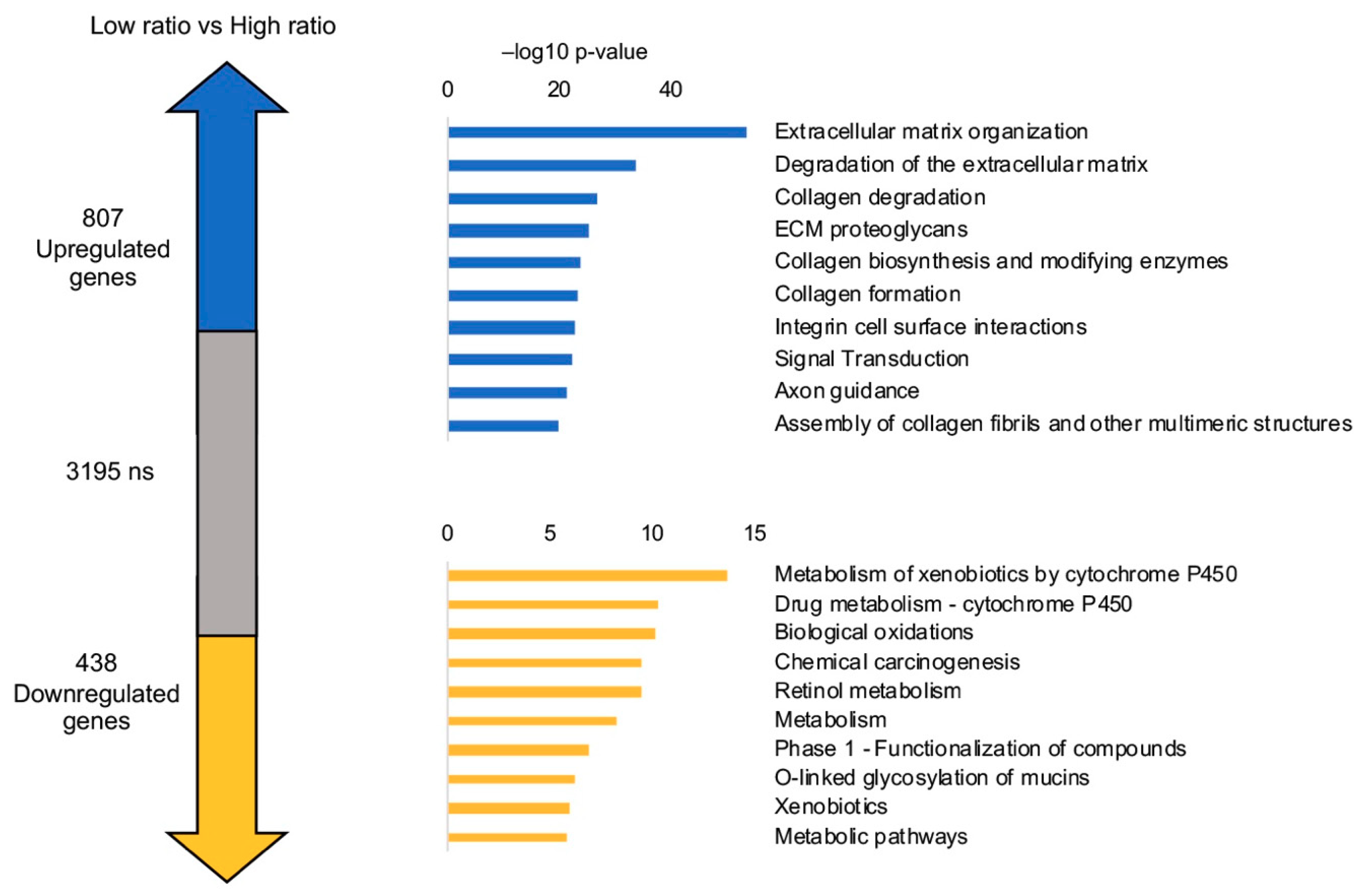
3.9. LUSC Tumors with Low NF-YAs/NF-YAl Ratio Have a Distinct Gene Signature
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levine, M.; Cattoglio, C.; Tjian, R. Looping back to leap forward: Transcription enters a new era. Cell 2014, 157, 13–25. [Google Scholar] [CrossRef]
- Nardini, M.; Gnesutta, N.; Donati, G.; Gatta, R.; Forni, C.; Fossati, A.; Vonrhein, C.; Moras, D.; Romier, C.; Bolognesi, M.; et al. Sequence-specific transcription factor NF-Y displays histone-like DNA binding and H2B-like ubiquitination. Cell 2013, 152, 132–143. [Google Scholar] [CrossRef]
- Li, X.Y.; Hooft van Huijsduijnen, R.; Mantovani, R.; Benoist, C.; Mathis, D. Intron-exon organization of the NF-Y genes. Tissue-specific splicing modifies an activation domain. J. Biol. Chem. 1992, 267, 8984–8990. [Google Scholar]
- Ceribelli, M.; Benatti, P.; Imbriano, C.; Mantovani, R. NF-YC complexity is generated by dual promoters and alternative splicing. J. Biol. Chem. 2009, 284, 34189–34200. [Google Scholar] [CrossRef]
- Gurtner, A.; Manni, I.; Piaggio, G. NF-Y in cancer: Impact on cell transformation of a gene essential for proliferation. Biochim. Biophys. Acta 2017, 1860, 604–616. [Google Scholar] [CrossRef]
- Goodarzi, H.; Elemento, O.; Tavazoie, S. Revealing global regulatory perturbations across human cancers. Mol. Cell. 2009, 36, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Derow, C.K.; Zhang, B. Co-expression module analysis reveals biological processes, genomic gain, and regulatory mechanisms associated with breast cancer progression. BMC Syst. Biol. 2010, 4, 74. [Google Scholar] [CrossRef] [PubMed]
- Gusev, Y.; Riggins, R.B.; Bhuvaneshwar, K.; Gauba, R.; Sheahan, L.; Clarke, R.; Madhavan, S. In silico discovery of mitosis regulation networks associated with early distant metastases in estrogen receptor positive breast cancers. Cancer Inform. 2013, 12, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Andrews, E.; Wang, Y.; Xia, T.; Cheng, W.; Cheng, C. Contextual Refinement of Regulatory Targets Reveals Effects on Breast Cancer Prognosis of the Regulome. PLoS Comput. Biol. 2017, 13, e1005340. [Google Scholar] [CrossRef]
- Zuo, Z.G.; Zhang, X.F.; Ye, X.Z.; Zhou, Z.H.; Wu, X.B.; Ni, S.C.; Song, H.Y. Bioinformatic analysis of RNA-seq data unveiled critical genes in rectal adenocarcinoma. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3017–3025. [Google Scholar]
- Dolfini, D.; Zambelli, F.; Pavesi, G.; Mantovani, R. A perspective of promoter architecture from the CCAAT box. Cell Cycle. 2009, 8, 4127–4137. [Google Scholar] [CrossRef] [PubMed]
- Bisikirska, B.; Bansal, M.; Shen, Y.; Teruya-Feldstein, J.; Chaganti, R.; Califano, A. Elucidation and Pharmacological Targeting of Novel Molecular Drivers of Follicular Lymphoma Progression. Cancer Res. 2016, 76, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Merino, D.; Nimmervoll, B.; Gupta, K.; Wang, Y.D.; Finkelstein, D.; Dalton, J.; Ellison, D.W.; Ma, X.; Zhang, J.; et al. Cross-Species Genomics Identifies TAF12, NFYC, and RAD54L as Choroid Plexus Carcinoma Oncogenes. Cancer Cell. 2015, 27, 712–727. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.D.; Pavesi, G.; Benatti, P.; Imbriano, C.; Mantovani, R.; Struhl, K. NF-Y coassociates with FOS at promoters, enhancers, repetitive elements, and inactive chromatin regions, and is stereo-positioned with growth-controlling transcription factors. Genome Res. 2013, 23, 1195–1209. [Google Scholar] [CrossRef]
- Xie, D.; Boyle, A.P.; Wu, L.; Zhai, J.; Kawli, T.; Snyder, M. Dynamic trans-acting factor colocalization in human cells. Cell 2013, 155, 713–724. [Google Scholar] [CrossRef]
- Mamat, S.; Ikeda, J.; Tian, T.; Wang, Y.; Luo, W.; Aozasa, K.; Morii, E. Transcriptional Regulation of Aldehyde Dehydrogenase 1A1 Gene by Alternative Spliced Forms of Nuclear Factor Y in Tumorigenic Population of Endometrial Adenocarcinoma. Genes Cancer 2011, 2, 979–984. [Google Scholar] [CrossRef]
- Cicchillitti, L.; Corrado, G.; Carosi, M.; Dabrowska, M.E.; Loria, R.; Falcioni, R.; Cutillo, G.; Piaggio, G.; Vizza, E. Prognostic role of NF-YA splicing isoforms and Lamin A status in low grade endometrial cancer. Oncotarget 2017, 8, 7935–7945. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, X.; Cui, N.; Liang, Y. Cadherins Associate with Distinct Stem Cell-Related Transcription Factors to Coordinate the Maintenance of Stemness in Triple-Negative Breast Cancer. Stem Cells Int. 2017, 2017, 509–541. [Google Scholar] [CrossRef]
- Cao, B.; Zhao, Y.; Zhang, Z.; Li, H.; Xing, J.; Guo, S.; Qiu, X.; Zhang, S.; Min, L.; Zhu, S. Gene regulatory network construction identified NFYA as a diffuse subtype-specific prognostic factor in gastric cancer. Int. J. Oncol. 2018, 53, 1857–1868. [Google Scholar] [CrossRef]
- Bie, L.Y.; Li, D.; Mu, Y.; Wang, S.; Chen, B.B.; Lyu, H.F.; Han, L.L.; Nie, C.Y.; Yang, C.C.; Wang, L.; et al. Analysis of cyclin E co-expression genes reveals nuclear transcription factor Y subunit α is an oncogene in gastric cancer. Chronic Dis. Transl. Med. 2018, 5, 44–52. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, M.; Wang, Y.; Wang, Y. NF-YC in glioma cell proliferation and tumor growth and its role as an independent predictor of patient survival. Neurosci. Lett. 2016, 631, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kottorou, A.E.; Antonacopoulou, A.G.; Dimitrakopoulos, F.I.; Tsamandas, A.C.; Scopa, C.D.; Petsas, T.; Kalofonos, H.P. Altered expression of NFY-C and RORA in colorectal adenocarcinomas. Acta Histochem. 2012, 114, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Relli, V.; Trerotola, M.; Guerra, E.; Alberti, S. Abandoning the Notion of Non-Small Cell Lung Cancer. Trends Mol. Med. 2019, 25, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, M.D.; Yin, X.; Hoadley, K.A.; Liu, Y.; Hayward, M.C.; Cabanski, C.R.; Muldrew, K.; Miller, C.R.; Randell, S.H.; Socinski, M.A.; et al. Lung squamous cell carcinoma mRNA expression subtypes are reproducible, clinically important, and correspond to normal cell types. Clin. Cancer Res. 2010, 16, 4864–4875. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive genomic characterization of squamous cell lung Cancers. Nature 2012, 489, 519–525. [Google Scholar] [CrossRef]
- Mezheyeuski, A.; Bergsland, C.H.; Backman, M.; Djureinovic, D.; Sjöblom, T.; Bruun, J.; Micke, P. Multispectral imaging for quantitative and compartment-specific immune infiltrates reveals distinct immune profiles that classify lung cancer patients. J. Pathol. 2018, 244, 421–431. [Google Scholar] [CrossRef]
- Girard, L.; Rodriguez-Canales, J.; Behrens, C.; Thompson, D.M.; Botros, I.W.; Tang, H.; Xie, Y.; Rekhtman, N.; Travis, W.D.; Wistuba, I.I.; et al. An Expression Signature as an Aid to the Histologic Classification of Non-Small Cell Lung Cancer. Clin. Cancer Res. 2016, 22, 4880–4889. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Zambelli, F.; Pesole, G.; Pavesi, G. Pscan: Finding over-represented transcription factor binding site motifs in sequences from co-regulated or co-expressed genes. Nucleic Acids Res. 2009, 37, W247–W252. [Google Scholar] [CrossRef]
- Pavesi, G.; Mereghetti, P.; Mauri., G.; Pesole, G. Weeder Web: Discovery of transcription factor binding sites in a set of sequences from co-regulated genes. Nucleic Acids Res. 2004, 32, W199–W200. [Google Scholar] [CrossRef] [PubMed]
- Bembom, O. seqLogo: Sequence Logos for DNA Sequence Alignments. R package version 1.48.0. 2018. Available online: https://pypi.org/project/seqlogo/0.0.1/ (accessed on 1 September 2019).
- Borcherding, N.; Bormann, N.L.; Voigt, A.P.; Zhang, W. TRGAted: A web tool for survival analysis using protein data in the Cancer Genome Atlas. F1000Research 2018, 7, 1235. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T. Survival. Version 2.38. 2015. Available online: https://cran.r-project.org/web/packages/survival/citation.html (accessed on 1 September 2019).
- Dolfini, D.; Andrioletti, V.; Mantovani, R. Overexpression and alternative splicing of NF-YA in breast cancer. Sci. Rep. 2019, 9, 12955. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.K.; Son, C.H.; Lee, S.K.; Choi, P.J.; Lee, K.E.; Roh, M.S. Forkhead box M1 expression in pulmonary squamous cell carcinoma: Correlation with clinicopathologic features and its prognostic significance. Hum. Pathol. 2009, 40, 464–470. [Google Scholar] [CrossRef]
- Shan, L.; Zhao, M.; Lu, Y.; Ning, H.; Yang, S.; Song, Y.; Chai, W.; Shi, X. CENPE promotes lung adenocarcinoma proliferation and is directly regulated by FOXM1. Int. J. Oncol. 2019, 55, 257–266. [Google Scholar]
- Zhang, C.; Min, L.; Zhang, L.; Ma, Y.; Yang, Y.; Shou, C. Combined analysis identifies six genes correlated with augmented malignancy from non-small cell to small cell lung cancer. Tumour Biol. 2016, 37, 2193–2207. [Google Scholar] [CrossRef]
- Wen, P.; Chidanguro, T.; Shi, Z.; Gu, H.; Wang, N.; Wang, T.; Li, Y.; Gao, J. Identification of candidate biomarkers and pathways associated with SCLC by bioinformatics analysis. Mol. Med. Rep. 2018, 18, 1538–1550. [Google Scholar]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416. [Google Scholar] [CrossRef]
- Linhart, C.; Elkon, R.; Shiloh, Y.; Shamir, R. Deciphering transcriptional regulatory elements that encode specific cell cycle phasing by comparative genomics analysis. Cell Cycle 2005, 4, 1788–1797. [Google Scholar] [CrossRef]
- Halperin, Y.; Linhart, C.; Ulitsky, I.; Shamir, R. Allegro: Analyzing expression and sequence in concert to discover regulatory programs. Nucleic Acids Res. 2009, 37, 1566–1579. [Google Scholar] [CrossRef]
- Dolfini, D.; Zambelli, F.; Pedrazzoli, M.; Mantovani, R.; Pavesi, G. A high definition look at the NF-Y regulome reveals genome-wide associations with selected transcription factors. Nucleic Acids Res. 2016, 44, 4684–4702. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, J.; Iyer, S.; Lin, X.; Whitfield, T.W.; Greven, M.C.; Pierce, B.G.; Dong, X.; Kundaje, A.; Cheng, Y.; et al. Sequence features and chromatin structure around the genomic regions bound by 119 human transcription factors. Genome Res. 2012, 22, 1798–1812. [Google Scholar] [CrossRef]
- Eymin, B.; Gazzeri, S.; Brambilla, C.; Brambilla, E. Distinct pattern of E2F1 expression in human lung tumours: E2F1 is upregulated in small cell lung carcinoma. Oncogene 2001, 20, 1678–1687. [Google Scholar] [CrossRef]
- Huang, C.L.; Liu, D.; Nakano, J.; Yokomise, H.; Ueno, M.; Kadota, K.; Wada, H. E2F1 overexpression correlates with thymidylate synthase and survivin gene expressions and tumor proliferation in non small-cell lung cancer. Clin. Cancer Res. 2007, 13, 6938–6946. [Google Scholar] [CrossRef]
- Park, S.A.; Platt, J.; Lee, J.W.; López-Giráldez, F.; Herbst, R.S.; Koo, J.S. E2F8 as a Novel Therapeutic Target for Lung Cancer. J. Natl. Cancer Inst. 2015, 107, 151. [Google Scholar] [CrossRef]
- Gao, Z.; Shi, R.; Yuan, K.; Wang, Y. Expression and prognostic value of E2F activators in NSCLC and subtypes: A research based on bioinformatics analysis. Tumour Biol. 2016, 37, 14979–14987. [Google Scholar] [CrossRef]
- Farina, A.; Manni, I.; Fontemaggi, G.; Tiainen, M.; Cenciarelli, C.; Bellorini, M.; Mantovani, R.; Sacchi, A.; Piaggio, G. Down-regulation of cyclin B1 gene transcription in terminally differentiated skeletal muscle cells is associated with loss of functional CCAAT-binding NF-Y complex. Oncogene 1999, 18, 2818–2827. [Google Scholar] [CrossRef][Green Version]
- Marziali, G.; Perrotti, E.; Ilari, R.; Coccia, E.M.; Mantovani, R.; Testa, U.; Battistini, A. The activity of the CCAAT-box binding factor NF-Y is modulated through the regulated expression of its A subunit during monocyte to macrophage differentiation: Regulation of tissue-specific genes through a ubiquitous transcription factor. Blood 1999, 93, 519–526. [Google Scholar] [CrossRef]
- Gurtner, A.; Manni, I.; Fuschi, P.; Mantovani, R.; Guadagni, F.; Sacchi, A.; Piaggio, G. Requirement for down-regulation of the CCAAT-binding activity of the NF-Y transcription factor during skeletal muscle differentiation. Mol. Biol. Cell 2003, 14, 2706–2715. [Google Scholar] [CrossRef]
- Dolfini, D.; Minuzzo, M.; Pavesi, G.; Mantovani, R. The short isoform of NF-YA belongs to the embryonic stem cell transcription factor circuitry. Stem Cells 2012, 30, 2450–2459. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Joe, G.J.; Pompetti, R.; Emerson, S.G. NF-Ya activates multiple hematopoietic stem cell (HSC) regulatory genes and promotes HSC self-renewal. Proc. Natl. Acad. Sci. USA 2005, 102, 11728–11733. [Google Scholar] [CrossRef]
- Domashenko, A.D.; Danet-Desnoyers, G.; Aron, A.; Carroll, M.P.; Emerson, S.G. TAT-mediated transduction of NF-YA peptide induces the ex vivo proliferation and engraftment potential of human hematopoietic progenitor cells. Blood 2010, 116, 2676–2683. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezzecchi, E.; Ronzio, M.; Dolfini, D.; Mantovani, R. NF-YA Overexpression in Lung Cancer: LUSC. Genes 2019, 10, 937. https://doi.org/10.3390/genes10110937
Bezzecchi E, Ronzio M, Dolfini D, Mantovani R. NF-YA Overexpression in Lung Cancer: LUSC. Genes. 2019; 10(11):937. https://doi.org/10.3390/genes10110937
Chicago/Turabian StyleBezzecchi, Eugenia, Mirko Ronzio, Diletta Dolfini, and Roberto Mantovani. 2019. "NF-YA Overexpression in Lung Cancer: LUSC" Genes 10, no. 11: 937. https://doi.org/10.3390/genes10110937
APA StyleBezzecchi, E., Ronzio, M., Dolfini, D., & Mantovani, R. (2019). NF-YA Overexpression in Lung Cancer: LUSC. Genes, 10(11), 937. https://doi.org/10.3390/genes10110937





