The Role of Molecular Testing for the Indeterminate Thyroid FNA
Abstract
1. Introduction
2. Molecular Testing and the Indeterminate Categories of the Bethesda System
2.1. AUS/FLUS
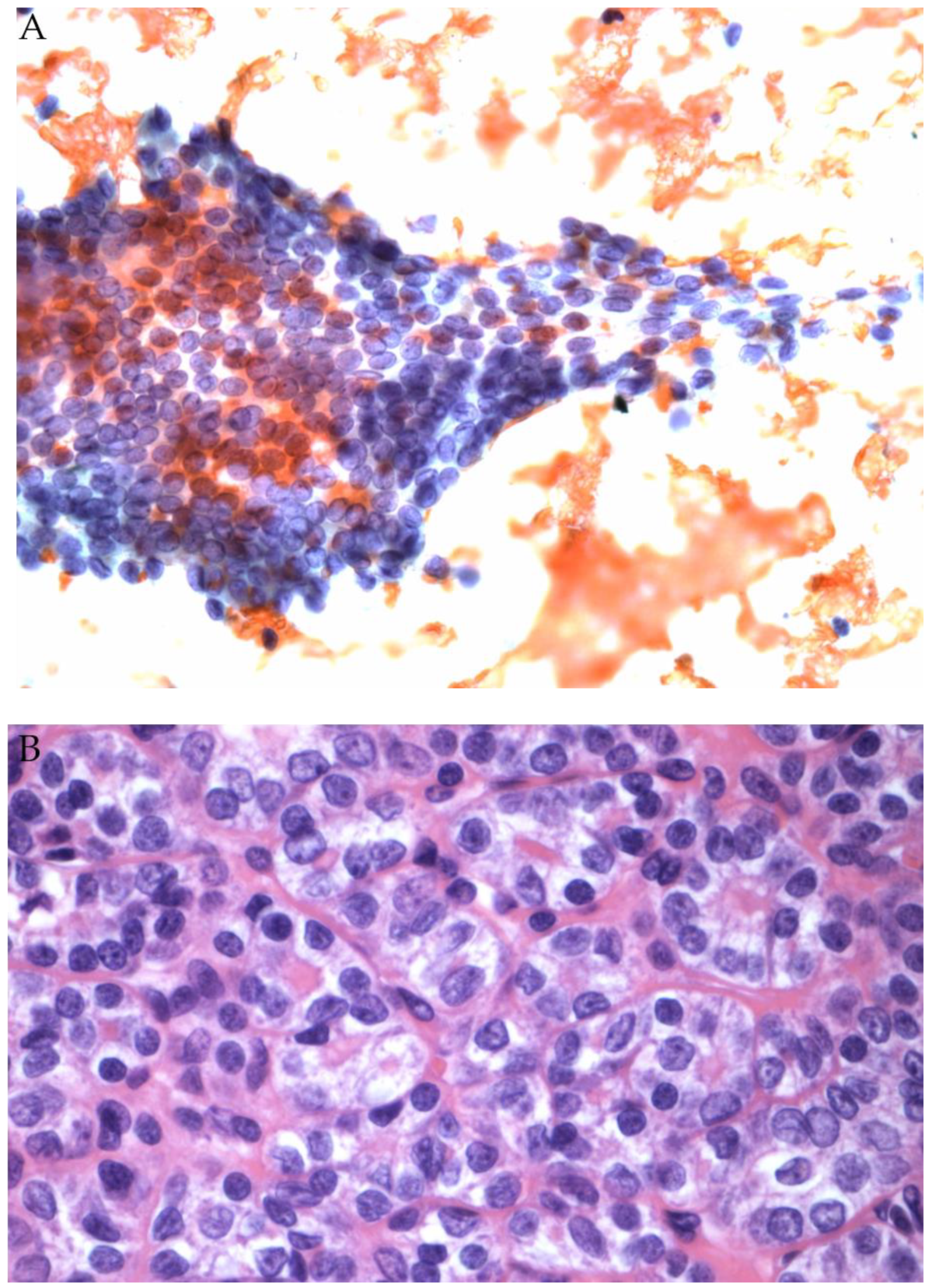
2.2. Follicular Neoplasm (FN/SFN)
2.3. The Application of Molecular Testing to NIFTP
3. Conclusions
Funding
Conflicts of Interest
References
- Cramer, H. Fine-needle aspiration cytology of the thyroid: An appraisal. Cancer 2000, 90, 325–329. [Google Scholar] [CrossRef]
- Gharib, H.; Papini, E.; Paschke, R. Thyroid nodules: A review of current guidelines, practices, and prospects. Eur. J. Endocrinol. 2008, 159, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Treglia, G.; Condorelli, E.; Romanelli, F.; Crescenzi, A.; Bongiovanni, M.; Giovanella, L. BRAF-mutated carcinomas among thyroid nodules with prior indeterminate FNA report: A systematic review and meta-analysis. Clin. Endocrinol. 2016, 84, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Broome, J.T.; Solorzano, C.C. The impact of atypia/follicular lesion of undetermined significance on the rate of malignancy in thyroid fine-needle aspiration: Evaluation of the Bethesda System for Reporting Thyroid Cytopathology. Surgery 2011, 150, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Ravetto, C.; Colombo, L.; Dottorini, M.E. Usefulness of fine-needle aspiration in the diagnosis of thyroid carcinoma: A retrospective study in 37,895 patients. Cancer 2000, 90, 357–363. [Google Scholar] [CrossRef]
- Poller, D.N.; Ibrahim, A.K.; Cummings, M.H.; Mikell, J.J.; Boote, D.; Perry, M. Fine-needle aspiration of the thyroid. Importance of an indeterminate diagnostic category. Cancer Cytopathol. 2000, 90, 239–244. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Sarti, E.E.; Jain, K.S.; Wang, H.; Nixon, I.J.; Shaha, A.R.; Shah, J.P.; Kraus, D.H.; Ghossein, R.; Fish, S.A.; et al. Malignancy Rate in Thyroid Nodules Classified as Bethesda Category III (AUS/FLUS). Thyroid 2014, 24, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Chu, Y.C.; Kim, L.; Park, I.S.; Han, J.Y.; Kim, J.M. Reclassifying Formerly Indeterminate Thyroid FNAs Using the Bethesda System Reduces the Number of Inconclusive Cases. Acta Cytol. 2012, 56, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ding, X.; Klein, M.; Sugrue, C.; Matano, S.; Edelman, M.; Wasserman, P. Thyroid fine-needle aspiration with atypia of undetermined significance: A necessary or optional category? Cancer 2009, 117, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Damiani, D.; Suciu, V.; Vielh, P. Cytopathology of Follicular Cell Nodules. Endocr. Pathol. 2015, 26, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Nagarkatti, S.S.; Faquin, W.C.; Lubitz, C.C.; Garcia, D.M.; Barbesino, G.; Ross, D.S.; Hodin, R.A.; Daniels, G.H.; Parangi, S. Management of Thyroid Nodules with Atypical Cytology on Fine-needle Aspiration Biopsy. Ann. Surg. Oncol. 2013, 20, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.T.; Clark, D.P.; Erozan, Y.S.; Ali, S.Z. Spectrum of Risk of Malignancy in Subcategories of ‘Atypia of Undetermined Significance’. Acta Cytol. 2011, 55, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Horne, M.J.; Chhieng, D.C.; Theoharis, C.; Schofield, K.; Kowalski, D.; Prasad, M.L.; Hammers, L.; Udelsman, R.; Adeniran, A.J. Thyroid follicular lesion of undetermined significance: Evaluation of the risk of malignancy using the two-tier sub-classification. Diagn. Cytopathol. 2012, 40, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, J.; Ahn, S.; Shin, J.H.; Oh, Y.L. The prediction of malignant risk in the category “atypia of undetermined significance/follicular lesion of undetermined significance” of the Bethesda System for Reporting Thyroid Cytopathology using subcategorization and BRAF mutation results. Cancer Cytopathol. 2014, 122, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Dincer, N.; Balci, S.; Yazgan, A.; Guney, G.; Ersoy, R.; Cakir, B.; Guler, G. Follow-up of atypia and follicular lesions of undetermined significance in thyroid fine needle aspiration cytology. Cytopathology 2013, 24, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Gocun, P.U.; Karakuş, E.; Bulutay, P.; Akturk, M.; Akin, M.; Poyraz, A.; Gocun, P.U. What is the malignancy risk for atypia of undetermined significance? Three years’ experience at a university hospital in Turkey. Cancer Cytopathol. 2014, 122, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Correia-Rodrigues, H.G.; Nogueira De Pontes, A.A.; Adan, L.F. Use of molecular markers in samples obtained from preoperative aspiration of thyroid. End. J. 2012, 59, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Baloch, Z.W.; Bernet, V.; Chen, A.; Fahey, T.J., 3rd; Ganly, I.; Hodak, S.P.; Kebebew, E.; Patel, K.N.; Shaha, A.; et al. For the American Thyroid Association Surgical Affairs Committee. American Thyroid Association Statement on Surgical Application of Molecular Profiling for Thyroid Nodules: Current Impact on Perioperative Decision Making. Thyroid 2015, 25, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Bartolazzi, A.; Orlandi, F.; Saggiorato, E.; Volante, M.; Arecco, F.; Rossetto, R.; Palestini, N.; Ghigo, E.; Papotti, M.; Bussolati, G.; et al. Italian Thyroid Cancer Study Group (ITCSG). Galectin-3 expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: A prospective multicentre study. Lancet Oncol. 2008, 9, 543–549. [Google Scholar] [CrossRef]
- Longatto-Filho, A.; Goncalves, A.E.; Martinho, O.; Schmitt, F.C.; Reis, R.M. Liquid based cytology in DNA-based molecular research. Anal. Quant. Cytol. Histol. 2009, 31, 395–400. [Google Scholar]
- Nikiforova, M.N.; Nikiforov, Y.E. Molecular Diagnostics and Predictors in Thyroid Cancer. Thyroid 2009, 19, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Steward, D.L.; Robinson-Smith, T.M.; Haugen, B.R.; Klopper, J.P.; Zhu, Z.; Fagin, J.A.; Falciglia, M.; Weber, K.; Nikiforova, M.N. Molecular Testing for Mutations in Improving the Fine-Needle Aspiration Diagnosis of Thyroid Nodules. J. Clin. Endocrinol. Metab. 2009, 94, 2092–2098. [Google Scholar] [CrossRef] [PubMed]
- Ohori, N.P.; Nikiforova, M.N.; Schoedel, K.E.; Lebeau, S.O.; Hodak, S.P.; Seethala, R.R.; Carty, S.E.; Ogilvie, J.B.; Yip, L.; Nikiforov, Y.E. Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance”. Cancer Cytopathol. 2010, 118, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Krane, J.F.; Cibas, E.S.; Alexander, E.K.; Paschke, R.; Eszlinger, M. Molecular analysis of residual ThinPrep material from thyroid FNAs increases diagnostic sensitivity. Cancer Cytopathol. 2015, 123, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.D.; Martini, M.; Capodimonti, S.; Lombardi, C.P.; Pontecorvi, A.; Vellone, V.G.; Zannoni, G.F.; Larocca, L.M.; Fadda, G. BRAF (v600e) mutation analysis on LBC-processed aspiration biopsies predicts bilaterality and nodal involvement in papillary thyroid microcarcinoma. Cancer Cytopathol. 2013, 121, 291–297. [Google Scholar] [CrossRef]
- Soares, P.; Trovisco, V.; Rocha, A.S.; Lima, J.; Castro, P.; Preto, A.; Máximo, V.; Botelho, T.; Seruca, R.; Sobrinho-Simões, M. BRAF mutations and RET/PTC rearrangements are alternative events in the ethiopathogenesis of PTC. Oncogene 2003, 22, 4578–4580. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.; Carydis, B.; Ezzat, S.; Bedard, Y.C.; Asa, S.L. Analysis of ret/PTC Gene Rearrangements Refines the Fine Needle Aspiration Diagnosis of Thyroid Cancer. J. Clin. Endocrinol. Metab. 2001, 86, 2187–2190. [Google Scholar] [CrossRef]
- Moses, W.; Weng, J.; Sansano, I.; Peng, M.; Khanafshar, E.; Ljung, B.-M.; Duh, Q.-Y.; Clark, O.H.; Kebebew, E. Molecular Testing for Somatic Mutations Improves the Accuracy of Thyroid Fine-needle Aspiration Biopsy. World J. Surg. 2010, 34, 2589–2594. [Google Scholar] [CrossRef]
- Musholt, T.J.; Fottner, C.; Weber, M.; Eichhorn, W.; Pohlenz, J.; Musholt, P.B.; Springer, E.; Schad, A. Detection of Papillary Thyroid Carcinoma by Analysis of BRAF and RET/PTC1 Mutations in Fine-needle Aspiration Biopsies of Thyroid Nodules. World J. Surg. 2010, 34, 2595–2603. [Google Scholar] [CrossRef]
- Colanta, A.; Lin, O.; Tafe, L.; Ghossein, R.; Nafa, K.; Mitchell, T.; Ladanyi, M.; Arcila, M. BRAF Mutation Analysis of Fine-Needle Aspiration Biopsies of Papillary Thyroid Carcinoma: Impact on Diagnosis and Prognosis. Acta Cytol. 2011, 55, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Ohori, N.P.; Hodak, S.P.; Carty, S.E.; Lebeau, S.O.; Ferris, R.L.; Yip, L.; Seethala, R.R.; Tublin, M.E.; Stang, M.T.; et al. Impact of Mutational Testing on the Diagnosis and Management of Patients with Cytologically Indeterminate Thyroid Nodules: A Prospective Analysis of 1056 FNA Samples. J. Clin. Endocrinol. Metab. 2011, 96, 3390–3397. [Google Scholar] [CrossRef] [PubMed]
- Radkay, L.; Chiosea, S.I.; Seethala, R.R.; Hodak, S.P.; LeBeau, S.O.; Yip, L.; McCoy, K.L.; Carty, S.E.; Schoedel, K.E.; Nikiforova, M.N.; et al. Thyroid nodules with KRAS mutations are different from nodules with NRAS and HRAS mutations with regard to cytopathologic and histopathologic outcome characteristics. Cancer Cytopathol. 2014, 122, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Wald, A.I.; Roy, S.; Durso, M.B.; Nikiforov, Y.E. Targeted next-generation sequencing panel (Thyro Seq) for detection of mutations in thyroid cancer. J. Clin. Endocrinol. Metab. 2013, 98, E1852–E1860. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Carty, S.E.; Chiosea, S.I.; Coyne, C.; Duvvuri, U.; Ferris, R.L.; Gooding, W.E.; Hodak, S.P.; LeBeau, S.O.; Ohori, N.P.; et al. Highly accurate diagnosis of cancer in thyroid nodules with follicolar neoplasm/suspicious for a follicular neoplasm cytology by Thyroseq v2 next generation sequencing assay. Cancer 2014, 120, 3627–3634. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Carty, S.E.; Chiosea, S.I.; Coyne, C.; Duvvuri, U.; Ferris, R.L.; Gooding, W.E.; Lebeau, S.O.; Ohori, N.P.; Seethala, R.R.; et al. Impact of the Multi-Gene ThyroSeq Next-Generation Sequencing Assay on Cancer Diagnosis in Thyroid Nodules with Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance Cytology. Thyroid 2015, 25, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.K.; Kennedy, G.C.; Baloch, Z.W.; Chudova, D.; Friedman, L.; Rosai, J.; Wilde, J.I.; Cibas, E.S.; Diggans, J.; Kloos, R.T.; et al. Preoperative Diagnosis of Benign Thyroid Nodules with Indeterminate Cytology. N. Engl. J. Med. 2012, 367, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. Braf mutationin papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr. Rev. 2007, 28, 742–762. [Google Scholar] [CrossRef] [PubMed]
- Puxeddu, E.; Durante, C.; Avenia, N.; Filetti, S.; Russo, D. Clinical implications of BRAF mutation in thyroid carcinoma. Trends Endocrinol. Metab. 2008, 19, 138–145. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Ali, S.; Cibas, E.S. The Bethesda System for Reporting Thyroid Cytopathology, 2nd ed.; Springer: Berlin, Germany, 2018. [Google Scholar]
- Hang, J.F.; Westra, W.H.; Cooper, D.S.; Ali, S.Z. The impact of noninvasive follicular thyroid neoplasm with papillary-like nuclear features on the performance of the Afirma gene expression classifier. Cancer 2017, 125, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Poller, D.N.; Glaysher, S. Molecular pathology and thyroid FNA. Cytopathology 2017, 28, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Dejmek, A.; Zendehrokh, N.; Tomaszewska, M.; Edsjö, A. Preparation of DNA from cytological material. Cancer Cytopathol. 2013, 121, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Catherwood, M.A.; Schmitt, F.; Salto-Tellez, M. Molecular diagnostics and the training of future tissue- and cell-based pathologists. Cytopathology 2012, 23, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Malapelle, U.; De Rosa, N.; Bellevicine, C.; Rocco, D.; Vitiello, F.; Piantedosi, F.V.; Illiano, A.; Nappi, O.; Troncone, G. EGFR mutations detection on liquid-based cytology: Is microscopy still necessary? J. Clin. Pathol. 2012, 65, 561–564. [Google Scholar] [CrossRef]
- Malapelle, U.; De Rosa, N.; Rocco, D.; Crispino, C.; Illiano, A.; Piantedosi, F.V.; Nappi, O.; Troncone, G. EGFR and KRAS mutations detection on lung liquid based cytology: A pilot study. J. Clin. Pathol. 2012, 65, 87–91. [Google Scholar] [CrossRef]
- Killian, J.K.; Walker, R.L.; Suuriniemi, M. Archival fine-needle aspiration cytopathology (FNAC) samples: Untapped resource for clinical molecular profiling. J. Mol. Diagn. 2010, 12, 739–745. [Google Scholar] [CrossRef]
- Fadda, G.; Rossi, E.D. Liquid-Based Cytology in Fine-Needle Aspiration Biopsies of the Thyroid Gland. Acta Cytol. 2011, 55, 389–400. [Google Scholar] [CrossRef]
- Rossi, E.D.; Schmitt, F. Pre-analytic steps for molecular testing on thyroid fine-needle aspirations: The goal of good results. CytoJournal 2013, 28, 10–24. [Google Scholar] [CrossRef]
- Chang, H.; Lee, H.; Yoon, S.O.; Kim, H.; Kim, A.; Kim, B.H. BRAF (V600E) mutation analysis of liquid-based preparation-processed fine needle aspiration sample improves the diagnostic rate of papillary thyroid carcinoma. Hum. Pathol. 2012, 43, 89–95. [Google Scholar] [CrossRef]
- Kwon, H.J.; Kim, E.-K.; Kwak, J.Y. Cytomorphologic features in thyroid nodules read as “suspicious for malignancy” on cytology may predict thyroid cancers with the BRAF mutation. Pathol. Res. Pr. 2015, 211, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Krane, J.F.; Vanderlaan, P.A.; Faquin, W.C.; Renshaw, A.A. The atypia of undetermined significance/follicular lesion of undetermined significance:malignant ratio. Cancer Cytopathol. 2012, 120, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Fulciniti, F.; Zilioli, V.; Ceriani, L.; Giovanella, L. Accuracy of international ultrasound risk stratification systems in thyroid lesions cytologically classified as indeterminate. Diagn. Cytopathol. 2017, 45, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.R.; Barletta, J.A.; Wenig, B.M.; Al Ghuzlan, A.; Kakudo, K.; et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016, 2, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Maletta, F.; Massa, F.; Torregrossa, L.; Duregon, E.; Casadei, G.P.; Basolo, F.; Tallini, G.; Volante, M.; Nikiforov, Y.E.; Papotti, M. Cytological features of “invasive follicular thyroid neoplasm with papillary-like nuclear features” and their correlation with tumor histology. Hum. Pathol. 2016, 13, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Faquin, W.C.; Wong, L.Q.; Afrogheh, A.H.; Ali, S.Z.; Bishop, J.A.; Bongiovanni, M.; Pusztaszeri, M.P.; VandenBussche, C.J.; Gourmaud, J.; Vaickus, L.J.; et al. Impact of reclassifying noninvasive follicular variant of papillary thyroid carcinoma on the risk of malignancy in the Bethesda system for reporting thyroid cytopathology. Cancer Cytopathol. 2016, 124, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.S.; Angell, T.E.; Strickland, K.C.; Alexander, E.K.; Cibas, E.S.; Krane, J.F.; Barletta, J.A. Noninvasive Follicular Variant of Papillary Thyroid Carcinoma and the Afirma Gene-Expression Classifier. Thyroid 2016, 26, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Strickland, K.C.; Howitt, B.E.; Marqusee, E.; Alexander, E.K.; Cibas, E.S.; Krane, J.F.; Barletta, J.A. The Impact of Noninvasive Follicular Variant of Papillary Thyroid Carcinoma on Rates of Malignancy for Fine-Needle Aspiration Diagnostic Categories. Thyroid 2015, 25, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Baloch, Z.W.; Nayar, R.; Bizzarro, T.; Fadda, G.; Adhikari-Guragain, D.; Hatem, J.; Larocca, L.M.; Samolczyk, J.; Slade, J.; et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): Implications for the risk of malignancy (ROM) in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC). Cancer 2017, 126, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Mazeh, H.; Mizrahi, I.; Halle, D.; Ilyayev, N.; Stojadinovic, A.; Trink, B.; Mitrani-Rosenbaum, S.; Roistacher, M.; Ariel, I.; Eid, A.; et al. Development of a microRNA-based molecular assay for the detection of papillary thyroid carcinoma in aspiration biopsy samples. Thyroid 2011, 21, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, M.; Trimboli, P.; Rossi, E.D.; Fadda, G.; Nobile, A.; Giovanella, L. Diagnosis of endocrine disease: High-yield thyroid fine-needle aspiration cytology: An update focused on ancillary techniques improving its accuracy. Eur. J. Endocrinol. 2016, 174, R53–R63. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, M.; Giovanella, L.; Romanelli, F.; Trimboli, P. Cytological Diagnoses Associated with Noninvasive Follicular Thyroid Neoplasms with Papillary-Like Nuclear Features According to the Bethesda System for Reporting Thyroid Cytopathology: A Systematic Review and Meta-Analysis. Thyroid 2019, 29, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, M.; Faquin, W.; Giovanella, L.; Durante, C.; Kopp, P.; Trimboli, P. Impact of non-invasive follicular thyroid neoplasms with papillary-like nuclear features (NIFTP) on risk of malignancy in patients undergoing lobectomy/thyroidectomy for suspicious for malignancy or malignant fine-needle aspiration cytology findings: A systematic review and meta-analysis. Eur. J. Endocrinol. 2019. [Google Scholar] [CrossRef]
- Saiselet, M.; Pita, J.M.; Augenlicht, A.; Dom, G.; Tarabichi, M.; Fimereli, D.; Dumont, J.E.; Detours, V.; Maenhaut, C. miRNA expression and function in thyroid carcinomas: A comparative and critical analysis and a model for other cancers. Oncotarget 2016, 7, 52475–52492. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Liyanarachchi, S.; Li, W.; Wakely, P.E.; Saji, M.; Huang, J.; Nagy, R.; Farrell, T.; Ringel, M.D.; De La Chapelle, A.; et al. MicroRNA Signature in Thyroid Fine Needle Aspiration Cytology Applied to “Atypia of Undetermined Significance” Cases. Thyroid 2012, 22, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Chiosea, S.I.; Nikiforov, Y.E. MicroRNA expression profiles in thyroid microRNAs in thyroid fine needle aspiration biopsy samples. Thyroid 2012, 22, 285–291. [Google Scholar]
- Nikiforova, M.N.; Tseng, G.C.; Steward, D.; Diorio, D.; Nikiforov, Y.E. MicroRNA Expression Profiling of Thyroid Tumors: Biological Significance and Diagnostic Utility. J. Clin. Endocrinol. Metab. 2008, 93, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Mazeh, H.; Levy, Y.; Mizrahi, I.; Appelbaum, L.; Ilyayev, N.; Halle, D.; Freund, H.R.; Nissan, A. Differentiating benign from malignant thyroid nodules using micro ribonucleic acid amplification in residual cells obtained by fine needle aspiration biopsy. J. Surg. Res. 2013, 180, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Keutgen, X.M.; Filicori, F.; Fahey, T.J., 3rd. Molecular diagnosis for indeterminate thyroid nodules on fine needle aspiration: Advances and limitations. Expert Rev. Mol. Diagn. 2013, 13, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Pallante, P.; Visone, R.; Ferracin, M.; Ferraro, A.; Berlingieri, M.T.; Troncone, G.; Chiappetta, G.; Liu, C.G.; Santoro, M.; Negrini, M.; et al. MircoRNA deregulation in human thyroid papillary carcinomas. Endocr. Relat. Cancer 2006, 13, 497–508. [Google Scholar] [CrossRef]
- Agretti, P.; Ferrarini, E.; Rago, T.; Candelieri, A.; De Marco, G.; Dimida, A.; Niccolai, F.; Molinaro, A.; Di Coscio, G.; Pinchera, A.; et al. MicroRNA expression profile helps to distinguish benign nodules from papillary thyroid carcinomas starting from cells of fine-needle aspiration. Eur. J. Endocrinol. 2012, 167, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhong, Q.; Chen, X.; Fang, J.; Huang, Z. Diagnostic value of microRNAs in discriminating malignant thyroid nodules from benign ones on fine-needle aspiration samples. Tumor Boil. 2014, 35, 9343–9353. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, M.; Perren, A.; Moch, H.; Komminoth, P.; Nikiforov, Y.E.; Nikiforova, M.N. Comprehensive MicroRNA Expression Profiling Identifies Novel Markers in Follicular Variant of Papillary Thyroid Carcinoma. Thyroid 2013, 23, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, M.; Vogetseder, A.; Durso, M.B.; Moch, H.; Komminoth, P.; Perren, A.; Nikiforov, Y.E.; Nikiforova, M.N. MicroRNA expression array identifies novel diagnostic markers for conventional and oncocytic follicular thyroid carcinomas. J. Clin. Endocrinol. Metab. 2013, 98, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.D.; Martini, M.; Bizzarro, T.; Capodimonti, S.; Sarti, D.; Cenci, T.; Bilotta, M.; Fadda, G.; Larocca, L.M. The evaluation of miRNAs on thyroid FNAC: The promising role of miR-375 in follicular neoplasms. Endocrine 2016, 54, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Burch, H.B.; Burman, K.D.; Cooper, D.S.; Hennessey, J.V.; Vietor, N.O. A 2015 Survey of Clinical Practice Patterns in the Management of Thyroid Nodules. J. Clin. Endocrinol. Metab. 2016, 101, 2853–2862. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benjamin, H.; Schnitzer-Perlman, T.; Shtabsky, A.; Vandenbussche, C.J.; Ali, S.Z.; Kolar, Z.; Pagni, F.; Bar, D.; Meiri, E. Analytical validity of a microRNA-based assay for diagnosing indeterminate thyroid FNA smears from routinely prepared cytology slides. Cancer Cytopathol. 2016, 124, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Le Mercier, M.; D’Haene, N.; De Neve, N.; Blanchard, O.; Degand, C.; Rorive, S.; Salmon, I. Next-generation sequencing improves the diagnosis of thyroid FNA specimens with indeterminate cytology. Histopathology 2015, 66, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Baloch, Z.W. Clinical validation of the ThyroSeq V3 genomic classifier in thyroid nodules with indeterminate FNA cytology. Cancer Cytopathol. 2019, 127, 225–230. [Google Scholar] [CrossRef]
- Nikiforova, M.; Mercurio, S.; Wald, A.; Barbi de Moura, M.; Callenberg, K.; Santana-Santos, L.; Gooding, W.E.; Yip, L.; Ferris, R.L.; Nikiforov, Y.E. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer 2018, 124, 1682–1690. [Google Scholar] [CrossRef]
- Nishino, M. Molecular cytopathology for thyroid nodules: A review of methodology and test performance. Cancer 2016, 124, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.D.; LaRocca, L.M.; Pantanowitz, L. Ancillary molecular testing of indeterminate thyroid nodules. Cancer Cytopathol. 2018, 126, 654–671. [Google Scholar] [CrossRef] [PubMed]
- Ohori, N.P.; Landau, M.S.; Carty, S.P.; Yip, L.; Lebeau, S.O.; Manroa, P.; Seethala, R.R.; Schoedel, K.E.; Nikiforova, M.N.; Nikiforov, Y.E. Benign call rate and molecular test result distribution in ThyroSeq V3. Cancer Cytopathol. 2019, 127, 161–168. [Google Scholar] [CrossRef] [PubMed]
- McIver, B.; Castro, M.R.; Morris, J.C.; Bernet, V.; Smallridge, R.; Henry, M.; Kosok, L.; Reddi, H. An Independent Study of a Gene Expression Classifier (Afirma) in the Evaluation of Cytologically Indeterminate Thyroid Nodules. J. Clin. Endocrinol. Metab. 2014, 99, 4069–4077. [Google Scholar] [CrossRef] [PubMed]
- Dedhia, P.H.; Rubio, G.A.; Cohen, M.S.; Miller, B.S.; Gauger, P.G.; Hughes, D.T. Potential Effects of Molecular Testing of Indeterminate Thyroid Nodule Fine Needle Aspiration Biopsy on Thyroidectomy Volume. World J. Surg. 2014, 38, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Kloos, R.T. Molecular Profiling of Thyroid Nodules: Current Role for the Afirma Gene Expression Classifier on Clinical Decision Making. Mol. Imaging Radionucl. Ther. 2017, 26, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.; Sosa, J.A.; Jiang, X. Evaluation of the Afirma Gene Expression Classifier in Repeat Indeterminate Thyroid Nodules. Arch. Pathol. Lab. Med. 2017, 141, 985–989. [Google Scholar] [CrossRef]
- Baca, S.C.; Wong, K.S.; Strickland, K.C.; Heller, H.T.; Kim, M.I.; Barletta, J.A.; Cibas, E.S.; Krane, J.F.; Marqusee, E.; Angell, T.E. Qualifiers of atypia in the cytologic diagnosis of thyroid nodules are associated with different Afirma gene expression classifier results and clinical outcomes. Cancer Cytopathol. 2017, 125, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Marti, J.L.; Avadhani, V.; Donatelli, L.A.; Niyogi, S.; Wang, B.; Wong, R.J.; Shaha, A.R.; Ghossein, R.A.; Lin, O.; Morris, L.G.T.; et al. Wide Inter-institutional Variation in Performance of a Molecular Classifier for Indeterminate Thyroid Nodules. Ann. Surg. Oncol. 2015, 22, 3996–4001. [Google Scholar] [CrossRef]
- Balentine, C.J.; Vanness, D.I.; Schneider, D.F. Cost-effectiveness of lobectomy versus genetic testing (Afirma®) for indeterminate thyroid nodules: Considering the costs of surveillance. Surgery 2018, 163, 88–96. [Google Scholar] [CrossRef]
- Hao, Y.; Duh, Q.Y.; Kloos, R.T.; Babiarz, J.; Harrell, R.M.; Traweek, S.T.; Kim, S.Y.; Fedorowicz, G.; Walsh, P.S.; Sadow, P.M.; et al. Identification of Hürthle cell cancers: Solving a clinical challenge with genomic sequencing and a trio of machine learning algorithms. BMC Syst. Boil. 2019, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Angell, T.E.; Heller, H.T.; Cibas, E.S.; Barletta, J.A.; Kim, M.I.; Krane, J.F.; Marqusee, E. Independent Comparison of the Afirma Genomic Sequencing Classifier and Gene Expression Classifier for Cytologically Indeterminate Thyroid Nodules. Thyroid 2019, 29, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Nabhan, F.; Porter, K.; Roll, K.; Shirley, L.A.; Azaryan, I.; Tonkovich, D.; Perlick, J.; Ryan, L.E.; Khawaja, R. Afirma Gene Sequencing Classifier Compared with Gene Expression Classifier in Indeterminate Thyroid Nodules. Thyroid 2019, 29, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Brauner, E.; Holmes, B.J.; Krane, J.F.; Nishino, M.; Zurakowski, D.; Hennessey, J.V.; Faquin, W.C.; Parangi, S. Performance of the Afirma Gene Expression Classifier in Hürthle Cell Thyroid Nodules Differs from Other Indeterminate Thyroid Nodules. Thyroid 2015, 25, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Lithwick-Yanai, G.; Dromi, N.; Shtabsky, A.; Morgenstern, S.; Strenov, Y.; Feinmesser, M.; Kravtsov, V.; Leon, M.E.; Hajdúch, M.; Ali, S.Z. Multicentre validation of a microRNA-based assay for diagnosing indeterminate thyroid nodules utilizing fine needle aspirate smears. J. Clin. Pathol. 2017, 70, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Keutgen, X.M.; Filicori, F.; Crowley, M.J.; Wang, Y.; Scognamiglio, T.; Hoda, R.; Buitrago, D.; Cooper, D.; Zeiger, M.A.; Zarnegar, R.; et al. A Panel of Four miRNAs Accurately Differentiates Malignant from Benign Indeterminate Thyroid Lesions on Fine Needle Aspiration. Clin. Cancer Res. 2012, 18, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Labourier, E.; Shifrin, A.; Busseniers, A.E.; Lupo, M.A.; Manganelli, M.L.; Andruss, B.; Wylie, D.; Beaudenon-Huibregtse, S. Molecular Testing for miRNA, mRNA, and DNA on Fine-Needle Aspiration Improves the Preoperative Diagnosis of Thyroid Nodules With Indeterminate Cytology. J. Clin. Endocrinol. Metab. 2015, 100, 2743–2750. [Google Scholar] [CrossRef]
- Wylie, D.; Beaudenon-Huibregtse, S.; Haynes, B.C.; Giordano, T.J.; Labourier, E. Molecular classification of thyroid lesions by combined testing for miRNA gene expression and somatic gene alterations. J. Pathol. Clin. Res. 2016, 2, 93–103. [Google Scholar] [CrossRef]
- Ohori, N.P.; Wolfe, J.; Hodak, S.P.; LeBeau, S.O.; Yip, L.; Carty, S.E.; Duvvuri, U.; Schoedel, K.E.; Nikiforova, M.N.; Nikiforov, Y.E. “Colloid-rich” follicular neoplasm/suspicious for follicular neoplasm thyroid fine-needle aspiration specimens: Cytologic, histologic, and molecular basis for considering an alternate view. Cancer Cytopathol. 2013, 121, 718–728. [Google Scholar] [CrossRef]
- Paskas, S.; Jankovic, J.; Živaljević, V.; Tatic, S.; Bozic, V.; Nikolić, A.; Radojkovic, D.; Savin, S.; Cvejiċ, D. Malignant risk stratification of thyroid FNA specimens with indeterminate cytology based on molecular testing. Cancer Cytopathol. 2015, 123, 471–479. [Google Scholar] [CrossRef]
- Rossi, E.D.; Bizzarro, T.; Martini, M.; Larocca, L.M.; Schmitt, F.; Vielh, P. Cytopathology of Follicular Cell Nodules. Adv Anat Pathol. 2017, 24, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Straccia, P.; Rossi, E.D.; Bizzarro, T.; Brunelli, C.; Cianfrini, F.; Damiani, D.; Fadda, G. A meta-analytic review of the Bethesda System for Reporting Thyroid Cytopathology: Has the rate of malignancy in indeterminate lesions been underestimated? Cancer Cytopathol. 2015, 123, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Jara, S.M.; Bhatnagar, R.; Guan, H.; Gocke, C.D.; Ali, S.Z.; Tufano, R.P. Utility of BRAF mutation detection in fine-needle aspiration biopsy samples read as “suspicious for papillary thyroid carcinoma”. Head Neck 2015, 37, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- Lau, R.P.; Paulsen, J.D.; Brandler, T.C.; Liu, C.Z.; Simsir, A.; Zhou, F. Impact of the Reclassification of “Noninvasive Encapsulated Follicular Variant of Papillary Thyroid Carcinoma” to “Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features” on the Bethesda System for Reporting Thyroid Cytopathology: A Large Academic Institution’s Experience. Am. J. Clin. Pathol. 2017. [Google Scholar] [CrossRef]
- Esebua, M.; Kannuswamy, R.; Layfield, L.J.; Baloch, Z.W.; Schmidt, R.L. Impact of the Reclassification of the Non-Invasive Follicular Variant of Papillary Carcinoma as Benign on the Malignancy Risk of the Bethesda System for Reporting Thyroid Cytopathology: A Meta-Analysis Study. Acta Cytol. 2017, 61, 187–193. [Google Scholar]
- Shahi, M.; Yousaf, H.; Amin, K.; Li, F. Impact of New Nomenclature “Non-Invasive Follicular Neoplasm with Papillary Like Nuclear Features” on the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC). An Institutional Experience. Mod. Pathol. 2017, 30, 116A. [Google Scholar]
- Bizzarro, T.; Martini, M.; Capodimonti, S.; Straccia, P.; Lombardi, C.P.; Pontecorvi, A.; LaRocca, L.M.; Rossi, E.D. Young investigator challenge: The morphologic analysis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features on liquid-based cytology: Some insights into their identification. Cancer Cytopathol. 2016, 124, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.C.H.; Fried, K.O.; Scognamiglio, T. Sonographic and cytologic differences of NIFTP from infiltrative or invasive encapsulated follicular variant of papillary thyroid carcinoma: A Review of 179 Cases. Diagn. Cytopathol. 2017, 45, 533–541. [Google Scholar] [CrossRef]
- Jiang, X.S.; Harrison, G.P.; Datto, M.B. Young Investigator Challenge: Molecular testing in noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Cancer Cytopathol. 2016, 124, 893–900. [Google Scholar] [CrossRef]
- Song, S.J.; LiVolsi, V.A.; Montone, K.; Baloch, Z. Pre-operative features of non-invasive follicular thyroid neoplasms with papillary-like nuclear features: An analysis of their cytological, Gene Expression Classifier and sonographic findings. Cytopathology 2017, 28, 488–494. [Google Scholar] [CrossRef]
- Basolo, F.; Macerola, E.; Ugolini, C.; Poller, D.N.; Baloch, Z. The molecular landscape of non invasive follicular thyroid neoplasm with papillary like-nuclear features (NIFTP): A literature review. Adv. Anat. Pathol. 2017, 24, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, M.; Kwon, A.Y.; Choe, J.H.; Kim, J.H.; Kim, J.S.; Hahn, S.Y.; Shin, J.H.; Chung, M.K.; Son, Y.I. Molecular genotyping of the non-invasive encapsulated follicular variant of papillary thyroid carcinoma. Histopathology 2017. [Google Scholar] [CrossRef] [PubMed]
- Steward, D.L.; Carty, S.E.; Sippel, R.S.; Yang, S.P.; Sosa, J.A.; Sipos, J.A.; Figge, J.J.; Mandel, S.; Haugen, B.R.; Burmen, K.D. Performance of a Multigene Genomic Classifier in Thyroid Nodules With Indeterminate Cytology: A Prospective Blinded Multicenter Study. JAMA Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sahli, Z.T.; Smith, P.W.; Umbricht, C.B.; Zeiger, M.A. Preoperative Molecular Markers in Thyroid Nodules. Front. Endocrinol. (Lausanne) 2018, 9, 179. [Google Scholar] [CrossRef] [PubMed]
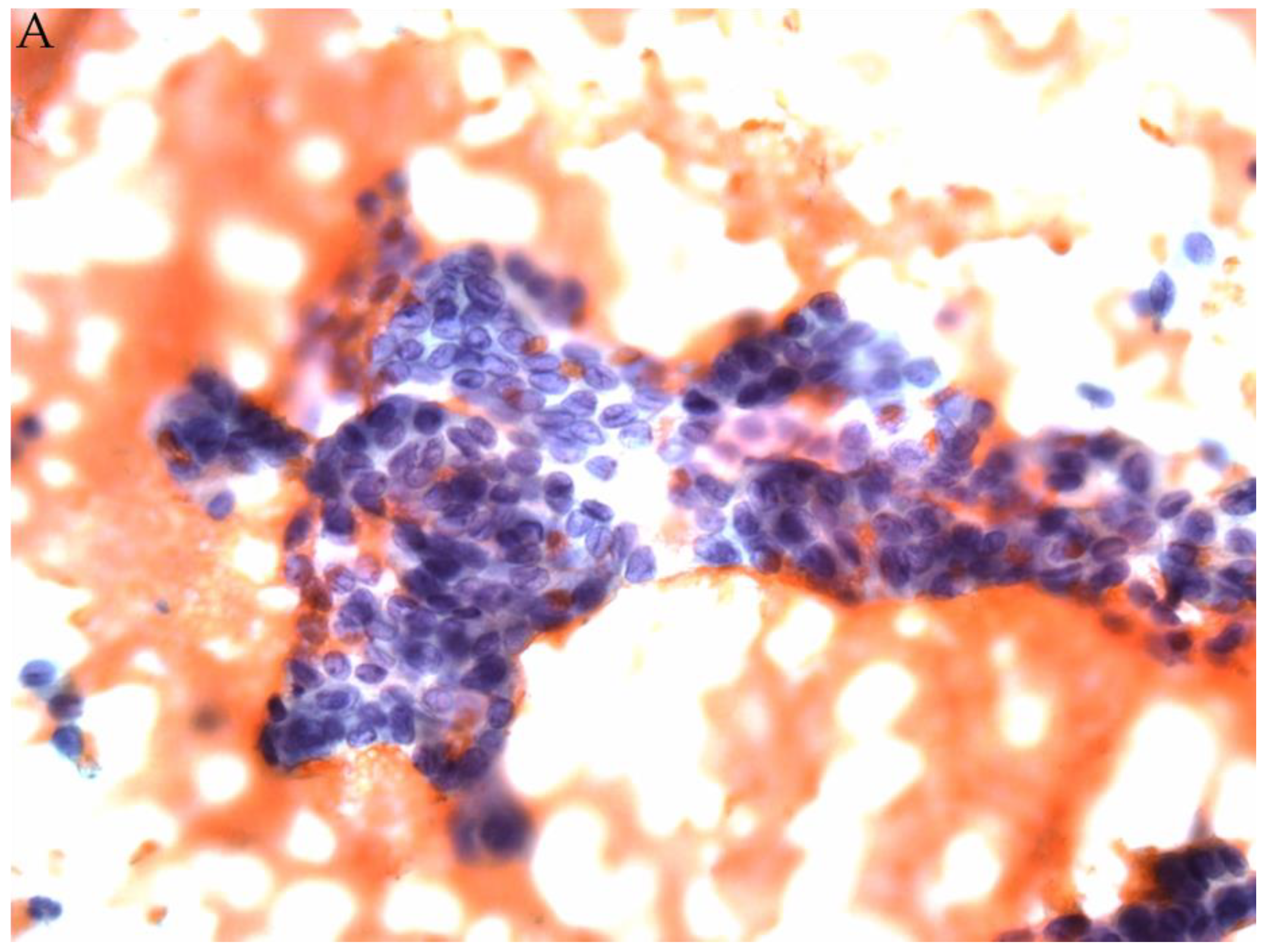
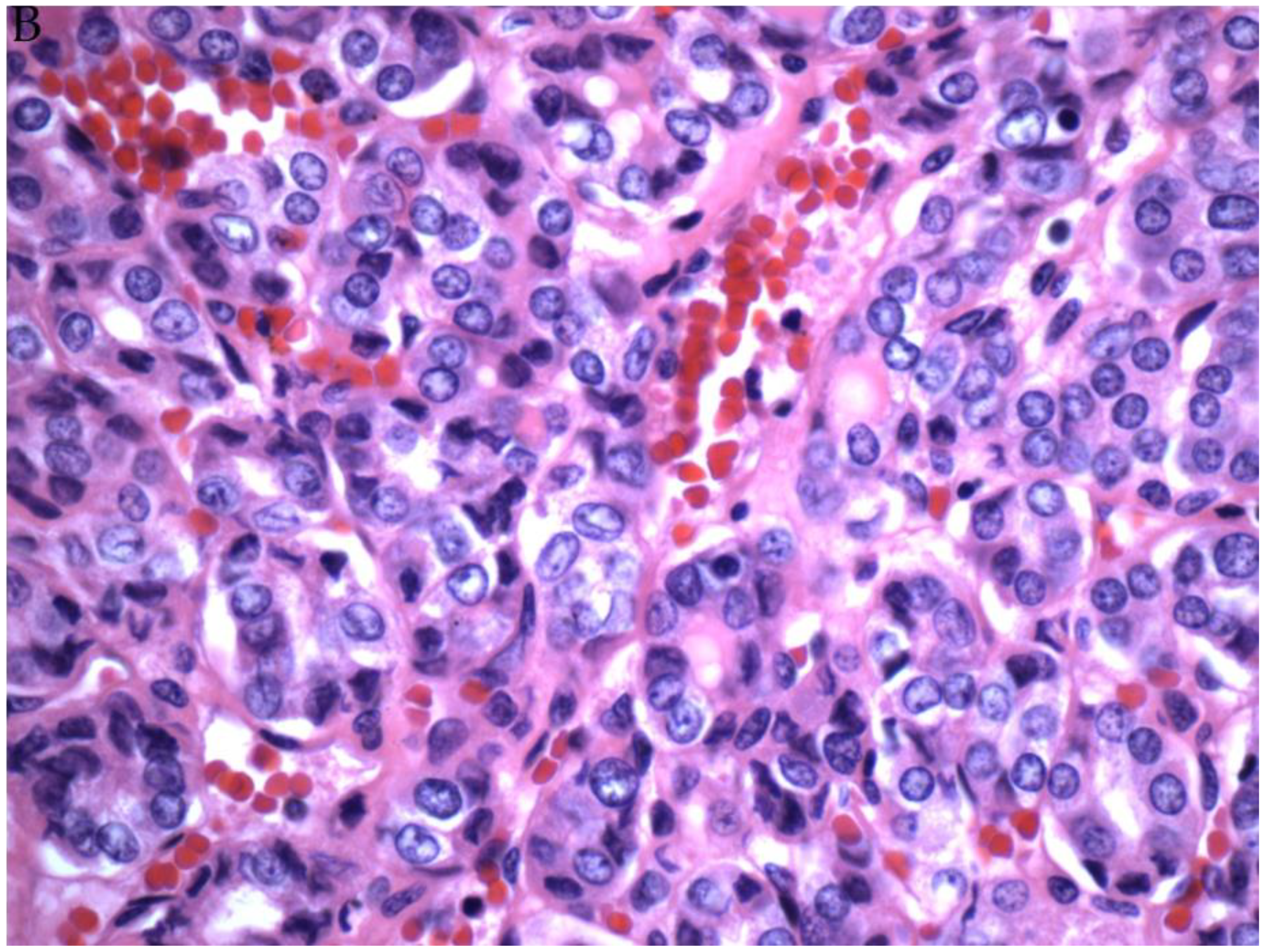
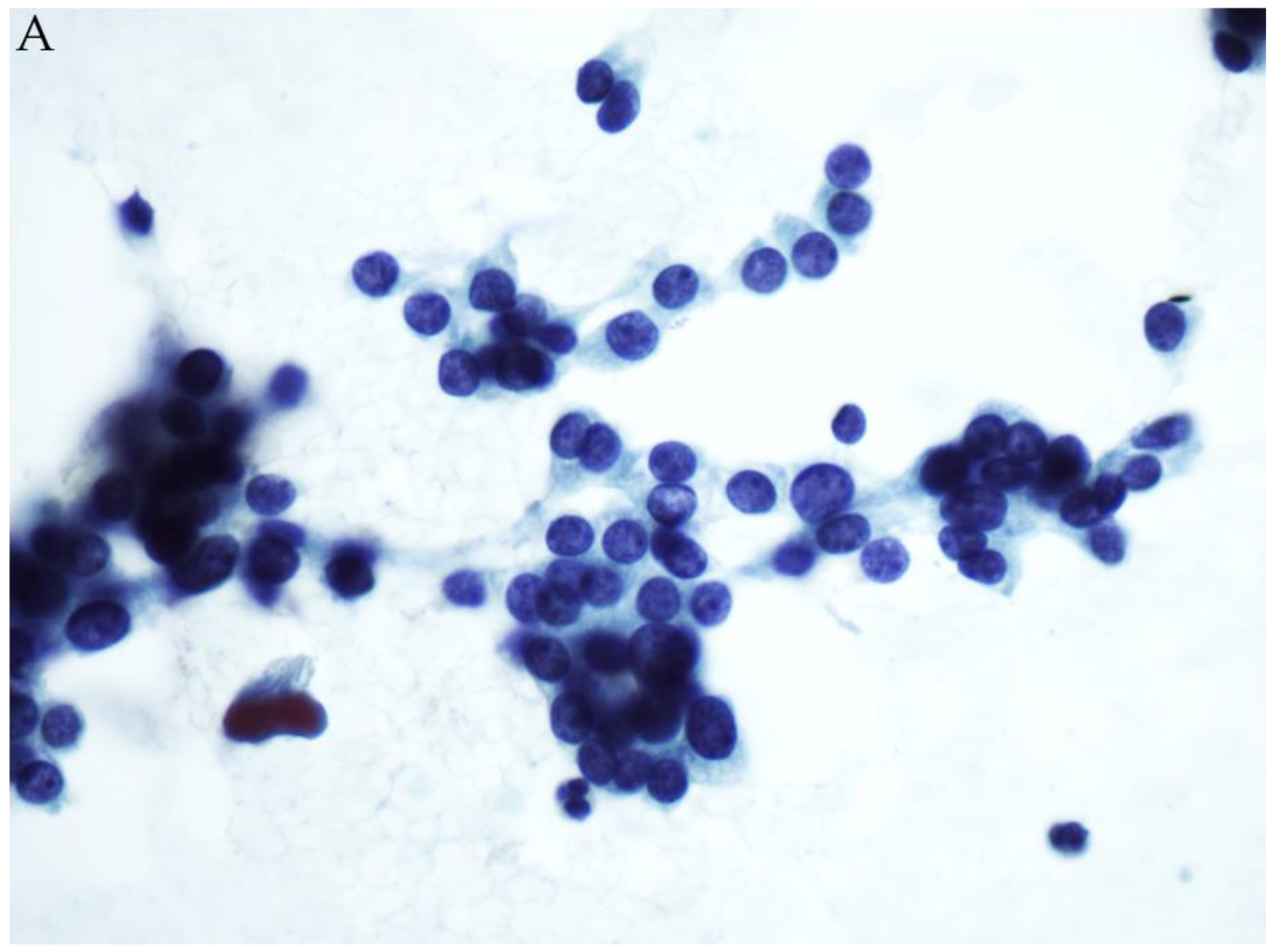
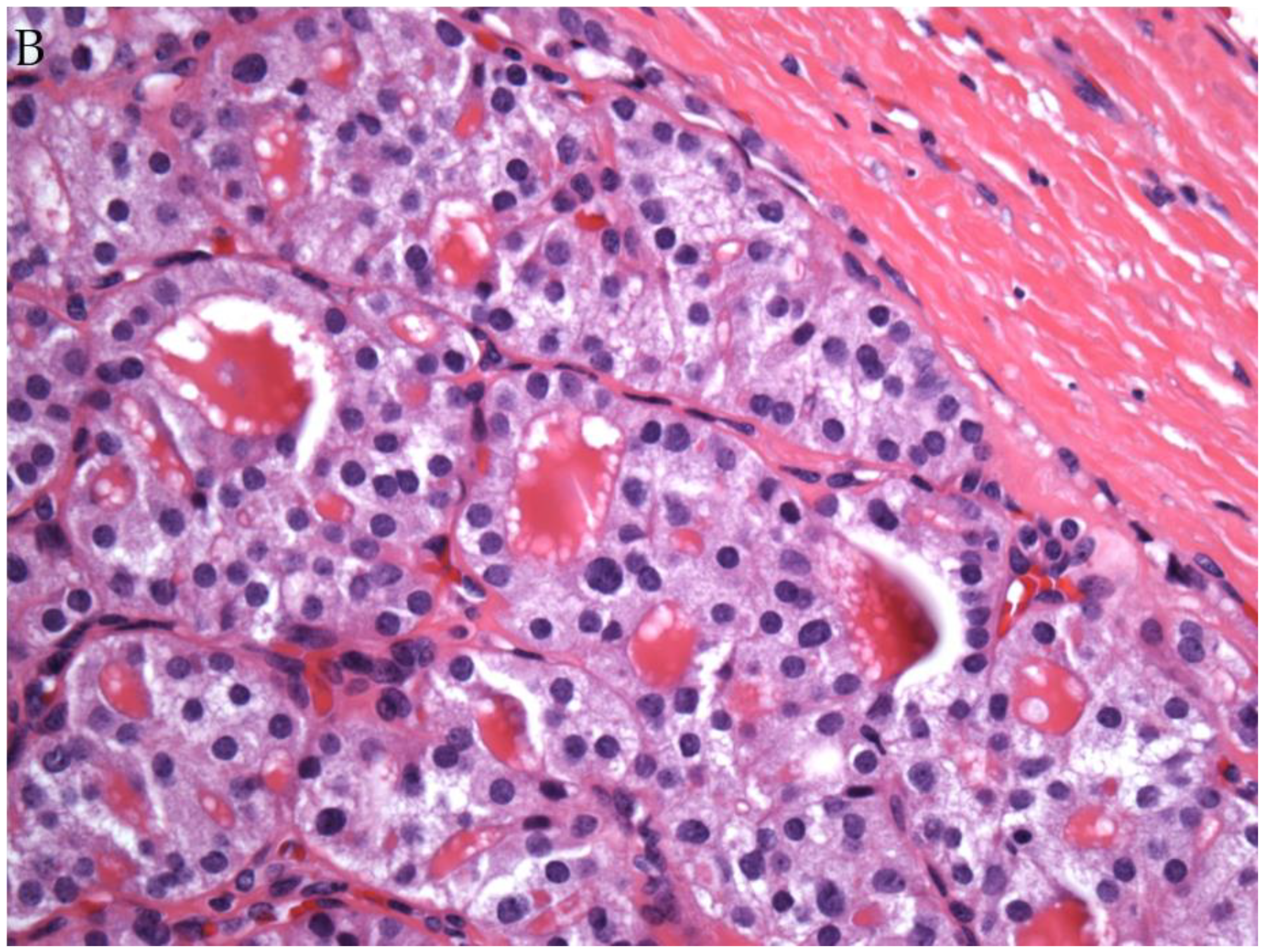
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, E.D.; Pantanowitz, L.; Faquin, W.C. The Role of Molecular Testing for the Indeterminate Thyroid FNA. Genes 2019, 10, 736. https://doi.org/10.3390/genes10100736
Rossi ED, Pantanowitz L, Faquin WC. The Role of Molecular Testing for the Indeterminate Thyroid FNA. Genes. 2019; 10(10):736. https://doi.org/10.3390/genes10100736
Chicago/Turabian StyleRossi, Esther Diana, Liron Pantanowitz, and William C. Faquin. 2019. "The Role of Molecular Testing for the Indeterminate Thyroid FNA" Genes 10, no. 10: 736. https://doi.org/10.3390/genes10100736
APA StyleRossi, E. D., Pantanowitz, L., & Faquin, W. C. (2019). The Role of Molecular Testing for the Indeterminate Thyroid FNA. Genes, 10(10), 736. https://doi.org/10.3390/genes10100736




