RITA Is Expressed in Trophoblastic Cells and Is Involved in Differentiation Processes of the Placenta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Collection
2.2. RNA Extraction and Real-Time PCR
2.3. Immunohistochemistry of Placental Tissue
2.4. Cell Culture, Transfection, and Treatment
2.5. Isolation and Purification of Primary Villous Cytotrophoblasts from Human Term Placental Tissue
2.6. Cell Viability and Invasion Assay
2.7. Cell Motility and Time-Lapse Microscopy
2.8. Western Blot Analysis
2.9. Immunofluorescence Staining and Measurement
2.10. Fusion Assay and β-hCG ELISA
2.11. Statistical Analysis
3. Results
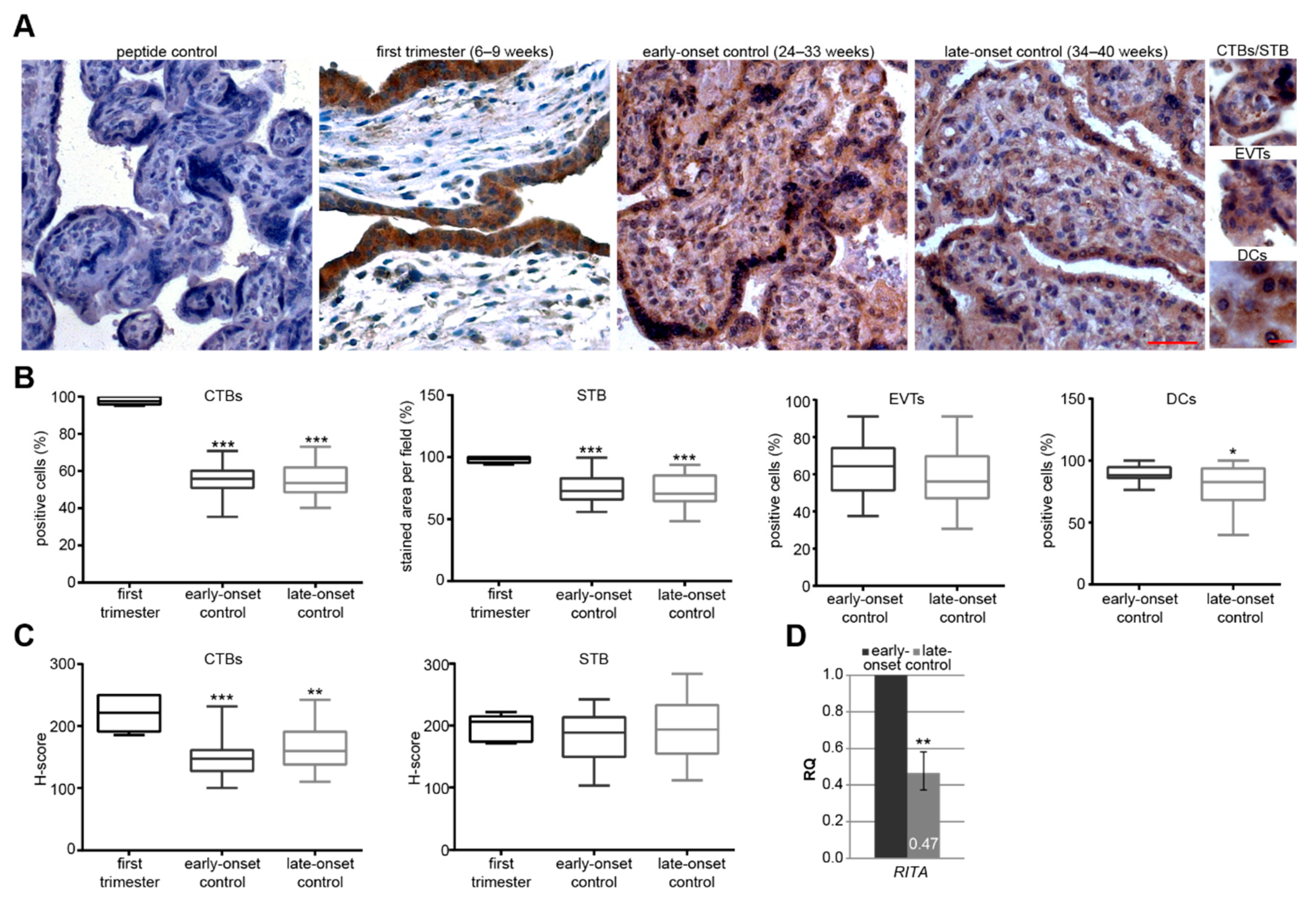
3.1. RITA is Specifically Expressed in Placental Tissue and its mRNA Level Decreases at Late Gestational Stages
3.2. The mRNA Level of RITA is Decreased in Early-Onset Preeclamptic Placentas
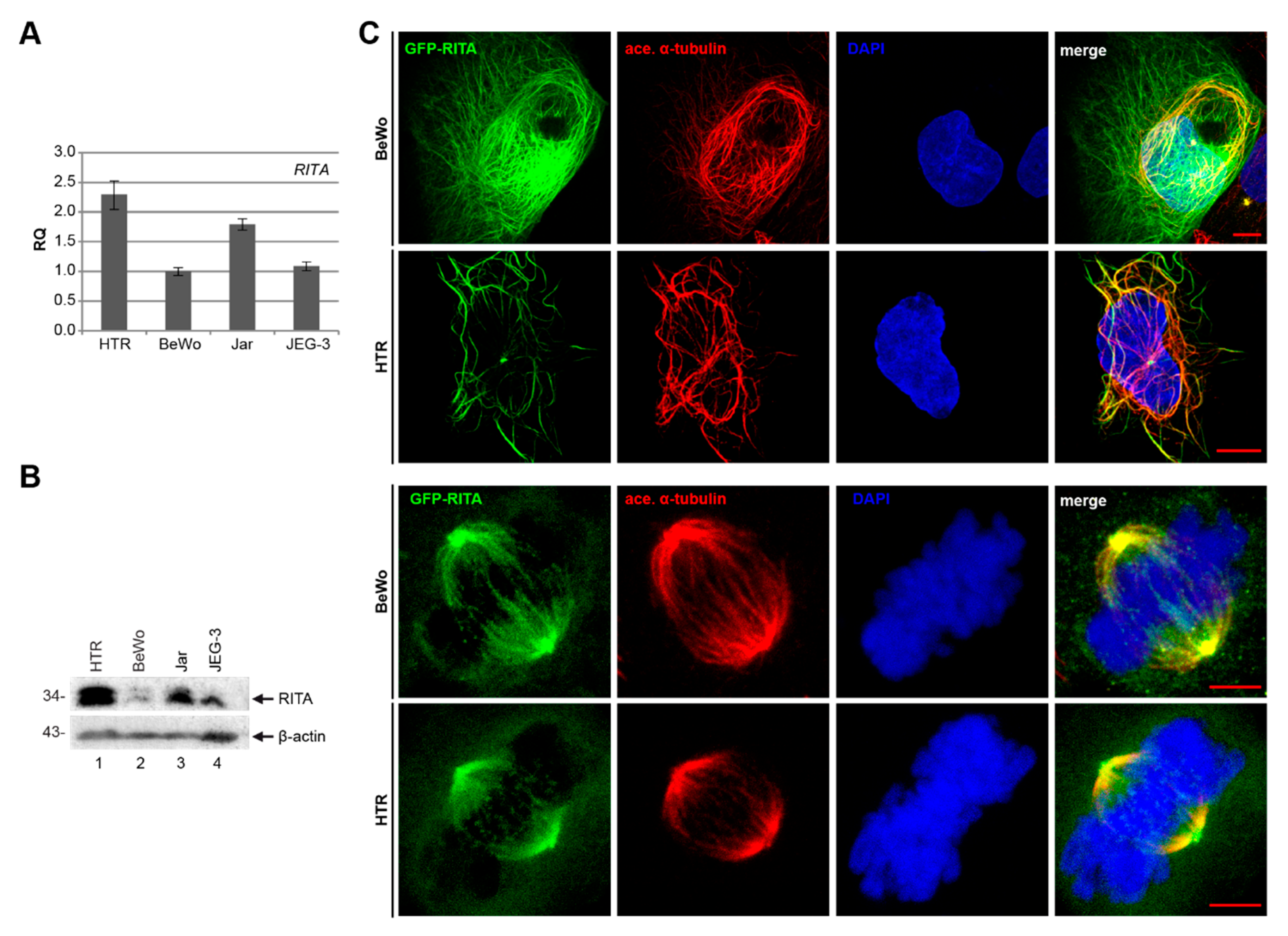
3.3. Expression and Localization of RITA in Trophoblastic Cell Lines
3.4. Gene Silencing of RITA Decreases Motility and Invasion Capability of Trophoblast Cells Derived from First Trimester Placentas
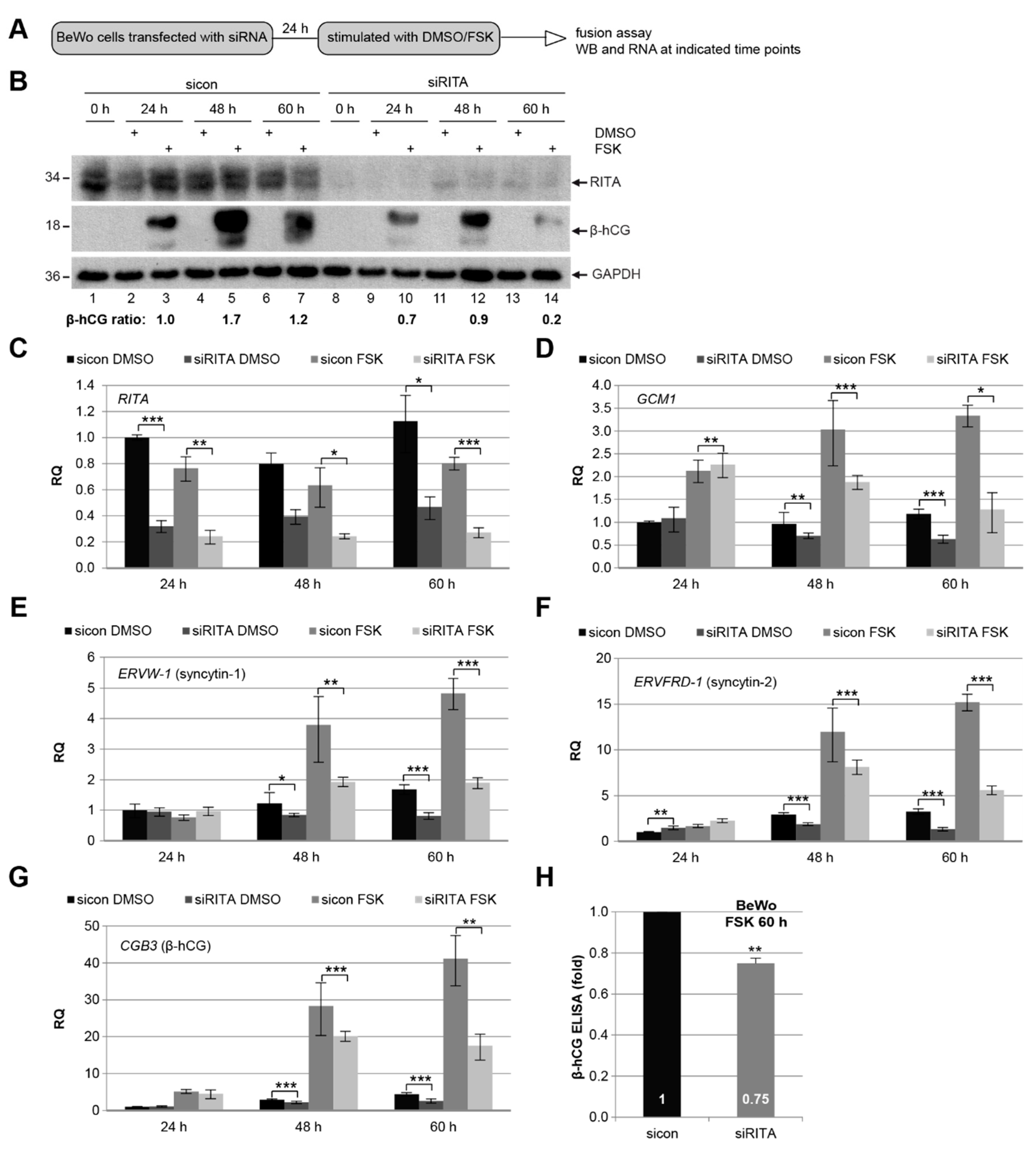
3.5. Knockdown of RITA Reduces the mRNA Level of Different Fusion-Related Molecules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roberts, J.M.; Hubel, C.A. The two stage model of preeclampsia: Variations on the theme. Placenta 2009, 30 (Suppl. A), S32–S37. [Google Scholar] [CrossRef]
- Steegers, E.A.; von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Sanchez-Aranguren, L.C.; Prada, C.E.; Riano-Medina, C.E.; Lopez, M. Endothelial dysfunction and preeclampsia: Role of oxidative stress. Front. Physiol. 2014, 5, 372. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Roberts, J.M. Preeclampsia: Syndrome or Disease? Curr. Hypertens. Rep. 2015, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Robillard, P.Y.; Dekker, G.; Chaouat, G.; Scioscia, M.; Iacobelli, S.; Hulsey, T.C. Historical evolution of ideas on eclampsia/preeclampsia: A proposed optimistic view of preeclampsia. J. Reprod. Immunol. 2017, 123, 72–77. [Google Scholar] [CrossRef]
- Ness, R.B.; Roberts, J.M. Heterogeneous causes constituting the single syndrome of preeclampsia: A hypothesis and its implications. Am. J. Obstet. Gynecol. 1996, 175, 1365–1370. [Google Scholar] [CrossRef]
- Phipps, E.; Prasanna, D.; Brima, W.; Jim, B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin. J. Am. Soc. Nephrol. CJASN 2016, 11, 1102–1113. [Google Scholar] [CrossRef]
- Wacker, S.A.; Alvarado, C.; von Wichert, G.; Knippschild, U.; Wiedenmann, J.; Clauss, K.; Nienhaus, G.U.; Hameister, H.; Baumann, B.; Borggrefe, T.; et al. RITA, a novel modulator of Notch signalling, acts via nuclear export of RBP-J. EMBO J. 2011, 30, 43–56. [Google Scholar] [CrossRef]
- Tabaja, N.; Yuan, Z.; Oswald, F.; Kovall, R.A. Structure-function analysis of RBP-J-interacting and tubulin-associated (RITA) reveals regions critical for repression of Notch target genes. J. Biol. Chem. 2017, 292, 10549–10563. [Google Scholar] [CrossRef]
- Haider, S.; Pollheimer, J.; Knofler, M. Notch signalling in placental development and gestational diseases. Placenta 2017, 56, 65–72. [Google Scholar] [CrossRef]
- Steinhauser, K.; Kloble, P.; Kreis, N.N.; Ritter, A.; Friemel, A.; Roth, S.; Reichel, J.M.; Michaelis, J.; Rieger, M.A.; Louwen, F.; et al. Deficiency of RITA results in multiple mitotic defects by affecting microtubule dynamics. Oncogene 2017, 36, 2146–2159. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.J.; Wordeman, L. The quantification and regulation of microtubule dynamics in the mitotic spindle. Curr. Opin. Cell Biol. 2019, 60, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kreis, N.N.; Steinhauser, K.; Ritter, A.; Kloble, P.; Hoock, S.C.; Roth, S.; Louwen, F.; Oswald, F.; Yuan, J. Potential involvement of RITA in the activation of Aurora A at spindle poles during mitosis. Oncogene 2019, 38, 4199. [Google Scholar] [CrossRef] [PubMed]
- Hoock, S.C.; Ritter, A.; Steinhauser, K.; Roth, S.; Behrends, C.; Oswald, F.; Solbach, C.; Louwen, F.; Kreis, N.N.; Yuan, J. RITA modulates cell migration and invasion by affecting focal adhesion dynamics. Mol. Oncol. 2019, 13, 2121–2141. [Google Scholar] [CrossRef] [PubMed]
- Rodel, F.; Steinhauser, K.; Kreis, N.N.; Friemel, A.; Martin, D.; Wieland, U.; Rave-Frank, M.; Balermpas, P.; Fokas, E.; Louwen, F.; et al. Prognostic impact of RITA expression in patients with anal squamous cell carcinoma treated with chemoradiotherapy. Radiother. Oncol. 2018, 126, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, G.; Wang, H.; Liu, C. RITA inhibits growth of human hepatocellular carcinoma through induction of apoptosis. Oncol. Res. 2013, 20, 437–445. [Google Scholar] [CrossRef]
- Milne, F.; Redman, C.; Walker, J.; Baker, P.; Bradley, J.; Cooper, C.; de Swiet, M.; Fletcher, G.; Jokinen, M.; Murphy, D.; et al. The pre-eclampsia community guideline (PRECOG): How to screen for and detect onset of pre-eclampsia in the community. BMJ 2005, 330, 576–580. [Google Scholar] [CrossRef]
- Meller, M.; Vadachkoria, S.; Luthy, D.A.; Williams, M.A. Evaluation of housekeeping genes in placental comparative expression studies. Placenta 2005, 26, 601–607. [Google Scholar] [CrossRef]
- Louwen, F.; Muschol-Steinmetz, C.; Reinhard, J.; Reitter, A.; Yuan, J. A lesson for cancer research: Placental microarray gene analysis in preeclampsia. Oncotarget 2012, 3, 759–773. [Google Scholar] [CrossRef]
- Muschol-Steinmetz, C.; Friemel, A.; Kreis, N.N.; Reinhard, J.; Yuan, J.; Louwen, F. Function of survivin in trophoblastic cells of the placenta. PLoS ONE 2013, 8, e73337. [Google Scholar] [CrossRef]
- Detre, S.; Saclani Jotti, G.; Dowsett, M. A “quickscore” method for immunohistochemical semiquantitation: Validation for oestrogen receptor in breast carcinomas. J. Clin. Pathol. 1995, 48, 876–878. [Google Scholar] [CrossRef] [PubMed]
- McClelland, R.A.; Finlay, P.; Walker, K.J.; Nicholson, D.; Robertson, J.F.; Blamey, R.W.; Nicholson, R.I. Automated quantitation of immunocytochemically localized estrogen receptors in human breast cancer. Cancer Res. 1990, 50, 3545–3550. [Google Scholar] [PubMed]
- Graham, C.H.; Hawley, T.S.; Hawley, R.G.; MacDougall, J.R.; Kerbel, R.S.; Khoo, N.; Lala, P.K. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp. Cell Res. 1993, 206, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.R.; Cartwright, J.E.; Whitley, G.S. Nitric oxide inhibits polyamine-induced apoptosis in the human extravillous trophoblast cell line SGHPL-4. Hum. Reprod. 2003, 18, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Kreis, N.N.; Friemel, A.; Zimmer, B.; Roth, S.; Rieger, M.A.; Rolle, U.; Louwen, F.; Yuan, J. Mitotic p21Cip1/CDKN1A is regulated by cyclin-dependent kinase 1 phosphorylation. Oncotarget 2016, 7, 50215–50228. [Google Scholar] [CrossRef] [PubMed]
- Petroff, M.G.; Phillips, T.A.; Ka, H.; Pace, J.L.; Hunt, J.S. Isolation and culture of term human trophoblast cells. Methods Mol. Med. 2006, 121, 203–217. [Google Scholar]
- Jasmer, B.; Muschol-Steinmetz, C.; Kreis, N.N.; Friemel, A.; Kielland-Kaisen, U.; Bruggmann, D.; Jennewein, L.; Allert, R.; Solbach, C.; Yuan, J.; et al. Involvement of the oncogene B-cell lymphoma 6 in the fusion and differentiation process of trophoblastic cells of the placenta. Oncotarget 2017, 8, 108643–108654. [Google Scholar] [CrossRef]
- Kreis, N.N.; Louwen, F.; Zimmer, B.; Yuan, J. Loss of p21Cip1/CDKN1A renders cancer cells susceptible to Polo-like kinase 1 inhibition. Oncotarget 2015, 6, 6611–6626. [Google Scholar] [CrossRef]
- Ritter, A.; Sanhaji, M.; Friemel, A.; Roth, S.; Rolle, U.; Louwen, F.; Yuan, J. Functional analysis of phosphorylation of the mitotic centromere-associated kinesin by Aurora B kinase in human tumor cells. Cell Cycle 2015, 14, 3755–3767. [Google Scholar] [CrossRef]
- Kreis, N.N.; Friemel, A.; Ritter, A.; Roth, S.; Rolle, U.; Louwen, F.; Yuan, J. Function of p21 (Cip1/Waf1/CDKN1A) in Migration and Invasion of Cancer and Trophoblastic Cells. Cancers 2019, 11, 989. [Google Scholar] [CrossRef]
- Ritter, A.; Friemel, A.; Kreis, N.N.; Louwen, F.; Yuan, J. Impact of Polo-like kinase 1 inhibitors on human adipose tissue-derived mesenchymal stem cells. Oncotarget 2016, 7, 84271–84285. [Google Scholar] [CrossRef] [PubMed]
- Kreis, N.N.; Sommer, K.; Sanhaji, M.; Kramer, A.; Matthess, Y.; Kaufmann, M.; Strebhardt, K.; Yuan, J. Long-term downregulation of Polo-like kinase 1 increases the cyclin-dependent kinase inhibitor p21(WAF1/CIP1). Cell Cycle 2009, 8, 460–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knofler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlohren, S.; Geusens, N.; Morton, J.; Verhaegen, I.; Hering, L.; Herse, F.; Dudenhausen, J.W.; Muller, D.N.; Luft, F.C.; Cartwright, J.E.; et al. Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy. Hypertension 2010, 56, 304–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loset, M.; Mundal, S.B.; Johnson, M.P.; Fenstad, M.H.; Freed, K.A.; Lian, I.A.; Eide, I.P.; Bjorge, L.; Blangero, J.; Moses, E.K.; et al. A transcriptional profile of the decidua in preeclampsia. Am. J. Obs. Gynecol. 2011, 204, 84.e1–84.e27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fragkiadaki, P.; Soulitzis, N.; Sifakis, S.; Koutroulakis, D.; Gourvas, V.; Vrachnis, N.; Spandidos, D.A. Downregulation of notch signaling pathway in late preterm and term placentas from pregnancies complicated by preeclampsia. PLoS ONE 2015, 10, e0126163. [Google Scholar] [CrossRef]
- James, J.; Tun, W.; Clark, A. Quantifying trophoblast migration: In vitro approaches to address in vivo situations. Cell Adhes. Migr. 2016, 10, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Baczyk, D.; Drewlo, S.; Proctor, L.; Dunk, C.; Lye, S.; Kingdom, J. Glial cell missing-1 transcription factor is required for the differentiation of the human trophoblast. Cell Death Differ. 2009, 16, 719–727. [Google Scholar] [CrossRef]
- Gry, M.; Rimini, R.; Stromberg, S.; Asplund, A.; Ponten, F.; Uhlen, M.; Nilsson, P. Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genom. 2009, 10, 365. [Google Scholar] [CrossRef] [Green Version]
- Efimov, A.; Schiefermeier, N.; Grigoriev, I.; Ohi, R.; Brown, M.C.; Turner, C.E.; Small, J.V.; Kaverina, I. Paxillin-dependent stimulation of microtubule catastrophes at focal adhesion sites. J. Cell Sci. 2008, 121, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Kaverina, I.; Krylyshkina, O.; Small, J.V. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J. Cell Biol. 1999, 146, 1033–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshie, M.; Kashima, H.; Bessho, T.; Takeichi, M.; Isaka, K.; Tamura, K. Expression of stathmin, a microtubule regulatory protein, is associated with the migration and differentiation of cultured early trophoblasts. Hum. Reprod. 2008, 23, 2766–2774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Librach, C.L.; Werb, Z.; Fitzgerald, M.L.; Chiu, K.; Corwin, N.M.; Esteves, R.A.; Grobelny, D.; Galardy, R.; Damsky, C.H.; Fisher, S.J. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J. Cell Biol. 1991, 113, 437–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staun-Ram, E.; Goldman, S.; Gabarin, D.; Shalev, E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod. Biol. Endocrinol. 2004, 2, 59. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.Y.; Pang, Z.J.; Yu, Y.H. Regulation of trophoblast invasion: The role of matrix metalloproteinases. Rev. Obs. Gynecol. 2012, 5, e137–e143. [Google Scholar]
- Espino, Y.S.S.; Flores-Pliego, A.; Espejel-Nunez, A.; Medina-Bastidas, D.; Vadillo-Ortega, F.; Zaga-Clavellina, V.; Estrada-Gutierrez, G. New Insights into the Role of Matrix Metalloproteinases in Preeclampsia. Int. J. Mol. Sci. 2017, 18, 1448. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Zhong, M.; Pang, Z.; Yu, Y. Dysregulated expression of matrix metalloproteinases and their inhibitors may participate in the pathogenesis of pre-eclampsia and fetal growth restriction. Early Hum. Dev. 2014, 90, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Rinkenberger, J.; Dai, J.; Flannery, M.; Sund, M.; Kanasaki, K.; Ni, W.; Kalluri, R.; Werb, Z. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc. Natl. Acad. Sci. USA 2013, 110, 11109–11114. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, A.; Omata, W.; Ackerman, W.E.t.; Takeshita, T.; Vandre, D.D.; Robinson, J.M. Cell fusion mediates dramatic alterations in the actin cytoskeleton, focal adhesions, and E-cadherin in trophoblastic cells. Cytoskeleton 2014, 71, 241–256. [Google Scholar] [CrossRef]
- Parast, M.M.; Aeder, S.; Sutherland, A.E. Trophoblast giant-cell differentiation involves changes in cytoskeleton and cell motility. Dev. Biol. 2001, 230, 43–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, G.C.; King, B.F. Colchicine inhibits human trophoblast differentiation in vitro. Placenta 1993, 14, 187–201. [Google Scholar] [CrossRef]
- Wang, R.; Yu, R.; Zhu, C.; Lin, H.Y.; Lu, X.; Wang, H. Tubulin detyrosination promotes human trophoblast syncytium formation. J. Mol. Cell Biol. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ersfeld, K.; Wehland, J.; Plessmann, U.; Dodemont, H.; Gerke, V.; Weber, K. Characterization of the tubulin-tyrosine ligase. J. Cell Biol. 1993, 120, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Orendi, K.; Gauster, M.; Moser, G.; Meiri, H.; Huppertz, B. The choriocarcinoma cell line BeWo: Syncytial fusion and expression of syncytium-specific proteins. Reproduction 2010, 140, 759–766. [Google Scholar] [CrossRef] [Green Version]
- Kudo, Y.; Boyd, C.A.; Sargent, I.L.; Redman, C.W. Hypoxia alters expression and function of syncytin and its receptor during trophoblast cell fusion of human placental BeWo cells: Implications for impaired trophoblast syncytialisation in pre-eclampsia. Biochim. Biophys. Acta 2003, 1638, 63–71. [Google Scholar] [CrossRef] [Green Version]



| n | Gestational Age (Weeks) | Body Mass Index (BMI) | Age | Birth Weight (g) | Systolic Blood Pressure (mmHg) | Diastolic Blood Pressure (mmHg) | Proteinuria (mg/24 h) | |
|---|---|---|---|---|---|---|---|---|
| Control | 21 | 28.8 ± 2.5 | 24.8 ± 3.7 | 29.6 ± 5.5 | 1295 ± 768 | 122 ± 11 | 73 ± 9 | n.d. |
| Early-Onset PE | 15 | 29.5 ± 2.7 | 25.8 ± 6.0 | 31.5 ± 5.4 | 1060 ± 328 | 165 ± 28 | 101 ± 17 | 1966 ± 1356 |
| p-Value | 0.428 | 0.582 | 0.318 | 0.223 | 0.00003 | 0.00001 | ||
| Control | 22 | 37.9 ± 1.8 | 24.2 ± 5.9 | 31.1 ± 3.4 | 3050 ± 650 | 124 ± 20 | 77 ± 17 | n.d. |
| Late-Onset PE | 16 | 37.6 ± 1.5 | 25.1 ± 7.2 | 31.7 ± 3.6 | 2253 ± 565 | 159 ± 24 | 102 ± 17 | 3363 ± 6820 |
| p-Value | 0.664 | 0.678 | 0.621 | 0.017 | 0.0001 | 0.0002 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wildner, J.M.; Friemel, A.; Jennewein, L.; Roth, S.; Ritter, A.; Schüttler, C.; Chen, Q.; Louwen, F.; Yuan, J.; Kreis, N.-N. RITA Is Expressed in Trophoblastic Cells and Is Involved in Differentiation Processes of the Placenta. Cells 2019, 8, 1484. https://doi.org/10.3390/cells8121484
Wildner JM, Friemel A, Jennewein L, Roth S, Ritter A, Schüttler C, Chen Q, Louwen F, Yuan J, Kreis N-N. RITA Is Expressed in Trophoblastic Cells and Is Involved in Differentiation Processes of the Placenta. Cells. 2019; 8(12):1484. https://doi.org/10.3390/cells8121484
Chicago/Turabian StyleWildner, Julia Maria, Alexandra Friemel, Lukas Jennewein, Susanne Roth, Andreas Ritter, Cornelia Schüttler, Qi Chen, Frank Louwen, Juping Yuan, and Nina-Naomi Kreis. 2019. "RITA Is Expressed in Trophoblastic Cells and Is Involved in Differentiation Processes of the Placenta" Cells 8, no. 12: 1484. https://doi.org/10.3390/cells8121484
APA StyleWildner, J. M., Friemel, A., Jennewein, L., Roth, S., Ritter, A., Schüttler, C., Chen, Q., Louwen, F., Yuan, J., & Kreis, N.-N. (2019). RITA Is Expressed in Trophoblastic Cells and Is Involved in Differentiation Processes of the Placenta. Cells, 8(12), 1484. https://doi.org/10.3390/cells8121484






