Human Mesenchymal Stromal Cell Secretome Promotes the Immunoregulatory Phenotype and Phagocytosis Activity in Human Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. hBMSC Culture and PUFA Supplementation
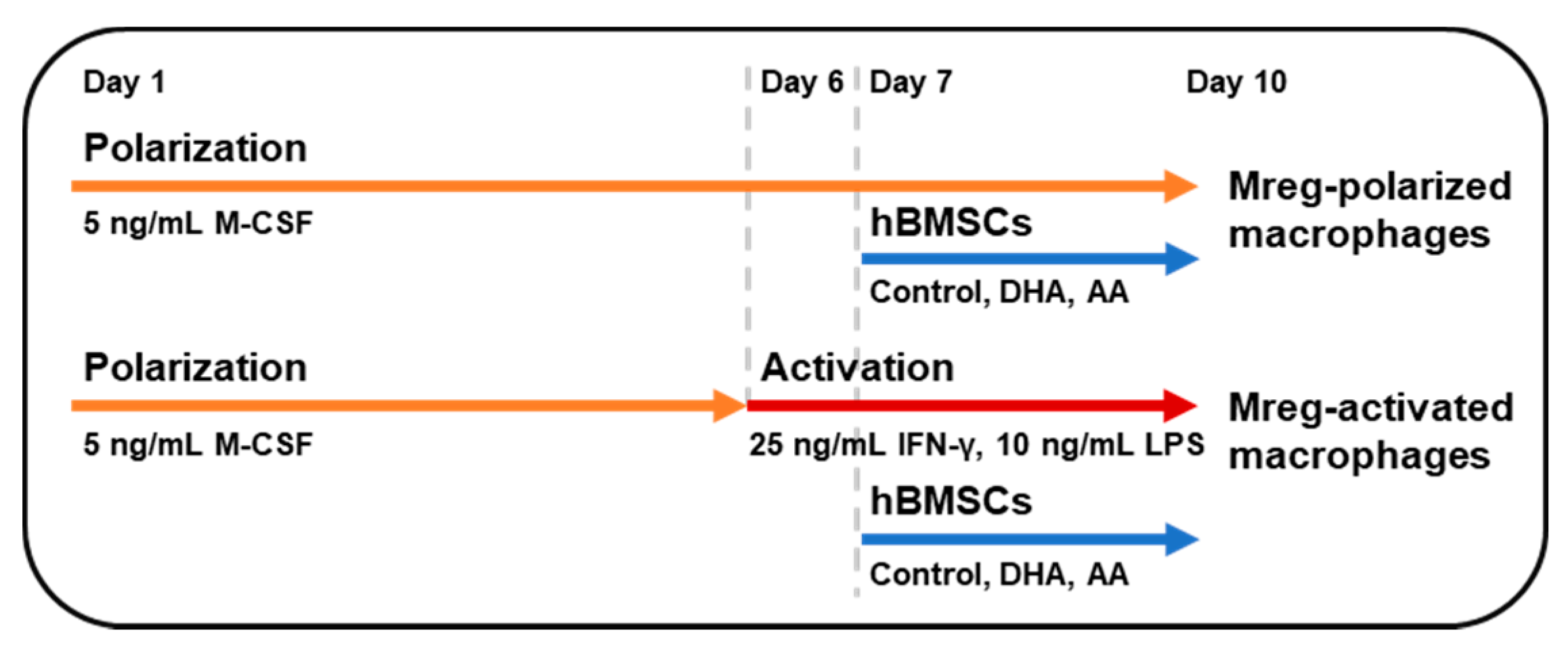
2.2. Mreg Polarization Assay
2.3. Determination of Cytokine Production
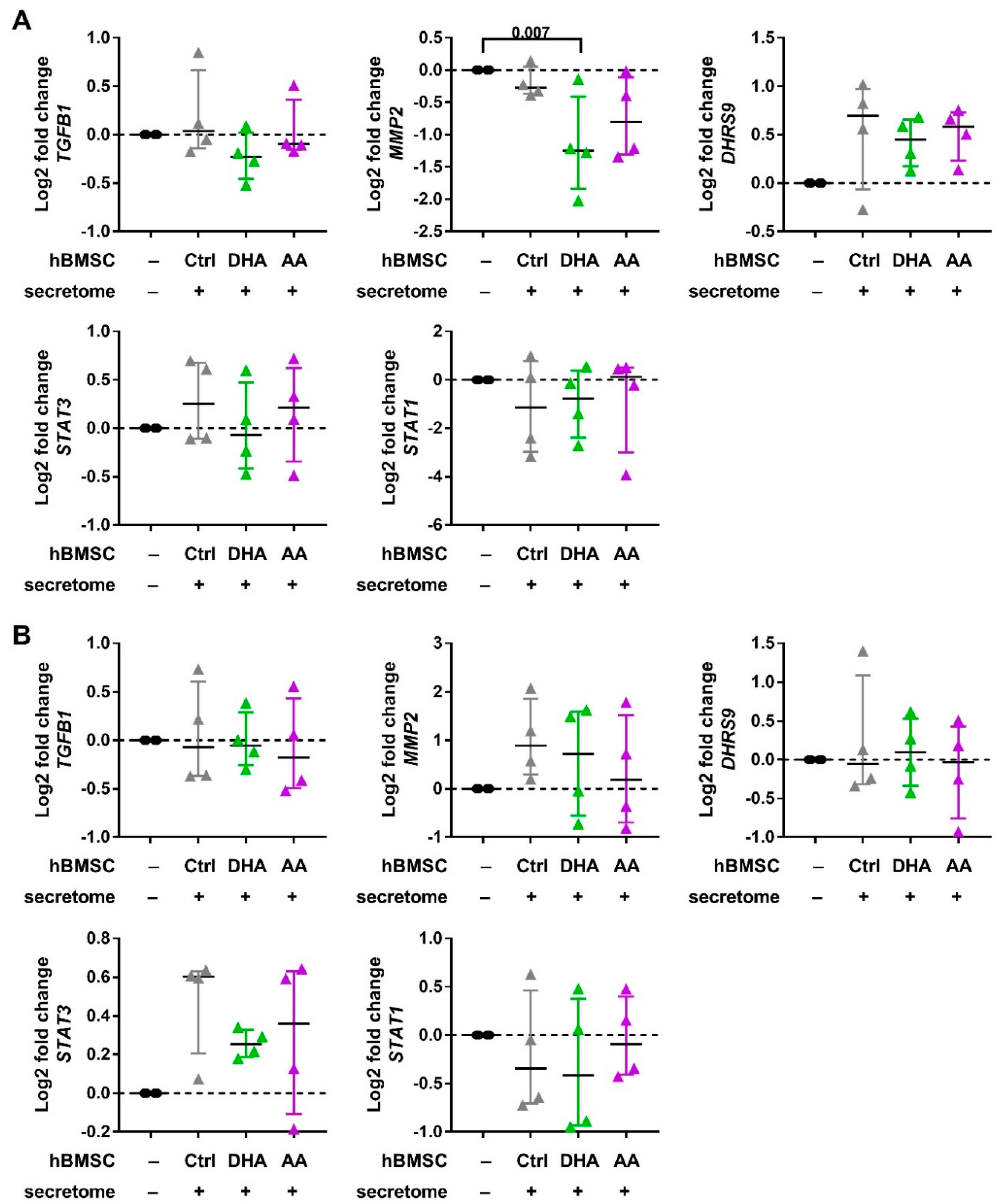
2.4. Macrophage Phenotyping with Real-Time Quantitative PCR
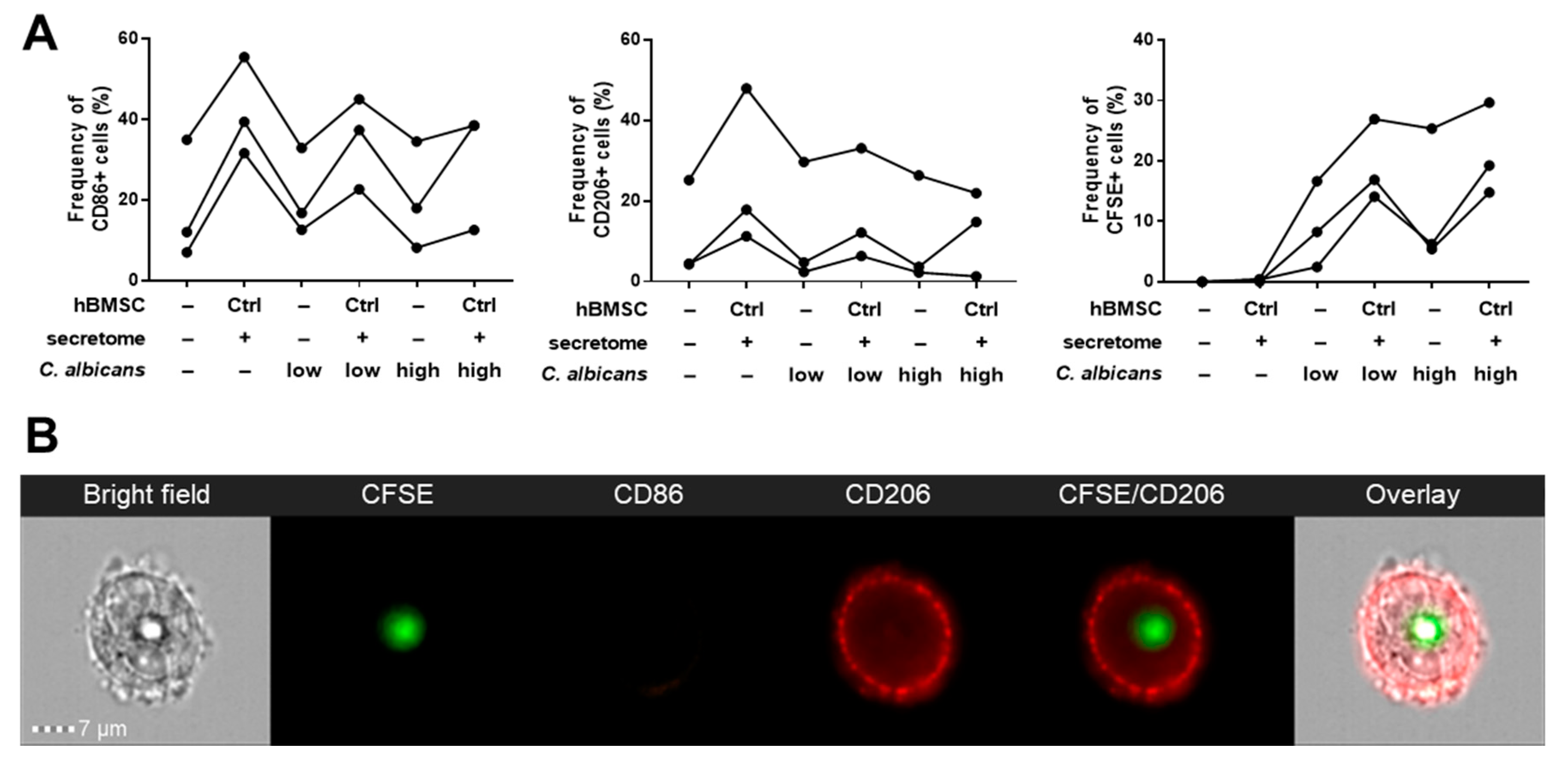
2.5. Macrophage Phenotyping with Flow Cytometry
2.6. Yeast Heat-Inactivation and CFSE-Staining
2.7. Phagocytosis Assay with Imaging Flow Cytometry
2.8. Statistical Analysis
3. Results
3.1. Phenotype of Polarized and Activated Macrophages
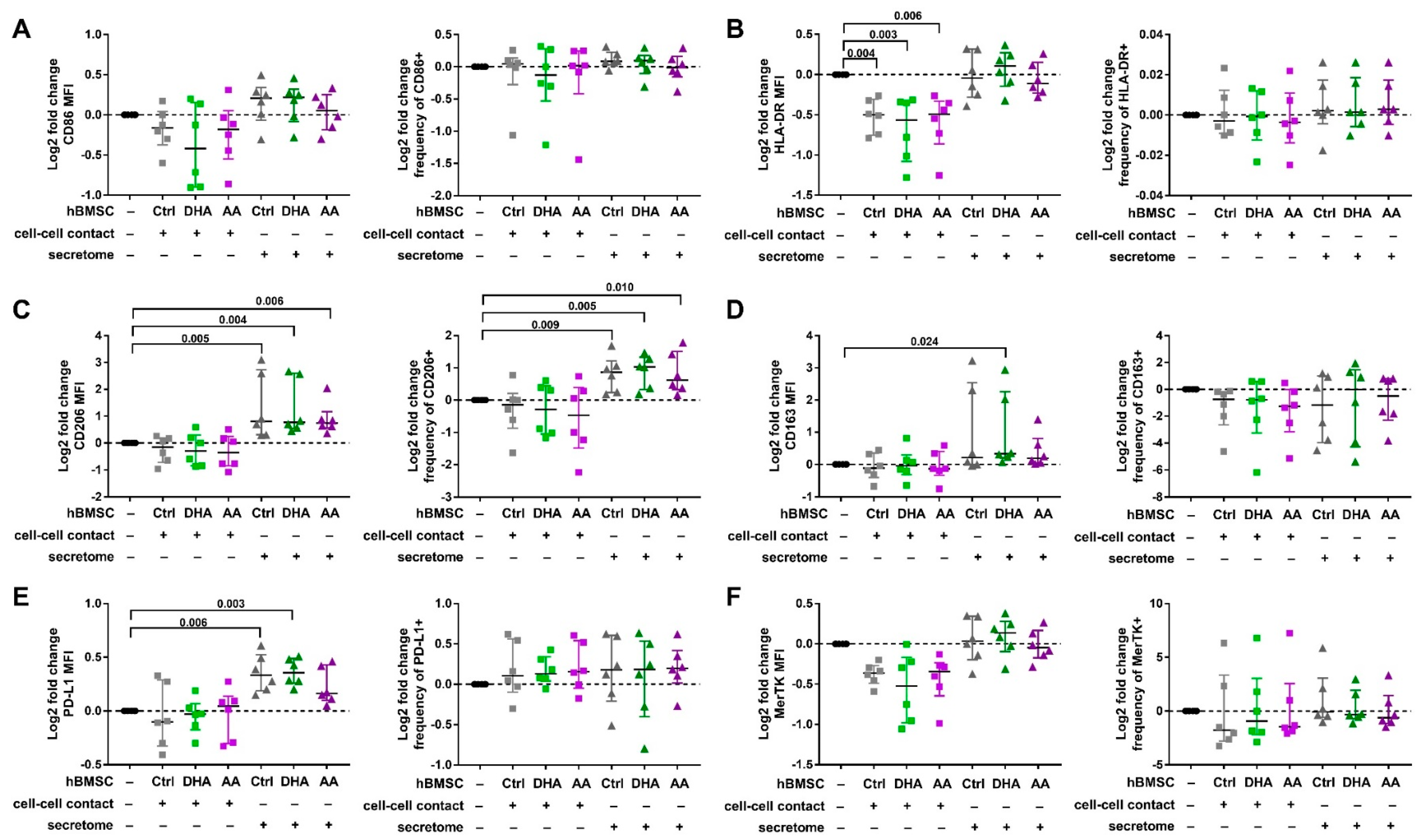
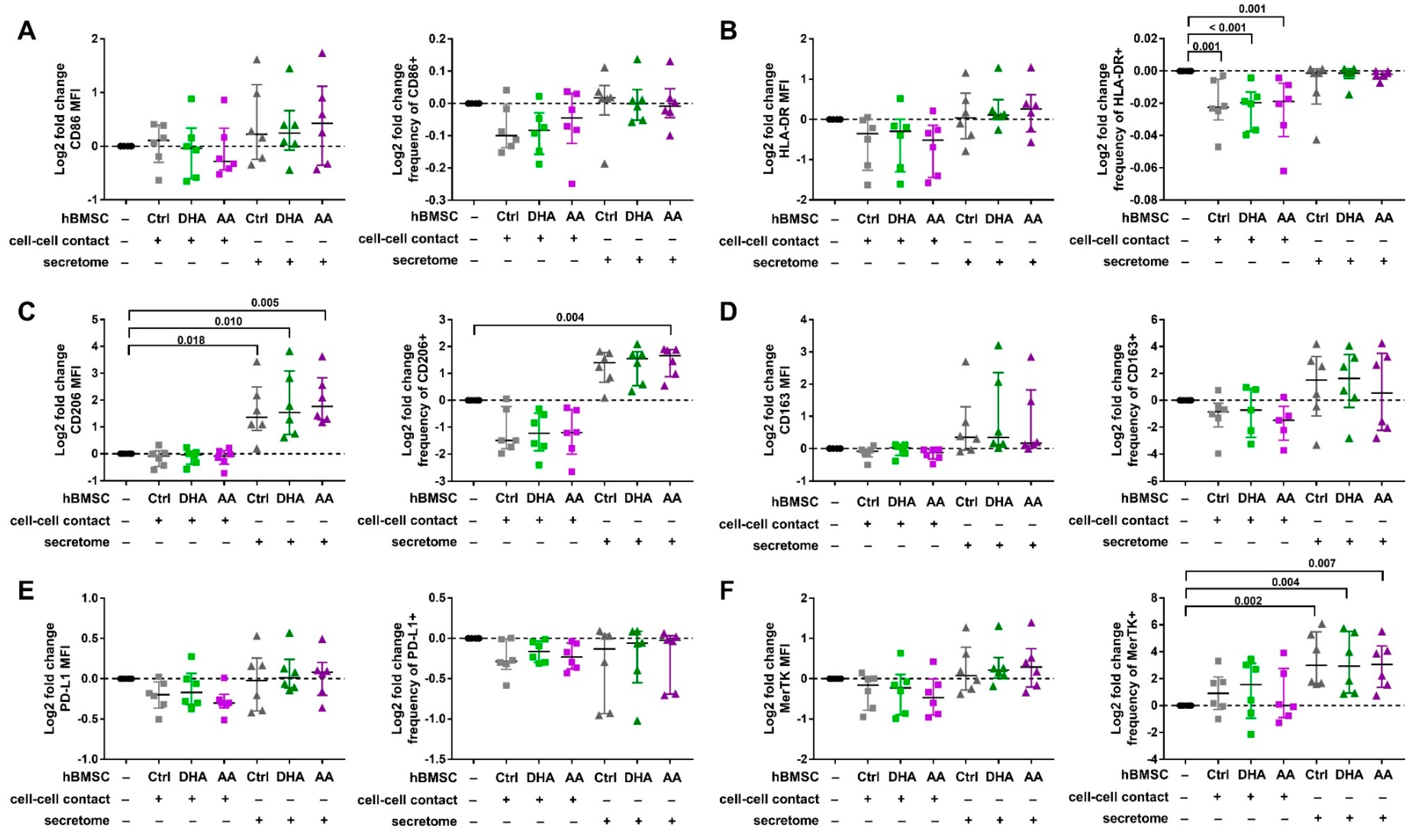
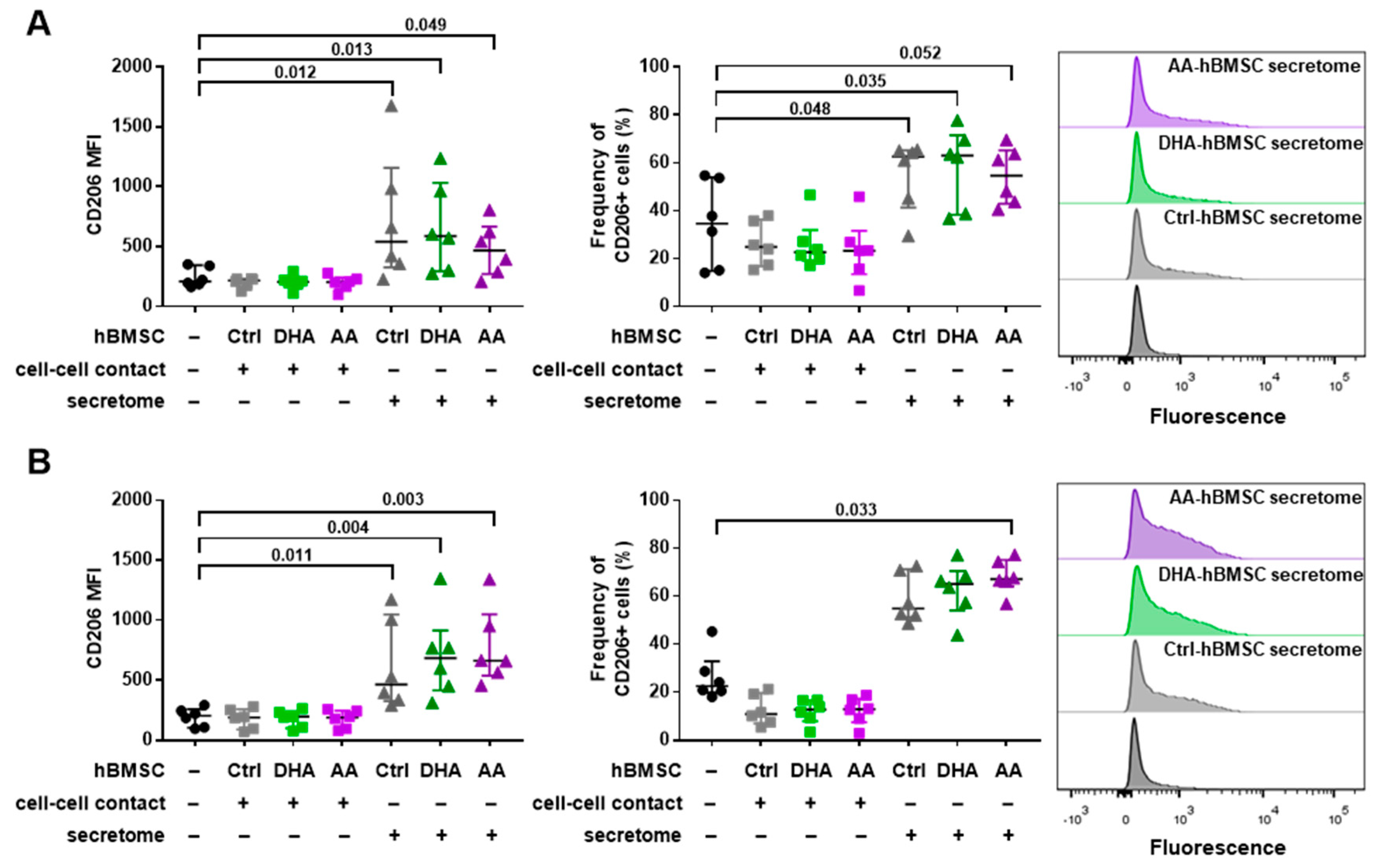
3.2. hBMSC Secretome Skews Mreg-Polarized and Mreg-Activated Macrophages toward an Anti-Inflammatory and Proresolving Phenotype
3.3. Phagocytosis Assay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salmenniemi, U.; Itälä-Remes, M.; Nystedt, J.; Putkonen, M.; Niittyvuopio, R.; Vettenranta, K.; Korhonen, M. Good responses but high TRM in adult patients after MSC therapy for GvHD. Bone Marrow Transplant. 2017, 52, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Kurtzberg, J.; Abdel-Azim, H.; Carpenter, P.; Chaudhury, S.; Horn, B.; Mahadeo, K.; Nemecek, E.; Neudorf, S.; Prasad, V.; Prockop, S.; et al. A Phase 3, single-arm, prospective study of Remestemcel-L, ex vivo culture-expanded adult human mesenchymal stromal cells for the treatment of pediatric patients who failed to respond to steroid treatment for acute graft-versus-host disease. Biol. Blood Marrow Transplant. 2020, 26, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Herreros, M.D.; Garcia-Arranz, M.; Guadalajara, H.; De-La-Quintana, P.; Garcia-Olmo, D. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: A phase III randomized clinical trial (FATT 1: Fistula advanced therapy trial 1) and long-term evaluation. Dis. Colon Rectum 2012, 55, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Transplantation 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meisel, R.; Zibert, A.; Laryea, M.; Göbel, U.; Däubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase–mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Yin, Y.; Lai, R.C.; Tan, S.S.; Choo, A.B.H.; Lim, S.K. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014, 23, 1233–1244. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Grisanti, S.; Gianni, A.M.; Matteucci, P.; Di Nicola, M.; Carlo-Stella, C.; et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Kim, J.; Hematti, P. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp. Hematol. 2009, 37, 1445–1453. [Google Scholar] [CrossRef] [Green Version]
- Abumaree, M.H.; Al Jumah, M.A.; Kalionis, B.; Jawdat, D.; Al Khaldi, A.; Abomaray, F.M.; Fatani, A.S.; Chamley, L.W.; Knawy, B.A. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 Macrophages. Stem Cell Rev. Rep. 2013, 9, 620–641. [Google Scholar] [CrossRef]
- Chiossone, L.; Conte, R.; Spaggiari, G.M.; Serra, M.; Romei, C.; Bellora, F.; Becchetti, F.; Andaloro, A.; Moretta, L.; Bottino, C. Mesenchymal stromal cells induce peculiar alternatively activated macrophages capable of dampening both innate and adaptive immune responses. Stem Cells 2016, 34, 1909–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, M.V.; Morrison, T.J.; Doherty, D.F.; McAuley, D.F.; Matthay, M.A.; Kissenpfennig, A.; O’Kane, C.M.; Krasnodembskaya, A.D. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells 2016, 34, 2210–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Broichhausen, C.; Riquelme, P.; Geissler, E.K.; Hutchinson, J.A. Regulatory macrophages as therapeutic targets and therapeutic agents in solid organ transplantation. Curr. Opin. Organ Transplant. 2012, 17, 332–342. [Google Scholar] [CrossRef]
- Riquelme, P.; Amodio, G.; Macedo, C.; Moreau, A.; Obermajer, N.; Brochhausen, C.; Ahrens, N.; Kekarainen, T.; Fändrich, F.; Cuturi, C.; et al. DHRS9 is a stable marker of human regulatory macrophages. Transplantation 2017, 101, 2731–2738. [Google Scholar] [CrossRef] [Green Version]
- Hyvärinen, K.; Holopainen, M.; Skirdenko, V.; Ruhanen, H.; Lehenkari, P.; Korhonen, M.; Käkelä, R.; Laitinen, S.; Kerkelä, E. Mesenchymal stromal cells and their extracellular vesicles enhance the anti-inflammatory phenotype of regulatory macrophages by downregulating the production of interleukin (IL)-23 and IL-22. Front. Immunol. 2018, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Ylöstalo, J.H.; Bartosh, T.J.; Coble, K.; Prockop, D.J. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells 2012, 30, 2283–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [Green Version]
- Doeppner, T.R.; Herz, J.; Görgens, A.; Schlechter, J.; Ludwig, A.-K.; Radtke, S.; De Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Gu, H.; Qin, D.; Yang, L.; Huang, W.; Essandoh, K.; Wang, Y.; Caldwell, C.C.; Peng, T.; Zingarelli, B.; et al. Exosomal miR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci. Rep. 2015, 5, 13721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- English, K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol. 2013, 91, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tigistu-Sahle, F.; Lampinen, M.; Kilpinen, L.; Holopainen, M.; Lehenkari, P.; Laitinen, S.; Käkelä, R. Metabolism and phospholipid assembly of polyunsaturated fatty acids in human bone marrow mesenchymal stromal cells. J. Lipid Res. 2017, 58, 92–110. [Google Scholar] [CrossRef] [Green Version]
- Holopainen, M.; Colas, R.A.; Valkonen, S.; Tigistu-sahle, F.; Hyvärinen, K.; Mazzacuva, F.; Lehenkari, P.; Käkelä, R.; Dalli, J.; Kerkelä, E.; et al. Polyunsaturated fatty acids modify the extracellular vesicle membranes and increase the production of proresolving lipid mediators of human mesenchymal stromal cells. BBA Mol. Cell Biol. Lipids 2019, 1864, 1350–1362. [Google Scholar] [CrossRef]
- Abreu, S.C.; Lopes-Pacheco, M.; Silva, A.L.; Xisto, D.G.; Oliveira, T.B.; Kitoko, J.Z.; Castro, L.L.; Amorim, N.R.; Martins, V.; Silva, L.H.A.; et al. Eicosapentaenoic acid enhances the effects of mesenchymal stromal cell therapy in experimental allergic asthma. Front. Immunol. 2018, 9, 1147. [Google Scholar] [CrossRef] [Green Version]
- Tsoyi, K.; Hall, S.R.R.; Dalli, J.; Colas, R.A.; Ghanta, S.; Ith, B.; Coronata, A.; Fredenburgh, L.E.; Baron, R.M.; Choi, A.M.K.; et al. Carbon monoxide improves efficacy of mesenchymal stromal cells during sepsis by production of specialized proresolving lipid mediators. Crit. Care Med. 2016, 44, e1236–e1245. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.D.; Lopes-Pacheco, M.; De Castro, L.L.; Kitoko, J.Z.; Trivelin, S.A.; Amorim, N.R.; Capelozzi, V.L.; Morales, M.M.; Gutfilen, B.; De Souza, S.A.L.; et al. Eicosapentaenoic acid potentiates the therapeutic effects of adipose tissue-derived mesenchymal stromal cells on lung and distal organ injury in experimental sepsis. Stem Cell Res. Ther. 2019, 10, 264. [Google Scholar] [CrossRef] [Green Version]
- Leskelä, H.V.; Risteli, J.; Niskanen, S.; Koivunen, J.; Ivaska, K.K.; Lehenkari, P. Osteoblast recruitment from stem cells does not decrease by age at late adulthood. Biochem. Biophys. Res. Commun. 2003, 311, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Diez-Orejas, R.; Feito, M.J.; Cicuéndez, M.; Rojo, J.M.; Portolés, M.T. Differential effects of graphene oxide nanosheets on Candida albicans phagocytosis by murine peritoneal macrophages. J. Colloid Interface Sci. 2018, 512, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Pohlert, T. The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR). Available online: https://CRAN.R-project.org/package=PMCMR (accessed on 21 September 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Hutchinson, J.A.; Riquelme, P.; Geissler, E.K.; Fändrich, F. Human regulatory macrophages. Methods Mol. Biol. 2011, 677, 181–192. [Google Scholar]
- Stahl, P.D.; Ezekowitz, R.A.B. The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 1998, 10, 50–55. [Google Scholar] [CrossRef]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Princepi, E.; Pascucci, L.; Berherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: Endorsement of macrophage polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef]
- Maródi, L.; Korchak, H.M.; Johnston, R.B., Jr. Mechanisms of host defense against Candida species. Phagocytosis by monocytes and monocyte-derived macrophage. J. Immunol. 1991, 146, 2783–2789. [Google Scholar]
- Deng, W.; Chen, W.; Zhang, Z.; Huang, S.; Kong, W.; Sun, Y.; Tang, X.; Yao, G.; Feng, X.; Chen, W.J.; et al. Mesenchymal stem cells promote CD206 expression and phagocytic activity of macrophages through IL-6 in systemic lupus erythematosus. Clin. Immunol. 2015, 161, 209–216. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and function of the PD-L1 checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [Green Version]
- Shouval, D.S.; Biswas, A.; Goettel, J.A.; McCann, K.; Conaway, E.; Redhu, N.S.; Mascanfroni, I.D.; AlAdham, Z.; Lavoie, S.; Ibourk, M.; et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 2014, 40, 706–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zizzo, G.; Hilliard, B.A.; Monestier, M.; Cohen, P.L. Efficient clearance of early apoptotic cells by human macrophages requires “M2c” polarization and MerTK induction. J. Immunol. 2012, 189, 3508–3520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, R.S.; McMahon, E.J.; Pop, S.M.; Reap, E.A.; Caricchio, R.; Cohen, P.L.; Shelton Earp, H.; Matsushima, G.K. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 2001, 411, 207–211. [Google Scholar] [CrossRef]
- Cai, B.; Thorp, E.B.; Doran, A.C.; Subramanian, M.; Sansbury, B.E.; Lin, C.S.; Spite, M.; Fredman, G.; Tabas, I. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc. Natl. Acad. Sci. USA 2016, 113, 6526–6531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Couto, G.; Jaghatspanyan, E.; Deberge, M.; Liu, W.; Luther, K.; Wang, Y.; Tang, J.; Thorp, E.B.; Marbán, E. Mechanism of enhanced MerTK-Dependent macrophage efferocytosis by extracellular vesicles. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2082–2096. [Google Scholar] [CrossRef] [PubMed]
- Giebel, B.; Kordelas, L.; Börger, V. Clinical potential of mesenchymal stem/stromal cell-derived extracellular vesicles. Stem Cell Investig. 2017, 4, 84. [Google Scholar] [CrossRef] [Green Version]
- Hanson, S.E.; King, S.N.; Kim, J.; Chen, X.; Thibeault, S.L.; Hematti, P. The effect of mesenchymal stromal cell-hyaluronic acid hydrogel constructs on immunophenotype of macrophages. Tissue Eng. Part A 2011, 17, 2463–2471. [Google Scholar] [CrossRef]
- Wise, A.F.; Williams, T.M.; Rudd, S.; Wells, C.A.; Kerr, P.G.; Ricardo, S.D. Human mesenchymal stem cells alter the gene profile of monocytes from patients with Type 2 diabetes and end-stage renal disease. Regen. Med. 2016, 11, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Etzerodt, A.; Moestrup, S.K. CD163 and inflammation: Biological, diagnostic, and therapeutic aspects. Antioxidants Redox Signal. 2013, 18, 2352–2363. [Google Scholar] [CrossRef] [Green Version]
- Parks, W.C.; Wilson, C.L.; López-Boado, Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004, 4, 617–629. [Google Scholar] [CrossRef]
- Hashizume, R.; Yamawaki-Ogata, A.; Ueda, Y.; Wagner, W.R.; Narita, Y. Mesenchymal stem cells attenuate angiotensin II-induced aortic aneurysm growth in apolipoprotein E-deficient mice. J. Vasc. Surg. 2011, 54, 1743–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamawaki-Ogata, A.; Fu, X.; Hashizume, R.; Fujimoto, K.L.; Araki, Y.; Oshima, H.; Narita, Y.; Usui, A. Therapeutic potential of bone marrow-derived mesenchymal stem cells in formed aortic aneurysms of a mouse model. Eur. J. Cardio Thorac. Surg. 2014, 45, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
| Concentration, pg/mL (IQR) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cell-Cell Contact | Cell-Cell Contact | Cell-Cell Contact | Secretome | Secretome | Secretome | |||
| Cytokine | Mreg-Polarized | Mreg-Polarized +Control-hBMSC | Mreg-Polarized +DHA-hBMSC | Mreg-Polarized +AA-hBMSC | Mreg-Polarized +Control-hBMSC | Mreg-Polarized +DHA-hBMSC | Mreg-Polarized +AA-hBMSC | p-value a |
| TNF-α | 123.5 (131.5) | 247.6 (199.7) | 165.4 (278.8) | 183.6 (177.2) | 181.9 (434.9) | 228.0 (273.8) | 192.8 (350.3) | 0.999 |
| IL-10 | 39.5 (81.5) | 48.0 (31.7) | 36.9 (55.4) | 36.1 (31.4) | 32.5 (40.5) | 29.1 (29.6) | 53.6 (27.7) | 0.975 |
| IL-23 | 0.0 (0.0) | 0.3 (0.9) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.015 |
| Mreg-activated | Mreg-activated +control-hBMSC | Mreg-activated +DHA-hBMSC | Mreg-activated +AA-hBMSC | Mreg-activated +control-hBMSC | Mreg-activated +DHA-hBMSC | Mreg-activated +AA-hBMSC | p-value a | |
| TNF-α | 1591.6 (1898.5) | 2106.7 (1825.5) | 1887.1 (2041.1) | 1899.1 (1672.3) | 2025.5 (1512.2) | 1950.5 (1895.9) | 1823.6 (1959.4) | 0.998 |
| IL-10 | 31.0 (69.9) | 45.5 (70.4) | 39.6 (54.4) | 33.9 (76.7) | 31.5 (88.2) | 45.0 (57.1) | 33.3 (69.6) | 0.871 |
| IL-23 | 0.0 (0.1) | 0.4 (2.7) | 0.4 (1.0) | 0.0 (0.2) | 0.1 (0.2) | 0.0 (0.4) | 0.1 (0.4) | 0.757 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holopainen, M.; Impola, U.; Lehenkari, P.; Laitinen, S.; Kerkelä, E. Human Mesenchymal Stromal Cell Secretome Promotes the Immunoregulatory Phenotype and Phagocytosis Activity in Human Macrophages. Cells 2020, 9, 2142. https://doi.org/10.3390/cells9092142
Holopainen M, Impola U, Lehenkari P, Laitinen S, Kerkelä E. Human Mesenchymal Stromal Cell Secretome Promotes the Immunoregulatory Phenotype and Phagocytosis Activity in Human Macrophages. Cells. 2020; 9(9):2142. https://doi.org/10.3390/cells9092142
Chicago/Turabian StyleHolopainen, Minna, Ulla Impola, Petri Lehenkari, Saara Laitinen, and Erja Kerkelä. 2020. "Human Mesenchymal Stromal Cell Secretome Promotes the Immunoregulatory Phenotype and Phagocytosis Activity in Human Macrophages" Cells 9, no. 9: 2142. https://doi.org/10.3390/cells9092142
APA StyleHolopainen, M., Impola, U., Lehenkari, P., Laitinen, S., & Kerkelä, E. (2020). Human Mesenchymal Stromal Cell Secretome Promotes the Immunoregulatory Phenotype and Phagocytosis Activity in Human Macrophages. Cells, 9(9), 2142. https://doi.org/10.3390/cells9092142





