Retinoic Acid Improves the Recovery of Replication-Competent Virus from Latent SIV Infected Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Blood Samples and Tissues
2.2. Simian Immunodeficiency Virus (SIV) Replication, Virus Expansion and Latent Reservoir Measurement Assays
2.2.1. SIV Replication Assays
2.2.2. Virus Expansion Assays
2.2.3. Latent Reservoir Quantification Assays
2.3. Retinoic Acid (RA) Treatment of RMs and Measurement in Plasma
2.3.1. Retinoic Acid (RA) Treatment of RMs
2.3.2. Retinoic Acid (RA) Measurement in Plasma
2.4. Isolation of Cells from Blood/Tissues
2.5. Cell Culture
2.6. Flow Cytometry
2.6.1. Quantification of Diverse Immune Activation Markers
2.6.2. Delineation of Memory and Differentiation Status
2.6.3. Data Acquisition and Analysis of Generated Flow Cytometry Data
2.7. Statistical Analysis
3. Results
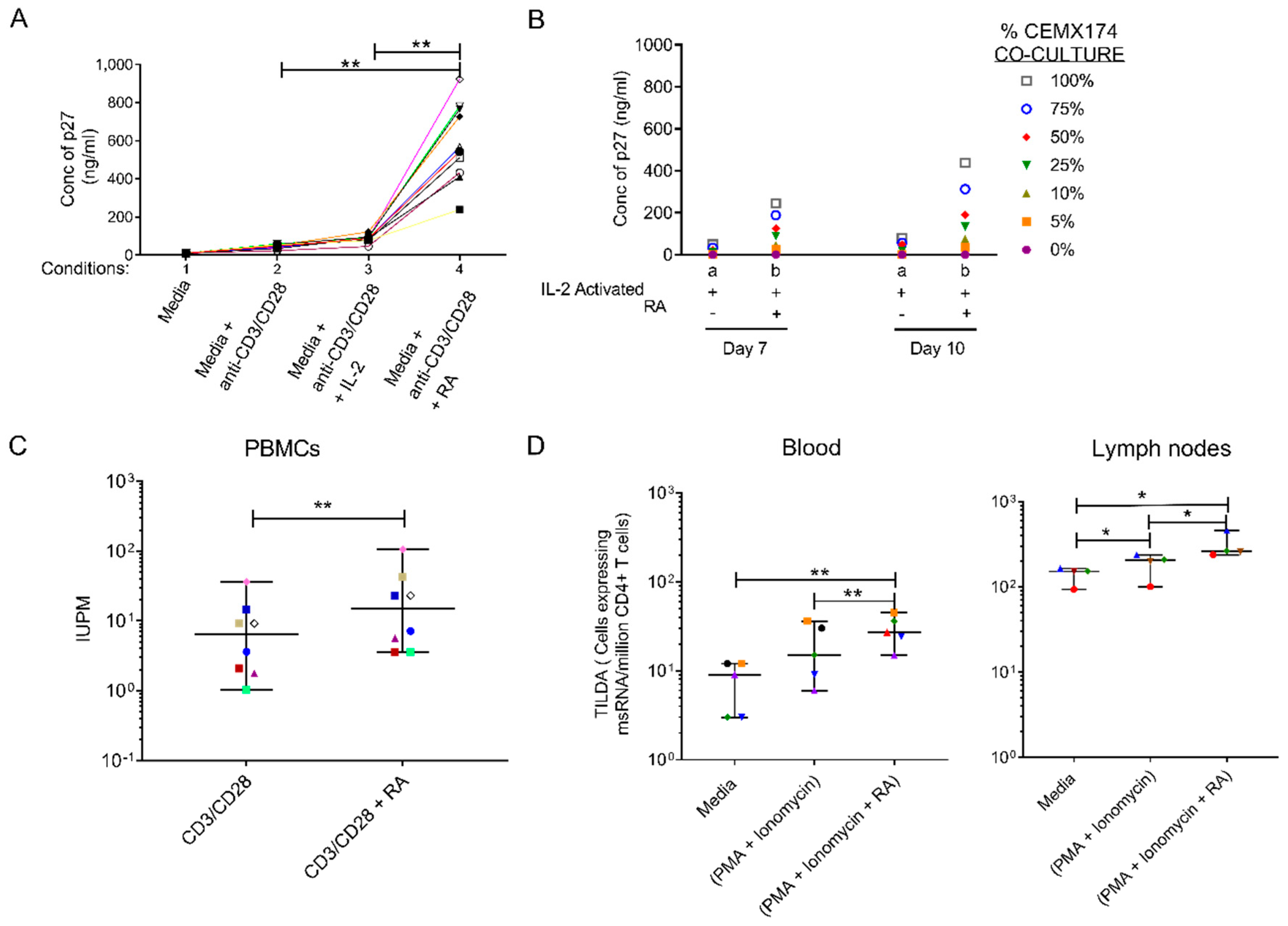
3.1. Retinoic Acid (RA) Enhances SIV Replication and Improves Latent SIV Quantitation
3.2. Oral Administration of All Trans RA to SIV Infected RMs Coincides with Increase in Viral Loads within the High Viral Load Group
3.3. Retinoic Acid (RA) Up-Regulates α4β7 Expression Levels and Induces a Distinct Immune Activation Profile
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, C. Second ‘Cured’ HIV Patient Goes Public. Available online: https://www.sciencemag.org/news/2020/03/second-cured-hiv-patient-goes-public (accessed on 25 June 2020).
- Gupta, R.K.; Abdul-Jawad, S.; McCoy, L.E.; Mok, H.P.; Peppa, D.; Salgado, M.; Martinez-Picado, J.; Nijhuis, M.; Wensing, A.M.J.; Lee, H.; et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 2019, 568, 244–248. [Google Scholar] [CrossRef]
- Henrich, T.J.; Hanhauser, E.; Marty, F.M.; Sirignano, M.N.; Keating, S.; Lee, T.H.; Robles, Y.P.; Davis, B.T.; Li, J.Z.; Heisey, A.; et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: Report of 2 cases. Ann. Intern. Med. 2014, 161, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Mavigner, M.; Watkins, B.; Lawson, B.; Lee, S.T.; Chahroudi, A.; Kean, L.; Silvestri, G. Persistence of virus reservoirs in ART-treated SHIV-infected rhesus macaques after autologous hematopoietic stem cell transplant. PLoS Pathog. 2014, 10, e1004406. [Google Scholar] [CrossRef] [PubMed]
- Bruner, K.M.; Hosmane, N.N.; Siliciano, R.F. Towards an HIV-1 cure: Measuring the latent reservoir. Trends Microbiol. 2015, 23, 192–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margolis, D.M.; Garcia, J.V.; Hazuda, D.J.; Haynes, B.F. Latency reversal and viral clearance to cure HIV-1. Science 2016, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhamel, J.; Bruggemans, A.; Debyser, Z. Establishment of latent HIV-1 reservoirs: What do we really know? J. Virus Erad. 2019, 5, 3–9. [Google Scholar] [CrossRef]
- Hosmane, N.N.; Kwon, K.J.; Bruner, K.M.; Capoferri, A.A.; Beg, S.; Rosenbloom, D.I.; Keele, B.F.; Ho, Y.C.; Siliciano, J.D.; Siliciano, R.F. Proliferation of latently infected CD4(+) T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J. Exp. Med. 2017, 214, 959–972. [Google Scholar] [CrossRef]
- Soriano-Sarabia, N.; Bateson, R.E.; Dahl, N.P.; Crooks, A.M.; Kuruc, J.D.; Margolis, D.M.; Archin, N.M. Quantitation of Replication-Competent HIV-1 in Populations of Resting CD4+ T Cells. J. Virol. 2014, 88, 14070–14077. [Google Scholar] [CrossRef] [Green Version]
- Hiener, B.; Horsburgh, B.A.; Eden, J.-S.; Barton, K.; Schlub, T.E.; Lee, E.; von Stockenstrom, S.; Odevall, L.; Milush, J.M.; Liegler, T. Identification of genetically intact HIV-1 proviruses in specific CD4+ T cells from effectively treated participants. Cell Rep. 2017, 21, 813–822. [Google Scholar] [CrossRef] [Green Version]
- Massanella, M.; Yek, C.; Lada, S.M.; Nakazawa, M.; Shefa, N.; Huang, K.; Richman, D.D. Improved assays to measure and characterize the inducible HIV reservoir. EBioMedicine 2018, 36, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Bruner, K.M.; Murray, A.J.; Pollack, R.A.; Soliman, M.G.; Laskey, S.B.; Capoferri, A.A.; Lai, J.; Strain, M.C.; Lada, S.M.; Hoh, R.; et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med. 2016, 22, 1043–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liszewski, M.K.; Yu, J.J.; O’Doherty, U. Detecting HIV-1 integration by repetitive-sampling Alu-gag PCR. Methods 2009, 47, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.; Ruggiero, A.; Procopio, F.A.; Pantaleo, G.; Paxton, W.A.; Pollakis, G. Comparative analysis and generation of a robust HIV-1 DNA quantification assay. J. Virol. Methods 2019, 263, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Yukl, S.A.; Kaiser, P.; Kim, P.; Telwatte, S.; Joshi, S.K.; Vu, M.; Lampiris, H.; Wong, J.K. HIV latency in isolated patient CD4+ T cells may be due to blocks in HIV transcriptional elongation, completion, and splicing. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Spivak, A.M.; Andrade, A.; Eisele, E.; Hoh, R.; Bacchetti, P.; Bumpus, N.N.; Emad, F.; Buckheit, R., III; McCance-Katz, E.F.; Lai, J. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1–infected adults on antiretroviral therapy. Clin. Infect. Dis. 2013, 58, 883–890. [Google Scholar] [CrossRef] [Green Version]
- Pardons, M.; Baxter, A.E.; Massanella, M.; Pagliuzza, A.; Fromentin, R.; Dufour, C.; Leyre, L.; Routy, J.-P.; Kaufmann, D.E.; Chomont, N. Single-cell characterization and quantification of translation-competent viral reservoirs in treated and untreated HIV infection. PLoS Pathog. 2019, 15, e1007619. [Google Scholar] [CrossRef]
- Bruner, K.M.; Wang, Z.; Simonetti, F.R.; Bender, A.M.; Kwon, K.J.; Sengupta, S.; Fray, E.J.; Beg, S.A.; Antar, A.A.R.; Jenike, K.M.; et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019, 566, 120–125. [Google Scholar] [CrossRef]
- Bender, A.M.; Simonetti, F.R.; Kumar, M.R.; Fray, E.J.; Bruner, K.M.; Timmons, A.E.; Tai, K.Y.; Jenike, K.M.; Antar, A.A.R.; Liu, P.T.; et al. The Landscape of Persistent Viral Genomes in ART-Treated SIV, SHIV, and HIV-2 Infections. Cell Host Microbe 2019, 26, 73–85. [Google Scholar] [CrossRef]
- Charlins, P.; Schmitt, K.; Remling-Mulder, L.; Hogan, L.E.; Hanhauser, E.; Hobbs, K.S.; Hecht, F.; Deeks, S.G.; Henrich, T.J.; Akkina, R. A humanized mouse-based HIV-1 viral outgrowth assay with higher sensitivity than in vitro qVOA in detecting latently infected cells from individuals on ART with undetectable viral loads. Virology 2017, 507, 135–139. [Google Scholar] [CrossRef]
- Zhang, Y.; Planas, D.; Raymond Marchand, L.; Massanella, M.; Chen, H.; Wacleche, V.S.; Gosselin, A.; Goulet, J.P.; Filion, M.; Routy, J.P.; et al. Improving HIV Outgrowth by Optimizing Cell-Culture Conditions and Supplementing With all-trans Retinoic Acid. Front. Microbiol. 2020, 11, 902. [Google Scholar] [CrossRef]
- Wightman, F.; Solomon, A.; Khoury, G.; Green, J.A.; Gray, L.; Gorry, P.R.; Ho, Y.S.; Saksena, N.K.; Hoy, J.; Crowe, S.M. Both CD31+ and CD31-naive CD4+ T cells are persistent HIV type 1-infected reservoirs in individuals receiving antiretroviral therapy. J. Infect. Dis. 2010, 202, 1738–1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.A.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.-R.; Ghattas, G.; Brenchley, J.M. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009, 15, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Khoury, G.; Anderson, J.L.; Fromentin, R.; Hartogensis, W.; Smith, M.Z.; Bacchetti, P.; Hecht, F.M.; Chomont, N.; Cameron, P.U.; Deeks, S.G. Persistence of integrated HIV DNA in CXCR3+ CCR6+ memory CD4+ T-cells in HIV-infected individuals on antiretroviral therapy. AIDS 2016, 30, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Buzon, M.J.; Sun, H.; Li, C.; Shaw, A.; Seiss, K.; Ouyang, Z.; Martin-Gayo, E.; Leng, J.; Henrich, T.J.; Li, J.Z. HIV-1 persistence in CD4+ T cells with stem cell–like properties. Nat. Med. 2014, 20, 139–142. [Google Scholar] [CrossRef]
- Cibrián, D.; Sánchez-Madrid, F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef]
- Saeidi, A.; Zandi, K.; Cheok, Y.Y.; Saeidi, H.; Wong, W.F.; Lee, C.Y.Q.; Cheong, H.C.; Yong, Y.K.; Larsson, M.; Shankar, E.M. T-Cell Exhaustion in Chronic Infections: Reversing the State of Exhaustion and Reinvigorating Optimal Protective Immune Responses. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Quarona, V.; Zaccarello, G.; Chillemi, A.; Brunetti, E.; Singh, V.K.; Ferrero, E.; Funaro, A.; Horenstein, A.L.; Malavasi, F. CD38 and CD157: A long journey from activation markers to multifunctional molecules. Cytometry B Clin. Cytom. 2013, 84, 207–217. [Google Scholar] [CrossRef]
- Buggert, M.; Frederiksen, J.; Lund, O.; Betts, M.R.; Biague, A.; Nielsen, M.; Tauriainen, J.; Norrgren, H.; Medstrand, P.; Karlsson, A.C.; et al. CD4+ T cells with an activated and exhausted phenotype distinguish immunodeficiency during aviremic HIV-2 infection. AIDS 2016, 30, 2415–2426. [Google Scholar] [CrossRef] [Green Version]
- Procopio, F.A.; Fromentin, R.; Kulpa, D.A.; Brehm, J.H.; Bebin, A.-G.; Strain, M.C.; Richman, D.D.; O’Doherty, U.; Palmer, S.; Hecht, F.M.; et al. A Novel Assay to Measure the Magnitude of the Inducible Viral Reservoir in HIV-infected Individuals. EBioMedicine 2015, 2, 874–883. [Google Scholar] [CrossRef] [Green Version]
- Frank, I.; Acharya, A.; Routhu, N.K.; Aravantinou, M.; Harper, J.L.; Maldonado, S.; Sole Cigoli, M.; Semova, S.; Mazel, S.; Paiardini, M.; et al. A Tat/Rev Induced Limiting Dilution Assay to Measure Viral Reservoirs in Non-Human Primate Models of HIV Infection. Sci. Rep. 2019, 9, 12078. [Google Scholar] [CrossRef] [Green Version]
- Sigal, A.; Kim, J.T.; Balazs, A.B.; Dekel, E.; Mayo, A.; Milo, R.; Baltimore, D. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature 2011, 477, 95–98. [Google Scholar] [CrossRef]
- Zerbato, J.M.; Serrao, E.; Lenzi, G.; Kim, B.; Ambrose, Z.; Watkins, S.C.; Engelman, A.N.; Sluis-Cremer, N. Establishment and reversal of HIV-1 latency in naive and central memory CD4+ T cells in vitro. J. Virol. 2016, 90, 8059–8073. [Google Scholar] [CrossRef] [Green Version]
- Rosenbloom, D.I.; Elliott, O.; Hill, A.L.; Henrich, T.J.; Siliciano, J.M.; Siliciano, R.F. Designing and Interpreting Limiting Dilution Assays: General Principles and Applications to the Latent Reservoir for Human Immunodeficiency Virus-1. Open Forum Infect. Dis. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Kraft, J.C.; Slikker, W.; Bailey, J.; Roberts, L.; Fischer, B.; Wittfoht, W.; Nau, H. Plasma pharmacokinetics and metabolism of 13-cis-and all-trans-retinoic acid in the cynomolgus monkey and the identification of 13-cis-and all-trans-retinoyl-beta-glucuronides. A comparison to one human case study with isotretinoin. Drug Metab. Dispos. 1991, 19, 317–324. [Google Scholar]
- Muindi, J.; Young, C.; Warrell, J.R. Clinical pharmacology of all-trans retinoic acid. Leukemia 1994, 8, 1807–1812. [Google Scholar]
- Jones, J.W.; Pierzchalski, K.; Yu, J.; Kane, M.A. Use of fast HPLC multiple reaction monitoring cubed for endogenous retinoic acid quantification in complex matrices. Anal. Chem. 2015, 87, 3222–3230. [Google Scholar] [CrossRef]
- Kane, M.A.; Napoli, J.L. Quantification of endogenous retinoids. Methods Mol Biol 2010, 652, 1–54. [Google Scholar] [CrossRef] [Green Version]
- Ansari, A.A.; Reimann, K.A.; Mayne, A.E.; Takahashi, Y.; Stephenson, S.T.; Wang, R.; Wang, X.; Li, J.; Price, A.A.; Little, D.M. Blocking of α4β7 gut-homing integrin during acute infection leads to decreased plasma and gastrointestinal tissue viral loads in simian immunodeficiency virus-infected rhesus macaques. J. Immunol. 2011, 186, 1044–1059. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Miyagi, T.; Chuang, L.F.; Doi, R.H.; Carlos, M.P.; Torres, J.V.; Chuang, R.Y. Morphine induces gene expression of CCR5 in human CEM x174 lymphocytes. J. Biol. Chem. 2000, 275, 31305–31310. [Google Scholar] [CrossRef] [Green Version]
- Byrareddy, S.N.; Sidell, N.; Arthos, J.; Cicala, C.; Zhao, C.; Little, D.M.; Dunbar, P.; Yang, G.X.; Pierzchalski, K.; Kane, M.A.; et al. Species-specific differences in the expression and regulation of alpha4beta7 integrin in various nonhuman primates. J. Immunol. 2015, 194, 5968–5979. [Google Scholar] [CrossRef] [PubMed]
- Bakdash, G.; Vogelpoel, L.T.; van Capel, T.M.; Kapsenberg, M.L.; de Jong, E.C. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015, 8, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cillo, A.R.; Sobolewski, M.D.; Bosch, R.J.; Fyne, E.; Piatak, M.; Coffin, J.M.; Mellors, J.W. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2014, 111, 7078–7083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, Y.-C.; Shan, L.; Hosmane, N.N.; Wang, J.; Laskey, S.B.; Rosenbloom, D.I.; Lai, J.; Blankson, J.N.; Siliciano, J.D.; Siliciano, R.F. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013, 155, 540–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beliakova-Bethell, N.; Hezareh, M.; Wong, J.K.; Strain, M.C.; Lewinski, M.K.; Richman, D.D.; Spina, C.A. Relative efficacy of T cell stimuli as inducers of productive HIV-1 replication in latently infected CD4 lymphocytes from patients on suppressive cART. Virology 2017, 508, 127–133. [Google Scholar] [CrossRef]
- Li, P.; Kaiser, P.; Lampiris, H.W.; Kim, P.; Yukl, S.A.; Havlir, D.V.; Greene, W.C.; Wong, J.K. Stimulating the RIG-I pathway to kill cells in the latent HIV reservoir following viral reactivation. Nat. Med. 2016, 22, 807–811. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, F.; Goes, L.R.; Ray, J.C.; Olowojesiku, R.; Sajani, A.; Ansari, A.A.; Perrone, I.; Hiatt, J.; Van Ryk, D.; Wei, D.; et al. MAdCAM costimulation through Integrin-α(4)β(7) promotes HIV replication. Mucosal Immunol. 2018, 11, 1342–1351. [Google Scholar] [CrossRef]
- Cicala, C.; Martinelli, E.; McNally, J.P.; Goode, D.J.; Gopaul, R.; Hiatt, J.; Jelicic, K.; Kottilil, S.; Macleod, K.; O’Shea, A.; et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc. Natl. Acad. Sci. USA 2009, 106, 20877–20882. [Google Scholar] [CrossRef] [Green Version]
- Shatrova, A.N.; Mityushova, E.V.; Vassilieva, I.O.; Aksenov, N.D.; Zenin, V.V.; Nikolsky, N.N.; Marakhova, I.I. Time-dependent regulation of IL-2R α-chain (CD25) expression by TCR signal strength and IL-2-induced STAT5 signaling in activated human blood T lymphocytes. PLoS ONE 2016, 11, e0167215. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, S.F.; Ramsdell, F.; Alderson, M.R. The activation antigen CD69. Stem Cells 1994, 12, 456–465. [Google Scholar] [CrossRef]
- Mehta, K.; Shahid, U.; Malavasi, F. Human CD38, a cell-surface protein with multiple functions. Faseb J. 1996, 10, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paley, M.A.; Kroy, D.C.; Odorizzi, P.M.; Johnnidis, J.B.; Dolfi, D.V.; Barnett, B.E.; Bikoff, E.K.; Robertson, E.J.; Lauer, G.M.; Reiner, S.L. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 2012, 338, 1220–1225. [Google Scholar] [CrossRef] [Green Version]
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-cell exhaustion in HIV infection. Immunol. Rev. 2019, 292, 149–163. [Google Scholar] [CrossRef]
- Evans, V.A.; van der Sluis, R.M.; Solomon, A.; Dantanarayana, A.; McNeil, C.; Garsia, R.; Palmer, S.; Fromentin, R.; Chomont, N.; Sékaly, R.-P.; et al. Programmed cell death-1 contributes to the establishment and maintenance of HIV-1 latency. AIDS 2018, 32, 1491–1497. [Google Scholar] [CrossRef]
- Ding, J.; Tasker, C.; Lespinasse, P.; Dai, J.; Fitzgerald-Bocarsly, P.; Lu, W.; Heller, D.; Chang, T.L.-Y. Integrin α4β7 Expression Increases HIV Susceptibility in Activated Cervical CD4+ T Cells by an HIV Attachment-Independent Mechanism. J. Acquir. Immune Defic. Syndr. 2015, 69, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Cutrona, G.; Tasso, P.; Dono, M.; Roncella, S.; Ulivi, M.; Carpaneto, E.M.; Fontana, V.; Comis, M.; Morabito, F.; Spinelli, M.; et al. CD10 is a marker for cycling cells with propensity to apoptosis in childhood ALL. Br. J. Cancer 2002, 86, 1776–1785. [Google Scholar] [CrossRef] [Green Version]
- Cutrona, G.; Leanza, N.; Ulivi, M.; Melioli, G.; Burgio, V.L.; Mazzarello, G.; Gabutti, G.; Roncella, S.; Ferrarini, M. Expression of CD10 by human T cells that undergo apoptosis both in vitro and in vivo. Blood 1999, 94, 3067–3076. [Google Scholar] [CrossRef]
- Tardif, M.R.; Tremblay, M.J. LFA-1 is a key determinant for preferential infection of memory CD4+ T cells by human immunodeficiency virus type 1. J. Virol. 2005, 79, 13714–13724. [Google Scholar] [CrossRef] [Green Version]
- Uzzan, M.; Tokuyama, M.; Rosenstein, A.K.; Tomescu, C.; SahBandar, I.N.; Ko, H.M.; Leyre, L.; Chokola, A.; Kaplan-Lewis, E.; Rodriguez, G.; et al. Anti-α4β7 therapy targets lymphoid aggregates in the gastrointestinal tract of HIV-1-infected individuals. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Vidal, E.; Castellví, M.; Pujantell, M.; Badia, R.; Jou, A.; Gomez, L.; Puig, T.; Clotet, B.; Ballana, E.; Riveira-Muñoz, E.; et al. Evaluation of the Innate Immune Modulator Acitretin as a Strategy To Clear the HIV Reservoir. Antimicrob. Agents Chemother. 2017, 61, e01368-17. [Google Scholar] [CrossRef] [Green Version]
- Gosselin, A.; Wiche Salinas, T.R.; Planas, D.; Wacleche, V.S.; Zhang, Y.; Fromentin, R.; Chomont, N.; Cohen, E.A.; Shacklett, B.; Mehraj, V.; et al. HIV persists in CCR6+CD4+ T cells from colon and blood during antiretroviral therapy. AIDS 2017, 31, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.O.; Hobbs, P.D.; Zhang, X.K.; Dawson, M.I.; Pfahl, M. A synthetic retinoid antagonist inhibits the human immunodeficiency virus type 1 promoter. Proc. Natl. Acad. Sci. USA 1994, 91, 5632–5636. [Google Scholar] [CrossRef] [Green Version]
- Richman, D.D.; Huang, K.; Lada, S.M.; Sun, X.; Jain, S.; Massanella, M.; Menke, B. Replication competence of virions induced from CD4+ lymphocytes latently infected with HIV. Retrovirology 2019, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Zerbato, J.M.; McMahon, D.K.; Sobolewski, M.D.; Mellors, J.W.; Sluis-Cremer, N. Naive CD4+ T Cells Harbor a Large Inducible Reservoir of Latent, Replication-competent Human Immunodeficiency Virus Type 1. Clin. Infect. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Tsunetsugu-Yokota, Y.; Kobayahi-Ishihara, M.; Wada, Y.; Terahara, K.; Takeyama, H.; Kawana-Tachikawa, A.; Tokunaga, K.; Yamagishi, M.; Martinez, J.P.; Meyerhans, A. Homeostatically Maintained Resting Naive CD4(+) T Cells Resist Latent HIV Reactivation. Front. Microbiol. 2016, 7, 1944. [Google Scholar] [CrossRef] [Green Version]
- Darcis, G.; Berkhout, B.; Pasternak, A.O. The Quest for Cellular Markers of HIV Reservoirs: Any Color You Like. Front. Immunol. 2019, 10, 2251. [Google Scholar] [CrossRef]
- Kuo, H.-H.; Lichterfeld, M. Recent progress in understanding HIV reservoirs. Curr. Opin. Hiv Aids 2018, 13, 137–142. [Google Scholar] [CrossRef]
- Honeycutt, J.B.; Thayer, W.O.; Baker, C.E.; Ribeiro, R.M.; Lada, S.M.; Cao, Y.; Cleary, R.A.; Hudgens, M.G.; Richman, D.D.; Garcia, J.V. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat. Med. 2017, 23, 638–643. [Google Scholar] [CrossRef]
- Eriksson, S.; Graf, E.H.; Dahl, V.; Strain, M.C.; Yukl, S.A.; Lysenko, E.S.; Bosch, R.J.; Lai, J.; Chioma, S.; Emad, F.; et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013, 9, e1003174. [Google Scholar] [CrossRef]
- Liu, Q.; Lusso, P. Integrin α4β7 in HIV-1 infection: A critical review. J. Leukoc. Biol. 2020, 108, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Houston, S.; Cerovic, V.; Thomson, C.; Brewer, J.; Mowat, A.; Milling, S. The lymph nodes draining the small intestine and colon are anatomically separate and immunologically distinct. Mucosal Immunol. 2016, 9, 468–478. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olwenyi, O.A.; Acharya, A.; Routhu, N.K.; Pierzchalski, K.; Jones, J.W.; Kane, M.A.; Sidell, N.; Mohan, M.; Byrareddy, S.N. Retinoic Acid Improves the Recovery of Replication-Competent Virus from Latent SIV Infected Cells. Cells 2020, 9, 2076. https://doi.org/10.3390/cells9092076
Olwenyi OA, Acharya A, Routhu NK, Pierzchalski K, Jones JW, Kane MA, Sidell N, Mohan M, Byrareddy SN. Retinoic Acid Improves the Recovery of Replication-Competent Virus from Latent SIV Infected Cells. Cells. 2020; 9(9):2076. https://doi.org/10.3390/cells9092076
Chicago/Turabian StyleOlwenyi, Omalla A., Arpan Acharya, Nanda Kishore Routhu, Keely Pierzchalski, Jace W. Jones, Maureen A. Kane, Neil Sidell, Mahesh Mohan, and Siddappa N. Byrareddy. 2020. "Retinoic Acid Improves the Recovery of Replication-Competent Virus from Latent SIV Infected Cells" Cells 9, no. 9: 2076. https://doi.org/10.3390/cells9092076
APA StyleOlwenyi, O. A., Acharya, A., Routhu, N. K., Pierzchalski, K., Jones, J. W., Kane, M. A., Sidell, N., Mohan, M., & Byrareddy, S. N. (2020). Retinoic Acid Improves the Recovery of Replication-Competent Virus from Latent SIV Infected Cells. Cells, 9(9), 2076. https://doi.org/10.3390/cells9092076




