Role of Jagged1-mediated Notch Signaling Activation in the Differentiation and Stratification of the Human Limbal Epithelium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Sclerocorneal Tissue
2.2. Isolation and Cultivation of Limbal Epithelial Cells
2.3. Notch Signaling Activation in the Limbal Epithelial Cells
2.4. Mitotic Arrest of the Stratified Limbal Epithelial Cultures and Analysis of Divisions
2.5. Immunohistochemistry
2.6. Quantitative RT-PCR (qRT-PCR)
2.7. Air-lifting Induction
2.8. Quantification and Statistical Analysis
3. Results
3.1. Jag1 is Expressed in the Limbal Epithelium and Activates Notch Signaling in LECs
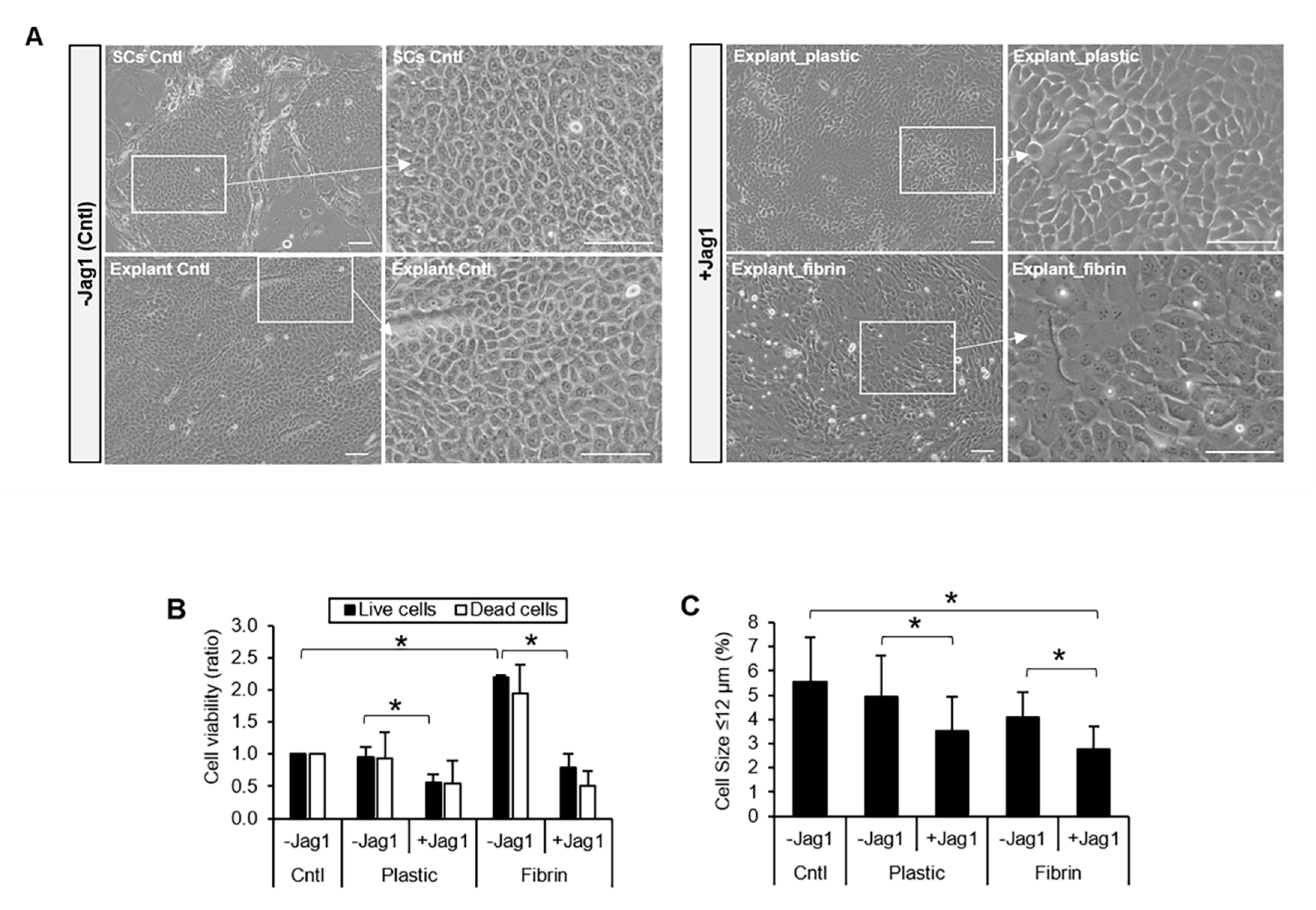
3.2. Jag1 Promoted Differentiation of LECs In Vitro
3.3. Jag1-Mediated Notch Activation Reduced Stratification and Promoted Differentiation of LECs upon Air-Lifting
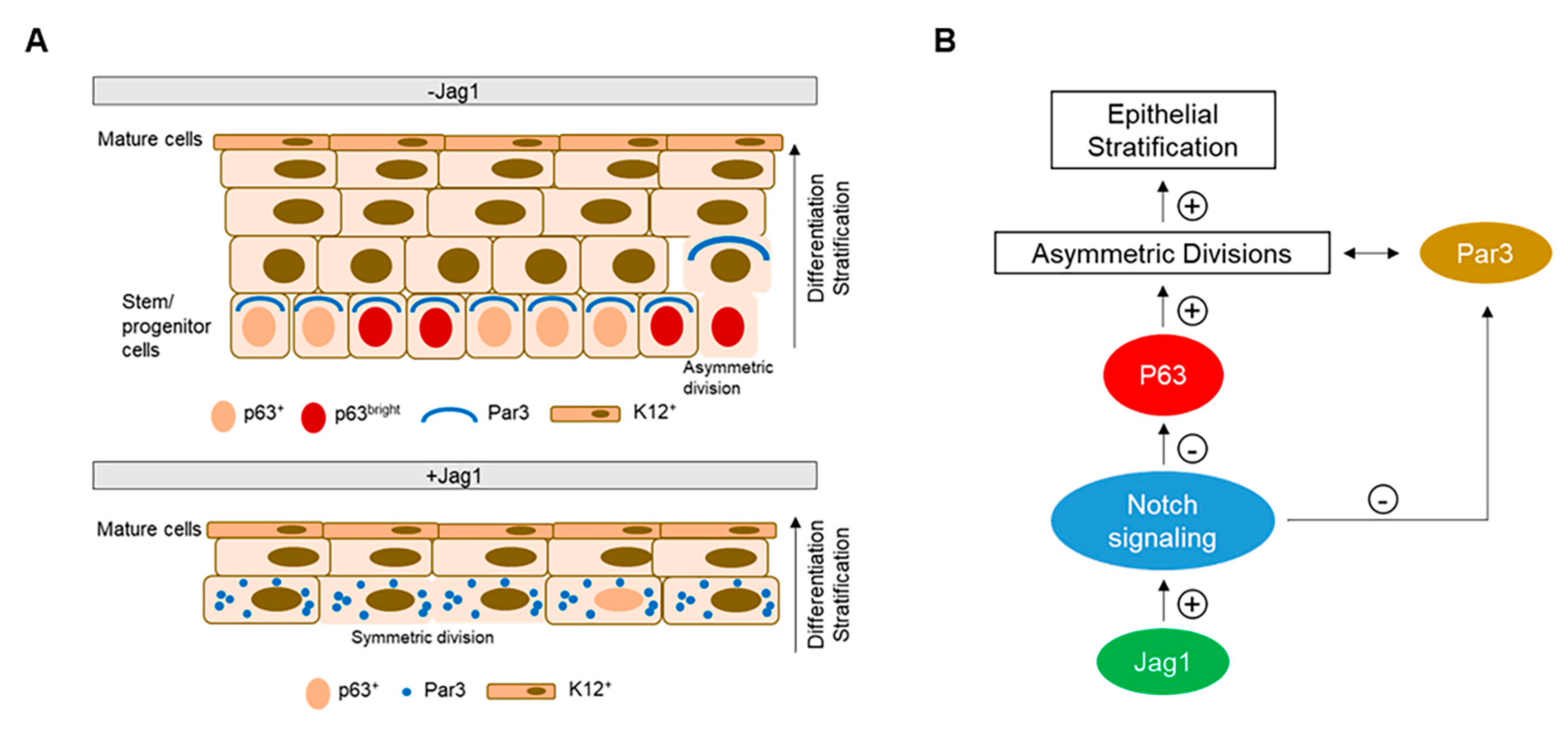
3.4. Jag1 Decreased Asymmetric Divisions in Basal LECs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daniels, J.T.; Harris, A.R.; Mason, C. Corneal epithelial stem cells in health and disease. Stem Cell Rev. 2006, 2, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hayashida, Y.; Chen, Y.T.; Tseng, S.C. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007, 17, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehrer, M.S.; Sun, T.T.; Lavker, R.M. Strategies of epithelial repair: Modulation of stem cell and transit amplifying cell proliferation. J. Cell Sci. 1998, 111 ( Pt. 19), 2867–2875. [Google Scholar]
- Sharma, A.; Coles, W.H. Kinetics of corneal epithelial maintenance and graft loss. A population balance model. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1962–1971. [Google Scholar]
- Thoft, R.A.; Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443. [Google Scholar]
- Fuchs, E. Epidermal differentiation and keratin gene expression. J. Cell Science. Suppl. 1993, 17, 197–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, E.R.; Sandberg, R.; Lendahl, U. Notch signaling: Simplicity in design, versatility in function. Development 2011, 138, 3593–3612. [Google Scholar] [CrossRef] [Green Version]
- Weinmaster, G. The ins and outs of notch signaling. Mol. Cell. Neurosci. 1997, 9, 91–102. [Google Scholar] [CrossRef]
- Ma, A.; Boulton, M.; Zhao, B.; Connon, C.; Cai, J.; Albon, J. A role for notch signaling in human corneal epithelial cell differentiation and proliferation. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3576–3585. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.B.; Liu, Y.H.; Zhuang, F.F.; Selvam, S.; Song, S.W.; Smith, R.E.; Trousdale, M.D.; Yiu, S.C. Identification of Notch-1 expression in the limbal basal epithelium. Mol. Vis. 2007, 13, 337–344. [Google Scholar]
- Djalilian, A.R.; Namavari, A.; Ito, A.; Balali, S.; Afshar, A.; Lavker, R.M.; Yue, B.Y. Down-regulation of Notch signaling during corneal epithelial proliferation. Mol. Vis. 2008, 14, 1041–1049. [Google Scholar] [PubMed]
- Nakamura, T.; Ohtsuka, T.; Sekiyama, E.; Cooper, L.J.; Kokubu, H.; Fullwood, N.J.; Barrandon, Y.; Kageyama, R.; Kinoshita, S. Hes1 regulates corneal development and the function of corneal epithelial stem/progenitor cells. Stem Cells 2008, 26, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, B.B.; Tighe, P.J.; Mohammed, I.; Yeung, A.M.; Powe, D.G.; Hopkinson, A.; Shanmuganathan, V.A.; Dua, H.S. Comparative transcriptional profiling of the limbal epithelial crypt demonstrates its putative stem cell niche characteristics. Bmc Genom. 2010, 11, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vauclair, S.; Majo, F.; Durham, A.D.; Ghyselinck, N.B.; Barrandon, Y.; Radtke, F. Corneal epithelial cell fate is maintained during repair by Notch1 signaling via the regulation of vitamin A metabolism. Dev. Cell 2007, 13, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Movahedan, A.; Afsharkhamseh, N.; Sagha, H.M.; Shah, J.R.; Milani, B.Y.; Milani, F.Y.; Logothetis, H.D.; Chan, C.C.; Djalilian, A.R. Loss of Notch1 disrupts the barrier repair in the corneal epithelium. PLoS ONE 2013, 8, e69113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, H.; Kaplan, N.; Hamanaka, R.B.; Katsnelson, J.; Blatt, H.; Yang, W.; Hao, L.; Bryar, P.J.; Johnson, R.S.; Getsios, S.; et al. microRNA-31/factor-inhibiting hypoxia-inducible factor 1 nexus regulates keratinocyte differentiation. Proc. Natl. Acad. Sci. United States Am. 2012, 109, 14030–14034. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, S.; Chen, L.; Deng, S.X. Comparative Study of Xenobiotic-Free Media for the Cultivation of Human Limbal Epithelial Stem/Progenitor Cells. Tissue Engineering. Part. C 2017, 23, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Mei, H.; Gonzalez, S.; Nakatsu, M.N.; Baclagon, E.R.; Chen, F.V.; Deng, S.X. Human adipose-derived stem cells support the growth of limbal stem/progenitor cells. PLoS ONE 2017, 12, e0186238. [Google Scholar] [CrossRef]
- Irvin, D.K.; Zurcher, S.D.; Nguyen, T.; Weinmaster, G.; Kornblum, H.I. Expression patterns of Notch1, Notch2, and Notch3 suggest multiple functional roles for the Notch-DSL signaling system during brain development. J. Comp. Neurol. 2001, 436, 167–181. [Google Scholar] [CrossRef]
- Chircop, M.; Sarcevic, B.; Larsen, M.R.; Malladi, C.S.; Chau, N.; Zavortink, M.; Smith, C.M.; Quan, A.; Anggono, V.; Hains, P.G.; et al. Phosphorylation of dynamin II at serine-764 is associated with cytokinesis. Biochim. Et Biophys. Acta 2011, 1813, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- McGeachie, A.B.; Odell, L.R.; Quan, A.; Daniel, J.A.; Chau, N.; Hill, T.A.; Gorgani, N.N.; Keating, D.J.; Cousin, M.A.; van Dam, E.M.; et al. Pyrimidyn compounds: Dual-action small molecule pyrimidine-based dynamin inhibitors. Acs Chem. Biol. 2013, 8, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, E.; Barbaro, V.; Ferrari, S.; Ortolani, C.; De Luca, M.; Pellegrini, G. Q-FIHC: Quantification of fluorescence immunohistochemistry to analyse p63 isoforms and cell cycle phases in human limbal stem cells. Microsc. Res. Tech. 2006, 69, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. New Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitkreutz, D.; Bohnert, A.; Herzmann, E.; Bowden, P.E.; Boukamp, P.; Fusenig, N.E. Differentiation specific functions in cultured and transplanted mouse keratinocytes: Environmental influences on ultrastructure and keratin expression. Differentiation 1984, 26, 154–169. [Google Scholar] [CrossRef]
- Rosdy, M.; Clauss, L.C. Terminal epidermal differentiation of human keratinocytes grown in chemically defined medium on inert filter substrates at the air-liquid interface. J. Investig. Dermatol. 1990, 95, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Pelletier, L. Pericentrin: Critical for spindle orientation. Curr. Biol. 2014, 24, R962–964. [Google Scholar] [CrossRef] [Green Version]
- Santoro, A.; Vlachou, T.; Carminati, M.; Pelicci, P.G.; Mapelli, M. Molecular mechanisms of asymmetric divisions in mammary stem cells. Embo Rep. 2016, 17, 1700–1720. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, M. The Par3/Par6/aPKC complex and epithelial cell polarity. Exp. Cell Res. 2013, 319, 1357–1364. [Google Scholar] [CrossRef]
- Lowell, S.; Jones, P.; Le Roux, I.; Dunne, J.; Watt, F.M. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr. Biol. 2000, 10, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Thelu, J.; Rossio, P.; Favier, B. Notch signalling is linked to epidermal cell differentiation level in basal cell carcinoma, psoriasis and wound healing. Bmc Dermatol. 2002, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, S.; Uhm, H.; Deng, S.X. Notch Inhibition Prevents Differentiation of Human Limbal Stem/Progenitor Cells in vitro. Sci. Rep. 2019, 9, 10373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickoloff, B.J.; Qin, J.Z.; Chaturvedi, V.; Denning, M.F.; Bonish, B.; Miele, L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 2002, 9, 842–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Loch, A.J.; Radtke, F.; Egan, S.E.; Xu, K. Jagged1 is the major regulator of Notch-dependent cell fate in proximal airways. Dev. Dyn. 2013, 242, 678–686. [Google Scholar] [CrossRef] [Green Version]
- Saravanamuthu, S.S.; Gao, C.Y.; Zelenka, P.S. Notch signaling is required for lateral induction of Jagged1 during FGF-induced lens fiber differentiation. Dev. Biol. 2009, 332, 166–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varnum-Finney, B.; Wu, L.; Yu, M.; Brashem-Stein, C.; Staats, S.; Flowers, D.; Griffin, J.D.; Bernstein, I.D. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J. Cell Sci. 2000, 113 Pt. 23, 4313–4318. [Google Scholar]
- Liu, L.; Wada, H.; Matsubara, N.; Hozumi, K.; Itoh, M. Identification of Domains for Efficient Notch Signaling Activity in Immobilized Notch Ligand Proteins. J. Cell. Biochem. 2017, 118, 785–796. [Google Scholar] [CrossRef]
- Koster, M.I.; Kim, S.; Mills, A.A.; DeMayo, F.J.; Roop, D.R. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004, 18, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Senoo, M.; Pinto, F.; Crum, C.P.; McKeon, F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell 2007, 129, 523–536. [Google Scholar] [CrossRef] [Green Version]
- Truong, A.B.; Kretz, M.; Ridky, T.W.; Kimmel, R.; Khavari, P.A. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 2006, 20, 3185–3197. [Google Scholar] [CrossRef] [Green Version]
- Tadeu, A.M.; Horsley, V. Notch signaling represses p63 expression in the developing surface ectoderm. Development 2013, 140, 3777–3786. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yeh, L.K.; Zhang, S.; Call, M.; Yuan, Y.; Yasunaga, M.; Kao, W.W.; Liu, C.Y. Wnt/beta-catenin signaling modulates corneal epithelium stratification via inhibition of Bmp4 during mouse development. Development 2015, 142, 3383–3393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culurgioni, S.; Mapelli, M. Going vertical: Functional role and working principles of the protein Inscuteable in asymmetric cell divisions. Cell. Mol. Life Sci. 2013, 70, 4039–4046. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Chen, T. A matter of life and death: Self-renewal in stem cells. Embo Rep. 2013, 14, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechler, T.; Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 2005, 437, 275–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, N.J.A.; Dias Gomes, M.; Bauer, R.; Brodesser, S.; Niemann, C.; Iden, S. Essential Role of Polarity Protein Par3 for Epidermal Homeostasis through Regulation of Barrier Function, Keratinocyte Differentiation, and Stem Cell Maintenance. J. Investig. Dermatol. 2016, 136, 2406–2416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bultje, R.S.; Castaneda-Castellanos, D.R.; Jan, L.Y.; Jan, Y.N.; Kriegstein, A.R.; Shi, S.H. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron 2009, 63, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Chenn, A.; McConnell, S.K. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell 1995, 82, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Cayouette, M.; Raff, M. Asymmetric segregation of Numb: A mechanism for neural specification from Drosophila to mammals. Nat. Neurosci. 2002, 5, 1265–1269. [Google Scholar] [CrossRef]
- Watt, F.M.; Estrach, S.; Ambler, C.A. Epidermal Notch signalling: Differentiation, cancer and adhesion. Curr. Opin. Cell Biol. 2008, 20, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Williams, S.E.; Beronja, S.; Pasolli, H.A.; Fuchs, E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature 2011, 470, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Perdigoto, C.N.; Schweisguth, F.; Bardin, A.J. Distinct levels of Notch activity for commitment and terminal differentiation of stem cells in the adult fly intestine. Development 2011, 138, 4585–4595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninov, N.; Borius, M.; Stainier, D.Y. Different levels of Notch signaling regulate quiescence, renewal and differentiation in pancreatic endocrine progenitors. Development 2012, 139, 1557–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, S.; Halabi, M.; Ju, D.; Tsai, M.; Deng, S.X. Role of Jagged1-mediated Notch Signaling Activation in the Differentiation and Stratification of the Human Limbal Epithelium. Cells 2020, 9, 1945. https://doi.org/10.3390/cells9091945
González S, Halabi M, Ju D, Tsai M, Deng SX. Role of Jagged1-mediated Notch Signaling Activation in the Differentiation and Stratification of the Human Limbal Epithelium. Cells. 2020; 9(9):1945. https://doi.org/10.3390/cells9091945
Chicago/Turabian StyleGonzález, Sheyla, Maximilian Halabi, David Ju, Matthew Tsai, and Sophie X. Deng. 2020. "Role of Jagged1-mediated Notch Signaling Activation in the Differentiation and Stratification of the Human Limbal Epithelium" Cells 9, no. 9: 1945. https://doi.org/10.3390/cells9091945
APA StyleGonzález, S., Halabi, M., Ju, D., Tsai, M., & Deng, S. X. (2020). Role of Jagged1-mediated Notch Signaling Activation in the Differentiation and Stratification of the Human Limbal Epithelium. Cells, 9(9), 1945. https://doi.org/10.3390/cells9091945




