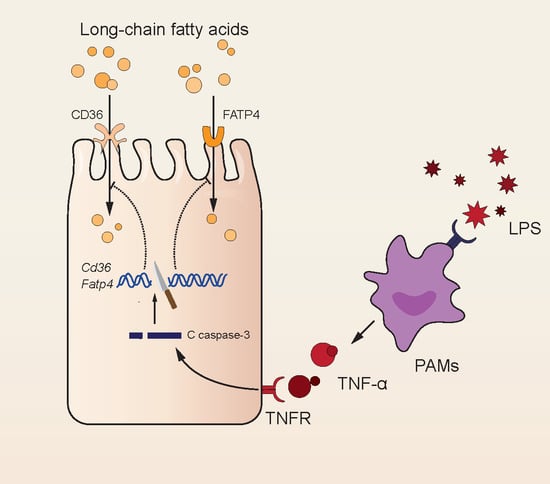
LPS Inhibits Fatty Acid Absorption in Enterocytes through TNF-α Secreted by Macrophages
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Design of In Vivo Experiments
2.2. Cell Culture and Design of In Vitro Experiments
2.3. Intestinal Morphology Analysis
2.4. Gut Permeability Assessment
2.5. Immunohistochemistry
2.6. Apoptosis and Pro-Inflammatory Cytokine Treatment on IPEC-J2 Cells
2.7. Western Blot Analysis
2.8. RNA Isolation and qRT-PCR
2.9. Fluorescence Microscopy
2.10. Statistical Analysis
3. Results
3.1. LPS Leading to FA Absorption Inhibition In Vivo
3.2. Inhibition of FA Absorption Caused by Apoptosis
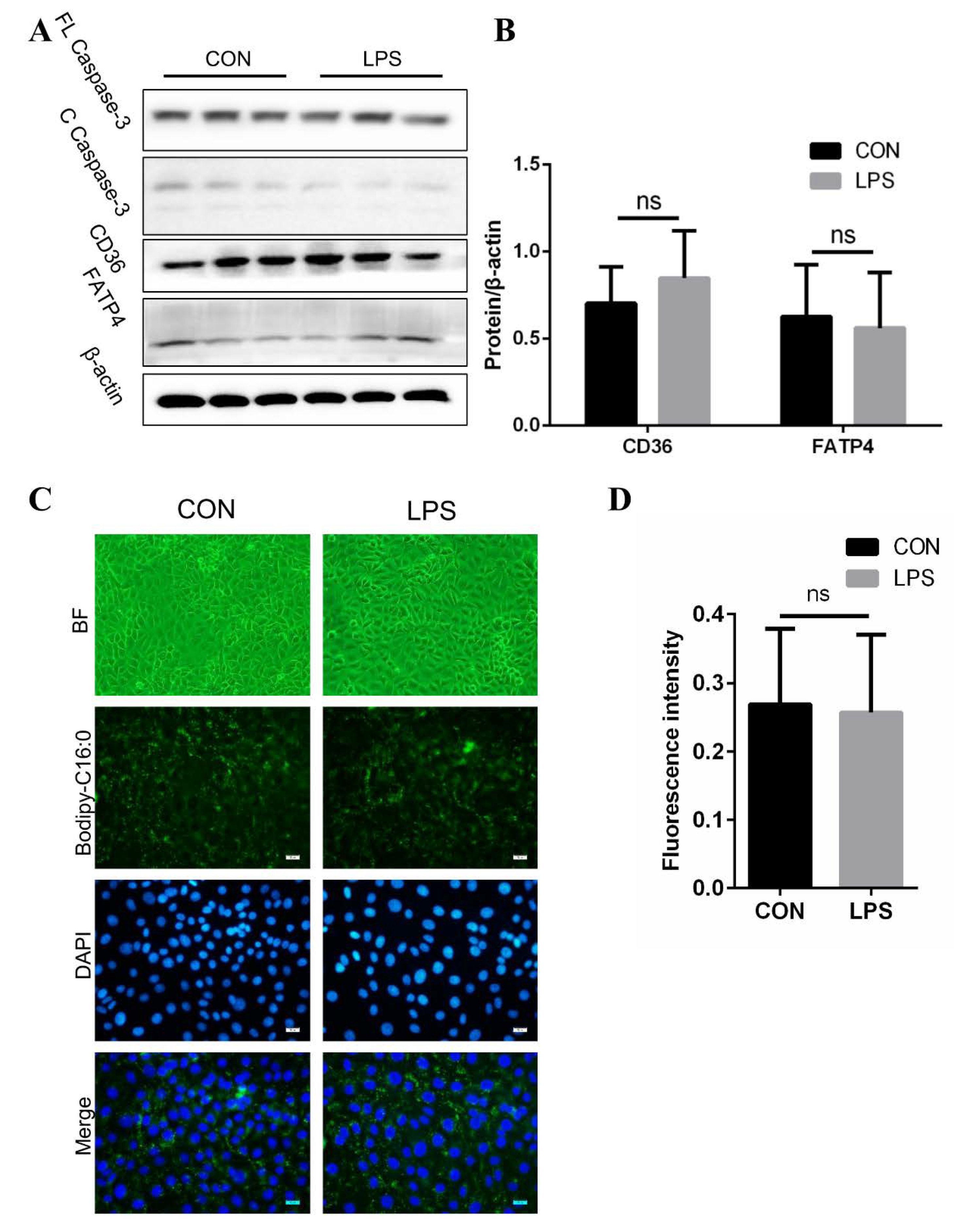
3.3. No Effect on Fatty Acid Absorption in IPEC-J2 after LPS Treatment
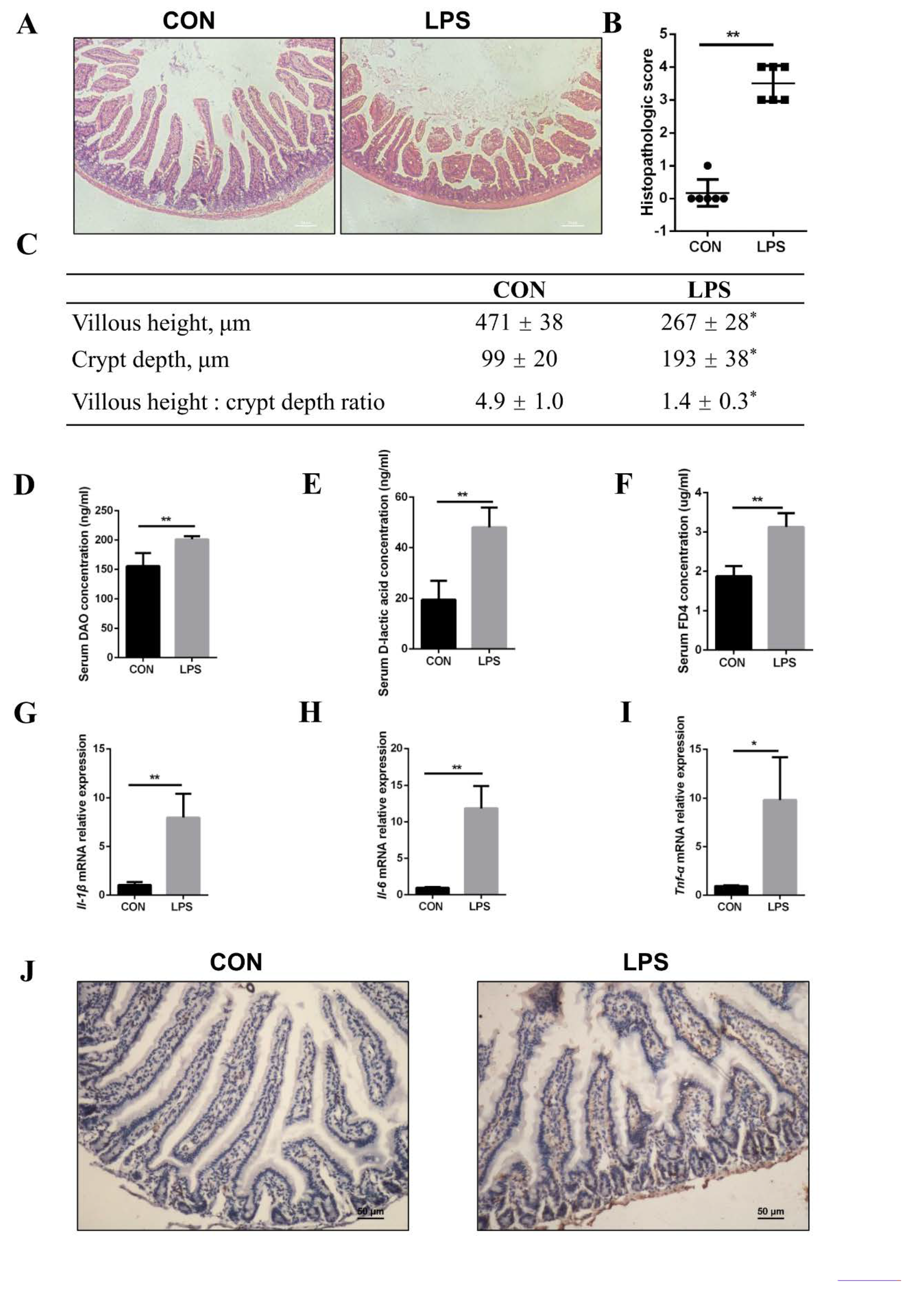
3.4. Injury of Jejunal Epithelium Caused by LPS Intraperitoneal Injection in Mice
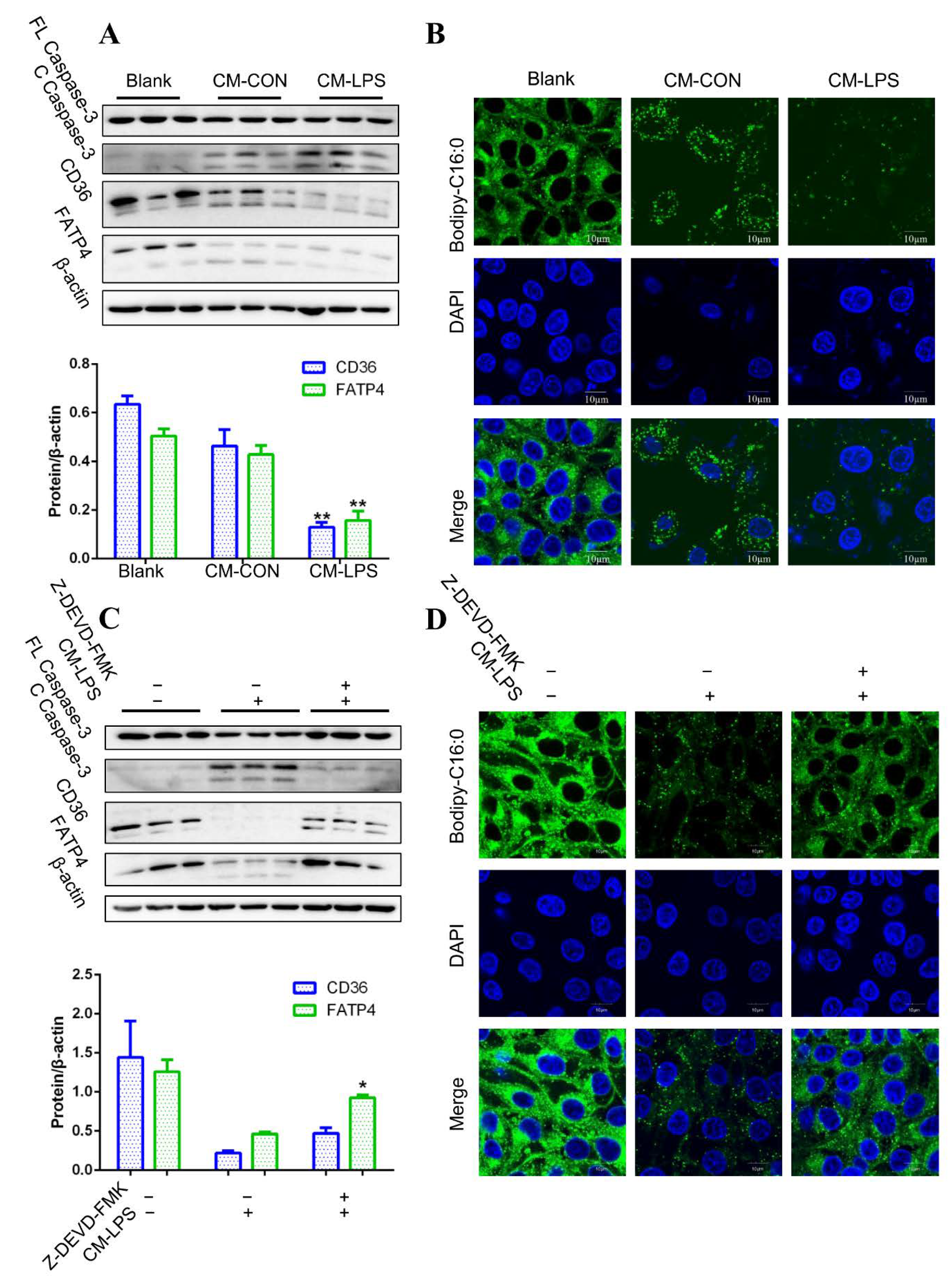
3.5. Apoptosis and Fatty Acid Absorption Inhibition in IPEC-J2 Induced by CM-LPS
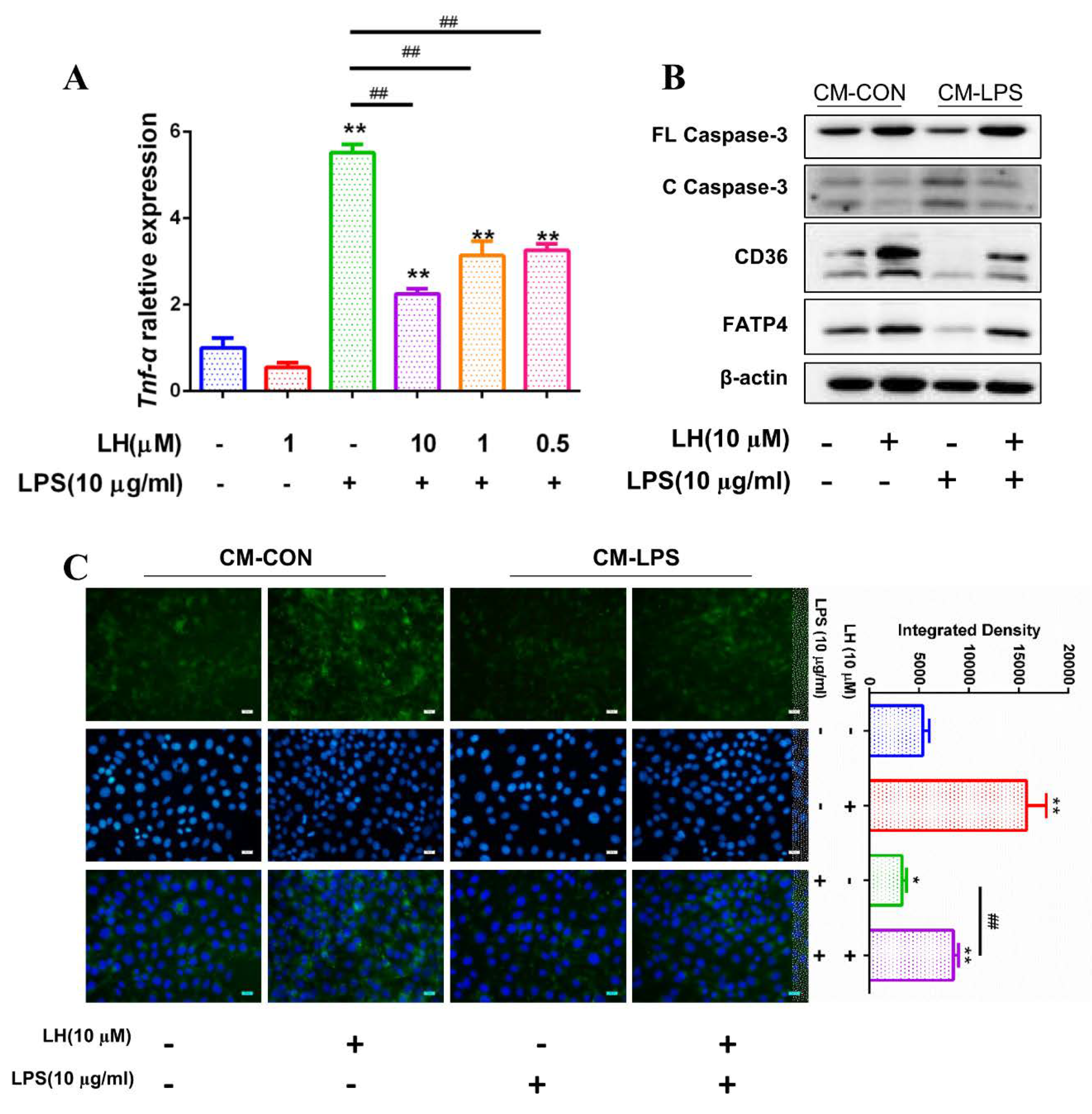
3.6. TNF-α Present in CM-LPS Inducing Apoptosis of IPEC-J2
3.7. Preventing the Secretion of TNF-α Leading to Better Fatty Acid Absorption
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, T.Y.; Liu, M.; Portincasa, P.; Wang, D.Q. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur. J. Clin. Investig. 2013, 43, 1203–1223. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Benitez-Paez, A.; de Palma, G.; Capilla, A.; Nova, E.; Castillejo, G.; Varea, V.; Marcos, A.; Garrote, J.A.; Polanco, I.; et al. Increased prevalence of pathogenic bacteria in the gut microbiota of infants at risk of developing celiac disease: The PROFICEL study. Gut Microbes 2018, 9, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Cheifetz, A.S. Oxford American Handbook of Gastroenterology and Hepatology; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Sutcliffe, I.C. A phylum level perspective on bacterial cell envelope architecture. Trends Microbiol. 2010, 18, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ribeiro, A.A.; Guan, Z.; McGrath, S.C.; Cotter, R.J.; Raetz, C.R. Structure and biosynthesis of free lipid A molecules that replace lipopolysaccharide in Francisella tularensis subsp. novicida. Biochemistry 2006, 45, 14427–14440. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Choi, S. Gram-negative marine bacteria: Structural features of lipopolysaccharides and their relevance for economically important diseases. Mar. Drugs 2014, 12, 2485–2514. [Google Scholar] [CrossRef]
- Kilar, A.; Dornyei, A.; Kocsis, B. Structural characterization of bacterial lipopolysaccharides with mass spectrometry and on- and off-line separation techniques. Mass Spectrom. Rev. 2013, 32, 90–117. [Google Scholar] [CrossRef]
- Lichtman, A.H.; Abbas, A.K. Basic Immunology: Functions and Disorders of the Immune System; Elsevier Saunders: Philadelphia, PA, USA, 2006. [Google Scholar]
- Opal, S.M. Endotoxins and other sepsis triggers. Contrib. Nephrol. 2010, 167, 14–24. [Google Scholar]
- Beutler, B.; Rietschel, E.T. Innate immune sensing and its roots: The story of endotoxin. Nat. Rev. Immunol. 2003, 3, 169–176. [Google Scholar] [CrossRef]
- Mandal, P.; Feng, Y.; Lyons, J.D.; Berger, S.B.; Otani, S.; DeLaney, A.; Tharp, G.K.; Maner-Smith, K.; Burd, E.M.; Schaeffer, M.; et al. Caspase-8 Collaborates with Caspase-11 to Drive Tissue Damage and Execution of Endotoxic Shock. Immunity 2018, 49, 42–55. [Google Scholar] [CrossRef]
- Tucureanu, M.M.; Rebleanu, D.; Constantinescu, C.A.; Deleanu, M.; Voicu, G.; Butoi, E.; Calin, M.; Manduteanu, I. Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of Gi-protein inhibitor. Int. J. Nanomed. 2018, 13, 63–76. [Google Scholar] [CrossRef]
- Tymoczko, J.L.; Stryer, L.; Stryer, L.; Berg, J.M. Biochemistry; W.H. Freeman: New York, NY, USA, 2007. [Google Scholar]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R. Lipid related consequences of intestinal malabsorption. Gut 1989, 30, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.H.; Rasmussen, K.; Niels-Christiansen, L.L.; Danielsen, E.M. Dietary free fatty acids form alkaline phosphatase-enriched microdomains in the intestinal brush border membrane. Mol. Membr. Biol. 2011, 28, 136–144. [Google Scholar] [CrossRef] [PubMed]
- van Bennekum, A.; Werder, M.; Thuahnai, S.T.; Han, C.H.; Duong, P.; Williams, D.L.; Wettstein, P.; Schulthess, G.; Phillips, M.C.; Hauser, H. Class B scavenger receptor-mediated intestinal absorption of dietary ss-carotene and cholesterol. Biochemistry 2005, 44, 4517–4525. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.L.; Febbraio, M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2009, 2, e3. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Hirsch, D.J.; Gimeno, R.E.; Punreddy, S.; Ge, P.; Watson, N.; Patel, S.; Kotler, M.; Raimondi, A.; Tartaglia, L.A.; et al. Identification of the major intestinal fatty acid transport protein. Mol. Cell 1999, 4, 299–308. [Google Scholar] [CrossRef]
- Buttet, M.; Traynard, V.; Tran, T.T.; Besnard, P.; Poirier, H.; Niot, I. From fatty-acid sensing to chylomicron synthesis: Role of intestinal lipid-binding proteins. Biochimie 2014, 96, 37–47. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef]
- Mattson, F.H.; Volpenhein, R.A. The digestion and absorption of triglycerides. J. Biol. Chem. 1964, 239, 2772–2777. [Google Scholar]
- Shen, H.; Howles, P.; Tso, P. From interaction of lipidic vehicles with intestinal epithelial cell membranes to the formation and secretion of chylomicrons. Adv. Drug Deliv. Rev. 2001, 50, S103–S125. [Google Scholar] [CrossRef]
- Dixon, J.B. Mechanisms of chylomicron uptake into lacteals. Ann. N. Y. Acad. Sci. 2010, 1207, E52–E57. [Google Scholar] [CrossRef] [PubMed]
- Brunzell, J.D.; Hazzard, W.R.; Porte, D.J.; Bierman, E.L. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. J. Clin. Investig. 1973, 52, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cao, X.; Wang, H.; Zhao, J.; Wang, X.; Wang, Y. Antimicrobial peptide KR-32 alleviates Escherichia coli K88-induced fatty acid malabsorption by improving expression of fatty acid transporter protein 4 (FATP4)1. J. Anim. Sci. 2019, 97, 2342–2356. [Google Scholar] [CrossRef]
- Wang, L.; Llorente, C.; Hartmann, P.; Yang, A.M.; Chen, P.; Schnabl, B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J. Immunol. Methods 2015, 421, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Liu, W.; Zuo, S.; Xie, T.; Deng, H.; Zhang, Q.; Zhong, B. Impaired function of the intestinal barrier in a novel sub-health rat model. Mol. Med. Rep. 2016, 13, 3459–3465. [Google Scholar] [CrossRef]
- Zong, X.; Hu, W.; Song, D.; Li, Z.; Du, H.; Lu, Z.; Wang, Y. Porcine lactoferrin-derived peptide LFP-20 protects intestinal barrier by maintaining tight junction complex and modulating inflammatory response. Biochem. Pharmacol. 2016, 104, 74–82. [Google Scholar] [CrossRef]
- Lorente, L.; Martin, M.M.; Perez-Cejas, A.; Gonzalez-Rivero, A.F.; Lopez, R.O.; Ferreres, J.; Solé-Violán, J.; Labarta, L.; Díaz, C.; Palmero, S.; et al. Sustained high serum caspase-3 concentrations and mortality in septic patients. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 281–288. [Google Scholar] [CrossRef]
- Fan, J.H.; Feng, G.G.; Huang, L.; Tang, G.D.; Jiang, H.X.; Xu, J. Naofen promotes TNF-alpha-mediated apoptosis of hepatocytes by activating caspase-3 in lipopolysaccharide-treated rats. World J. Gastroenterol. 2014, 20, 4963–4971. [Google Scholar] [CrossRef]
- Song, D.; Zong, X.; Zhang, H.; Wang, T.; Yi, H.; Luan, C.; Wang, Y. Antimicrobial peptide Cathelicidin-BF prevents intestinal barrier dysfunction in a mouse model of endotoxemia. Int. Immunopharmacol. 2015, 25, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Do-Umehara, H.C.; Chen, C.; Urich, D.; Zhou, L.; Qiu, J.; Jang, S.; Zander, A.; Baker, M.A.; Eilers, M.; Sporn, P.H.S.; et al. Suppression of inflammation and acute lung injury by Miz1 via repression of C/EBP-delta. Nat. Immunol. 2013, 14, 461–469. [Google Scholar] [CrossRef]
- Nabuurs, M.J. Weaning piglets as a model for studying pathophysiology of diarrhea. Vet. Q. 1998, 20, S42–S45. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, D.; Liu, D.; Liu, M.; Ge, Y.; Jiang, M.; Liu, Y.; Zheng, D. Tumor necrosis factor-related apoptosis-inducing ligand induces the expression of proinflammatory cytokines in macrophages and re-educates tumor-associated macrophages to an antitumor phenotype. Mol. Biol. Cell 2015, 26, 3178–3189. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.; Lee, T.; Yang, H.; Chen, C.; Au, Y.; Lu, Y.; Wu, L.; Wei, S.; Ni, Y.; Lin, B.; et al. LPS receptor subunits have antagonistic roles in epithelial apoptosis and colonic carcinogenesis. Cell Death Differ. 2015, 22, 1590–1604. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, L.; Tao, W.; Pei, X.; Wang, G.; Wang, M. Clostridium tyrobutyricum protect intestinal barrier function from LPS-induced apoptosis via p38/JNK signaling pathway in IPEC-J2 cells. J. Anim. Sci. 2018, 963, 166–167. [Google Scholar] [CrossRef]
- Boatright, K.M.; Salvesen, G.S. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 2003, 15, 725–731. [Google Scholar] [CrossRef]
- Walters, J.; Pop, C.; Scott, F.L.; Drag, M.; Swartz, P.; Mattos, C.; Salvesen, G.S.; Clark, A.C. A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis. Biochem. J. 2009, 424, 335–345. [Google Scholar] [CrossRef]
- Porter, A.G.; Janicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Ghoshal, S.; Witta, J.; Zhong, J.; de Villiers, W.; Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2008, 50, 90–97. [Google Scholar] [CrossRef]
- Plociennikowska, A.; Hromada-Judycka, A.; Borzecka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef]
- Gaur, U.; Aggarwal, B.B. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem. Pharmacol. 2003, 66, 1403–1408. [Google Scholar] [CrossRef]
- Thorburn, A. Death receptor-induced cell killing. Cell. Signal. 2004, 16, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Sykes, M.C.; Mowbray, A.L.; Jo, H. Reversible glutathiolation of caspase-3 by glutaredoxin as a novel redox signaling mechanism in tumor necrosis factor-alpha-induced cell death. Circ. Res. 2007, 100, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Bader, J.E.; Enos, R.T.; Velazquez, K.T.; Carson, M.S.; Nagarkatti, M.; Nagarkatti, P.S.; Chatzistamou, I.; Davis, J.M.; Carson, J.A.; Robinson, C.M.; et al. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G22–G31. [Google Scholar] [CrossRef]
- Fredriksson, L.; Herpers, B.; Benedetti, G.; Matadin, Q.; Puigvert, J.C.; de Bont, H.; Dragovic, S.; Vermeulen, N.P.E.; Commandeur, J.N.M.; Danen, E.; et al. Diclofenac inhibits tumor necrosis factor-alpha-induced nuclear factor-kappaB activation causing synergistic hepatocyte apoptosis. Hepatology 2011, 53, 2027–2041. [Google Scholar] [CrossRef]
- Kim, K.Y.; Stevens, M.V.; Akter, M.H.; Rusk, S.E.; Huang, R.J.; Cohen, A.; Noguchi, A.; Springer, D.; Bocharov, A.V.; Eggerman, T.L.; et al. Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J. Clin. Investig. 2011, 121, 3701–3712. [Google Scholar] [CrossRef]
- Dlugosz, E.; Basalaj, K.; Zawistowska-Deniziak, A. Cytokine production and signalling in human THP-1 macrophages is dependent on Toxocara canis glycans. Parasitol. Res. 2019, 118, 2925–2933. [Google Scholar] [CrossRef]
- Sharma, A.K.; Fernandez, L.G.; Awad, A.S.; Kron, I.L.; Laubach, V.E. Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage-produced TNF-alpha during pulmonary ischemia-reperfusion injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L105–L113. [Google Scholar] [CrossRef]
- Voortman, T.; van den Hooven, E.H.; Braun, K.V.; van den Broek, M.; Bramer, W.M.; Chowdhurry, R.; Franco, O.H. Effects of polyunsaturated fatty acid intake and status during pregnancy, lactation, and early childhood on cardiometabolic health: A systematic review. Prog. Lipid Res. 2015, 59, 67–87. [Google Scholar] [CrossRef]
- Neto, N.; Murari, A.; Oyama, L.M.; Otoch, J.P.; Alcantara, P.; Tokeshi, F.; Figuerêdo, R.G.; Alves, M.J.; Lima, G.D.C.C.; Matos-Neto, E.M.; et al. Peritumoural adipose tissue pro-inflammatory cytokines are associated with tumoural growth factors in cancer cachexia patients. J. Cachexia Sarcopenia Muscle 2018, 9, 1101–1108. [Google Scholar] [CrossRef]



| Gene | Forward Primer (5′-3′) | Forward Primer (5′-3′) |
|---|---|---|
| m *-Il-1β | TCCAGGATGAGGACATGAGCAC | GAACGTCACACACCAGCAGGTTA |
| m-Il-6 | CCACTTCACAAGTCGGAGGCTTA | CCAGTTTGGTAGCATCCATCATTTC |
| m-Tnf-α | TATGGCCCAGACCCTCACA | GGAGTAGACAAGGTACAACCCATC |
| m-Gapdh | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG |
| p #-Il-1β | GAGCTGAAGGCTCTCCACCTC | ATCGCTGTCATCTCCTTGCAC |
| p-Il-6 | TTCACCTCTCCGGACAAAAC | TCTGCCAGTACCTCCTTGCT |
| p-Tnf-α | TTCCAGCTGGCCCCTTGAGC | GAGGGCATTGGCATACCCAC |
| p-Cd36 | CCATACCCTATTCCTACCAC | AGGCTGCATCTGTACCATTA |
| p-Fatp4 | TATGGTGTGGAGGTGCCAGGAA | CCGCAGGTCTGTCTTCTGTAGC |
| p-Gapdh | CAAGGAGTAAGAGCCCCTGG | GGTACATGACGAGGCAGGTC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Kai, L.; Du, H.; Wang, X.; Wang, Y. LPS Inhibits Fatty Acid Absorption in Enterocytes through TNF-α Secreted by Macrophages. Cells 2019, 8, 1626. https://doi.org/10.3390/cells8121626
Liu H, Kai L, Du H, Wang X, Wang Y. LPS Inhibits Fatty Acid Absorption in Enterocytes through TNF-α Secreted by Macrophages. Cells. 2019; 8(12):1626. https://doi.org/10.3390/cells8121626
Chicago/Turabian StyleLiu, Heyuan, Lixia Kai, Huahua Du, Xinxia Wang, and Yizhen Wang. 2019. "LPS Inhibits Fatty Acid Absorption in Enterocytes through TNF-α Secreted by Macrophages" Cells 8, no. 12: 1626. https://doi.org/10.3390/cells8121626
APA StyleLiu, H., Kai, L., Du, H., Wang, X., & Wang, Y. (2019). LPS Inhibits Fatty Acid Absorption in Enterocytes through TNF-α Secreted by Macrophages. Cells, 8(12), 1626. https://doi.org/10.3390/cells8121626