Oxidative Stress-Responsive Apoptosis Inducing Protein (ORAIP) Plays a Critical Role in High Glucose-Induced Apoptosis in Rat Cardiac Myocytes and Murine Pancreatic β-Cells
Abstract
:1. Introduction
2. Materials and Methods
3. Results
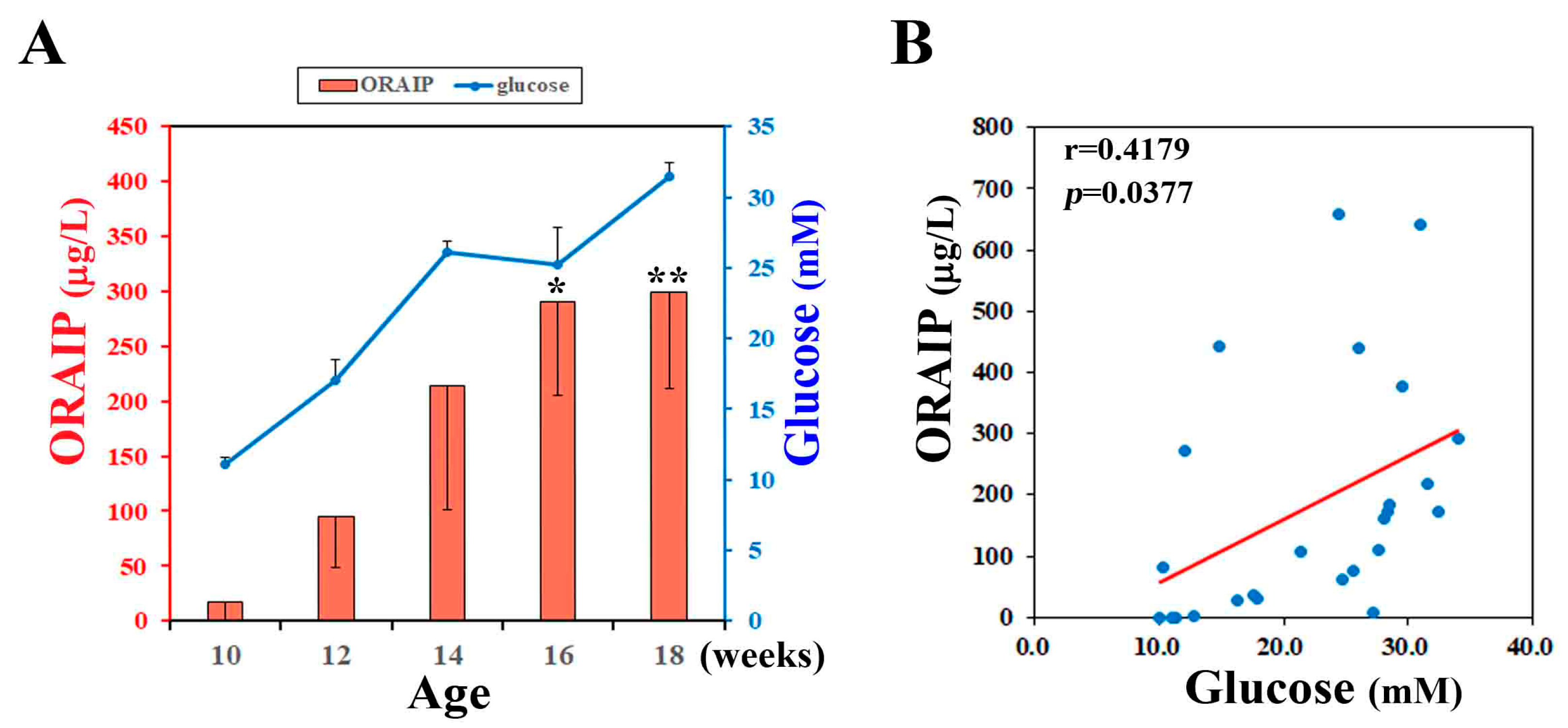
3.1. Hyperglycemia Markedly Increases Plasma ORAIP Levels
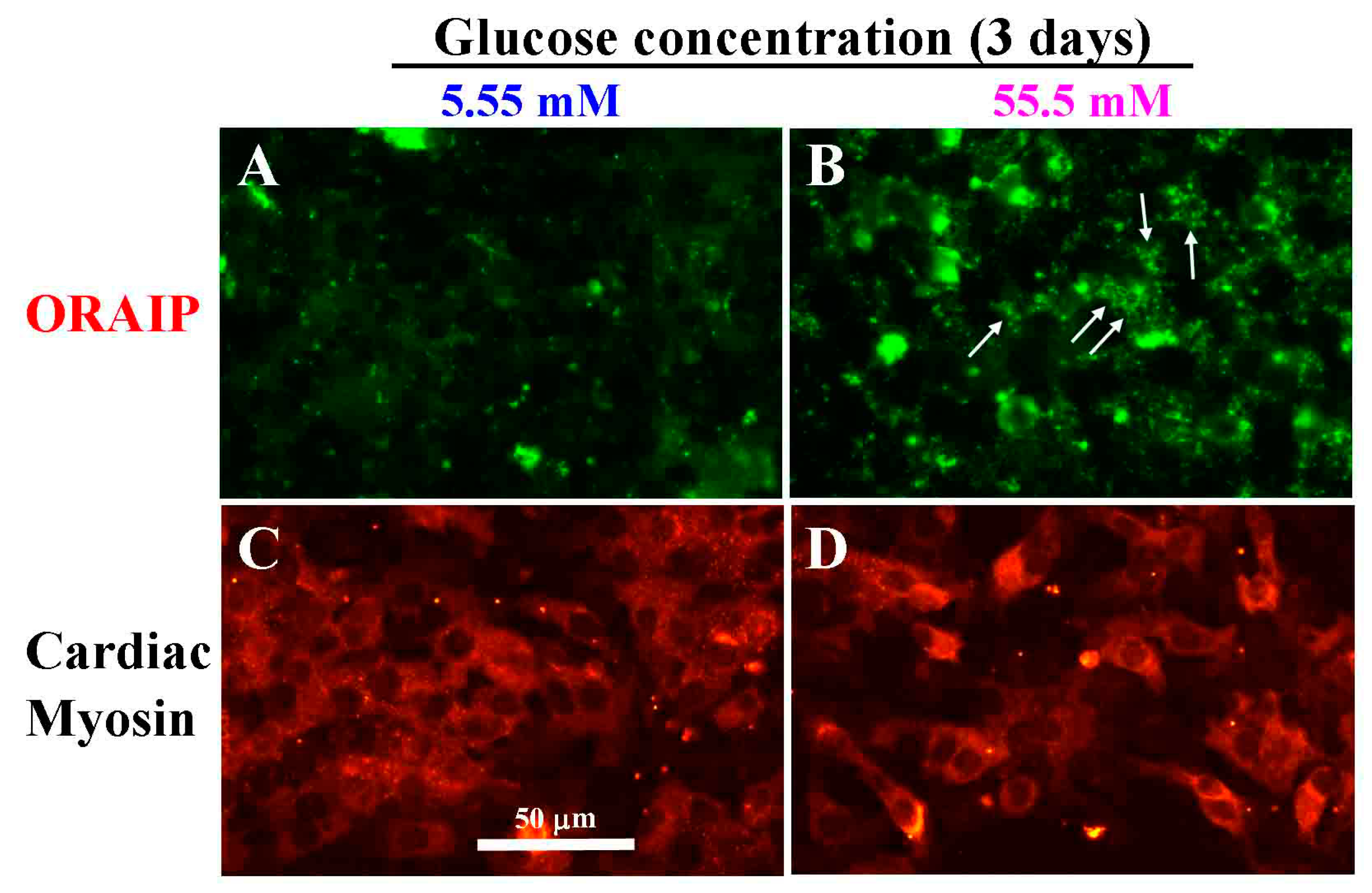
3.2. High Glucose Induces Expression of ORAIP in Cultured Cardiac Myocytes
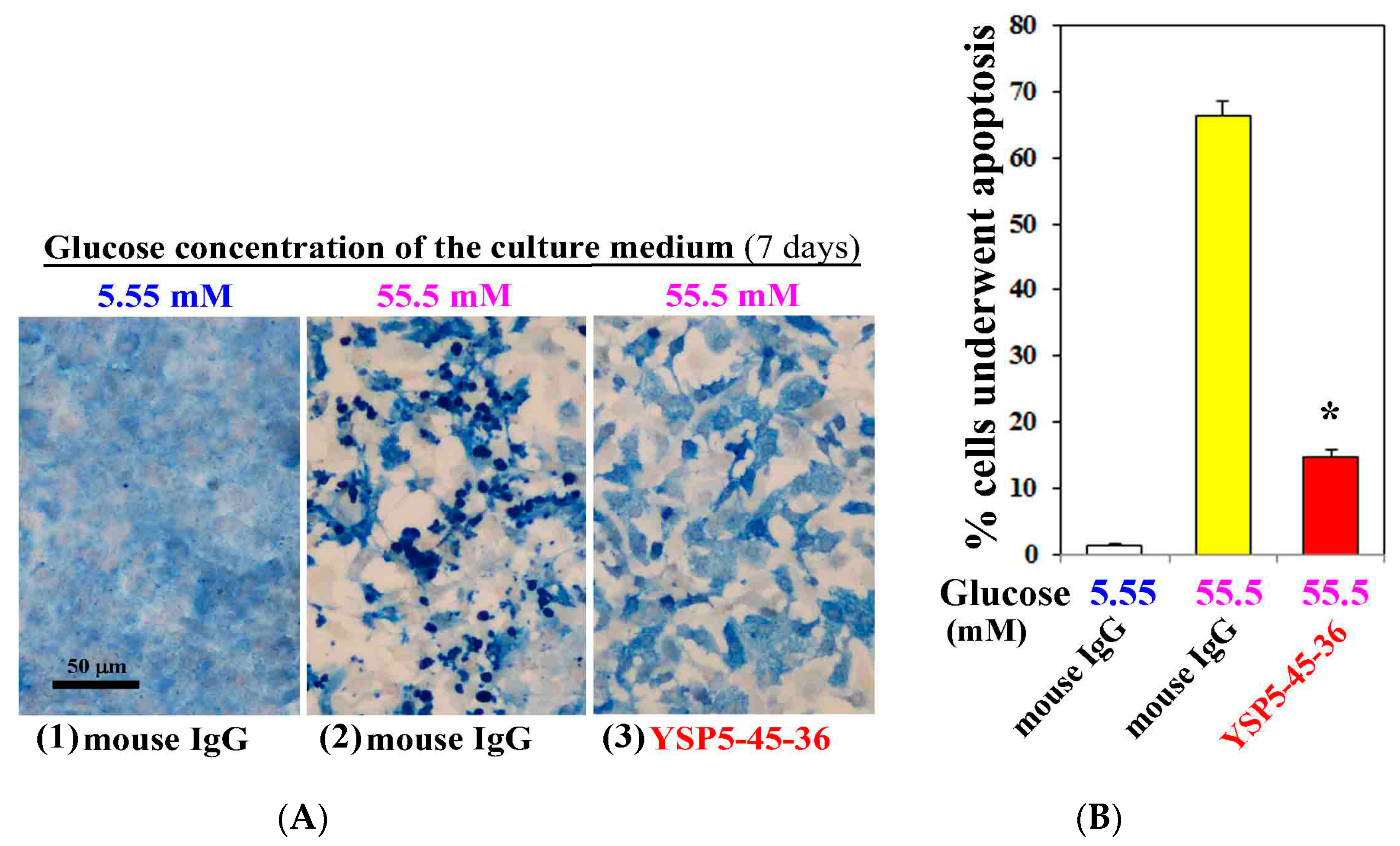
3.3. Anti-ORAIP mAb Mostly Suppressed High Glucose-Induced Apoptosis in Cultured Cardiac Myocytes
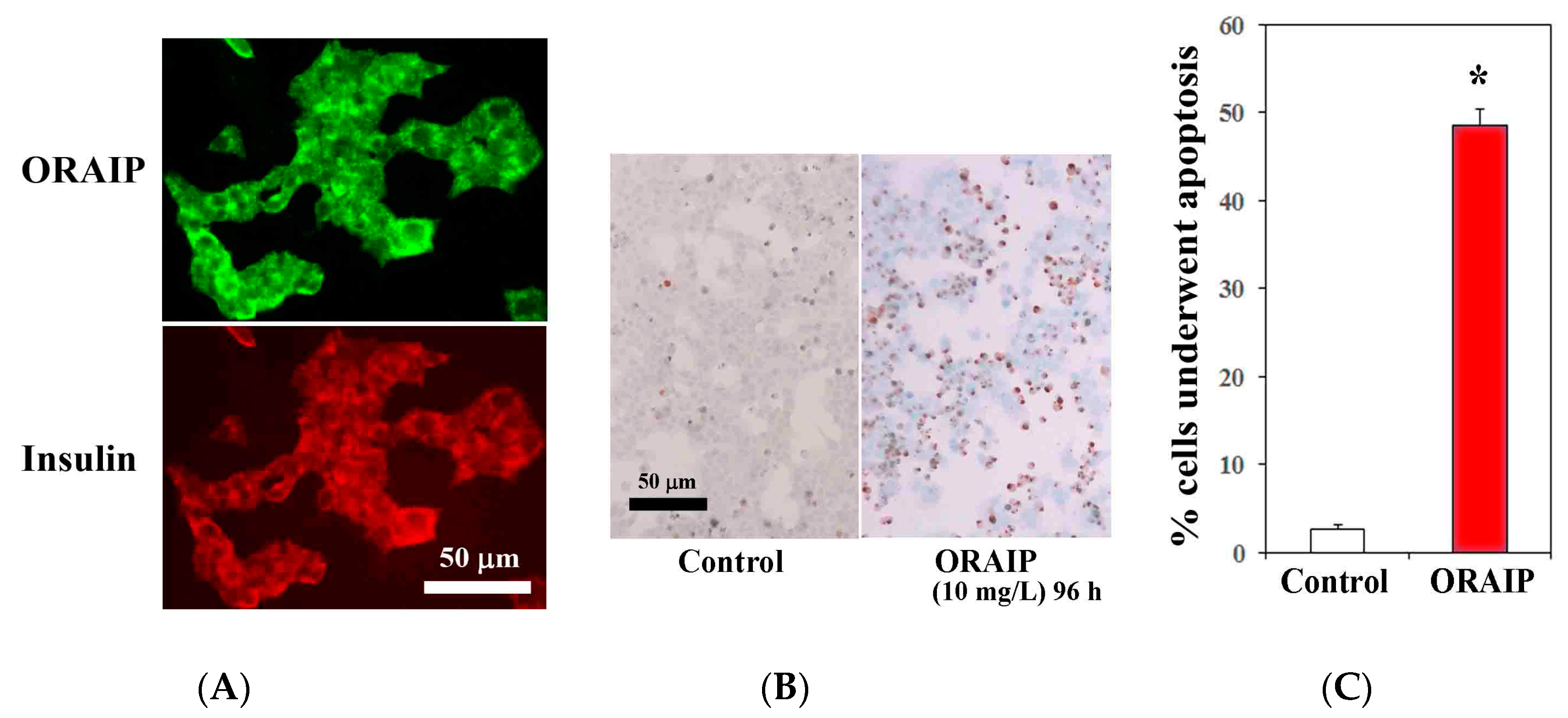
3.4. MIN6 Cells Strongly Express ORAIP and Recombinant-ORAIP Induces Apoptosis in MIN6 Cells In Vitro
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seko, Y.; Fujimura, T.; Yao, T.; Taka, H.; Mineki, R.; Okumura, K.; Murayama, K. Secreted tyrosine sulfated-eIF5A mediates oxidative stress-induced apoptosis. Sci. Rep. 2015, 5, 13737. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Fujimura, T.; Murayama, K.; Seko, Y. Plasma levels of oxidative stress-responsive apoptosis inducing protein (ORAIP) in rats subjected to various types of oxidative stress. Biosci. Rep. 2016, 36, e00317. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Yao, T.; Fujimura, T.; Murayama, K.; Fukuda, S.; Okumura, K.; Seko, Y. Marked elevation of plasma levels of oxidative stress-responsive apoptosis inducing protein in dialysis patients. Kidney Int. Rep. 2016, 1, 321–324. [Google Scholar] [CrossRef]
- Yao, T.; Tanaka, K.; Fujimura, T.; Murayama, K.; Fukuda, S.; Okumura, K.; Seko, Y. Plasma levels of oxidative stress-responsive apoptosis inducing protein (ORAIP) in patients with atrial fibrillation. Int. J. Cardiol. 2016, 222, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Gross, D.J.; Cerasi, E.; Kaiser, N. Hyperglycemia-induced β-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes 1999, 48, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T. Apoptosis in pancreatic β-islet cells in Type 2 diabetes. Bosn. J. Basic Med. Sci. 2016, 16, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Hoshino, M.; Hidaka, S.; Yoshida, E.; Beppu, M.; Hoshikawa, R.; Sudo, K.; Kawada, A.; Takagi, S.; Seino, S. A novel rat model of type 2 diabetes: The zucker fatty diabetes mellitus ZFDM rat. J. Diabetes Res. 2013, 2013, 103731. [Google Scholar] [CrossRef] [PubMed]
- Seko, Y.; Tobe, K.; Ueki, K.; Kadowaki, T.; Yazaki, Y. Hypoxia and hypoxia/reoxygenation activate Raf-1, mitogen-activated protein (MAP) kinase kinase, MAP kinases, and S6 kinase in cultured rat cardiac myocytes. Circ. Res. 1996, 78, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Araki, K.; Yamato, E.; Ikegami, H.; Asano, T.; Shibasaki, Y.; Oka, Y.; Yamamura, K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: Special reference to expression of glucose transporter isoforms. Endocrinology 1990, 127, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Seko, Y.; Takahashi, N.; Sabe, H.; Tobe, K.; Kadowaki, T.; Nagai, R. Hypoxia induces activation and subcellular translocation of focal adhesion kinase (p125FAK) in cultured rat cardiac myocytes. Biochem. Biophys. Res. Commun. 1999, 262, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, Y.; Tsuchimochi, H.; Kuro-o, M.; Kurabayashi, M.; Isobe, M.; Ueda, S.; Nagai, R.; Takaku, F. Distribution of myosin isozymes in human atrial and ventricular myocardium: Comparison in normal and overloaded heart. Eur. Heart J. 1984, 5, 103–110. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Ansley, D.M.; Wang, B. Oxidative stress and myocardial injury in the diabetic heart. J. Pathol. 2013, 229, 232–241. [Google Scholar] [CrossRef]
- Maiese, K.; Daniela Morhan, S.; Zhong Chong, Z. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr. Neurovasc. Res. 2007, 4, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, E.; Jha, J.C.; Sharma, A.; Wilkinson-Berka, J.L.; Jandeleit-Dahm, K.A.; de Haan, J.B. Are reactive oxygen species still the basis for diabetic complications? Clin. Sci. 2015, 129, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Dagenais, G.; Pogue, J.; Bosch, J.; Sleight, P. The heart outcomes prevention evaluation study investigators. Vitamin E supplementation and cardiovascular events in high-risk patients. N. Engl. J. Med. 2000, 342, 154–160. [Google Scholar] [PubMed]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Fortmann, S.P.; Burda, B.U.; Senger, C.A.; Lin, J.S.; Whitlock, E.P. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 159, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, K.; Fujui, J.; Seko, Y. Diabetes mellitus is important as a risk factor of atrial fibrillation. Integr. Mol. Med. 2014, 1, 73–75. [Google Scholar]
- Mandavia, C.H.; Aroor, A.R.; Demarco, V.G.; Sowers, J.R. Molecular and metabolic mechanisms of cardiac dysfunction in diabetes. Life Sci. 2013, 92, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Joe, M.K.; Lee, H.J.; Suh, Y.H.; Han, K.L.; Lim, J.H.; Song, J.; Seong, J.K.; Jung, M.H. Crucial roles of neuronatin in insulin secretion and high glucoseinduced apoptosis in pancreatic β-cells. Cell. Signal. 2008, 20, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Men, X.; Wang, H.; Li, M.; Cai, H.; Xu, S.; Zhang, W.; Xu, Y.; Ye, L.; Yang, W.; Wollheim, C.B.; et al. Dynamin-related protein 1 mediates high glucose induced pancreatic β cell apoptosis. Int. J. Biochem. Cell. Biol. 2009, 41, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Kimura, T.; Taniguchi, S.; Souma, M.; Kojima, Y.; Kimura, Y.; Kimura, H.; Niki, I. Glucose-induced production of hydrogen sulfide may protect the pancreatic beta-cells from apoptotic cell death by high glucose. FEBS. Lett. 2009, 583, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Prause, M.; Christensen, D.P.; Billestrup, N.; Mandrup-Poulsen, T. JNK1 protects against glucolipotoxicity-mediated β-cell apoptosis. PLoS ONE 2014, 9, e87067. [Google Scholar] [CrossRef] [PubMed]
- Broca, C.; Varin, E.; Armanet, M.; Tourrel-Cuzin, C.; Bosco, D.; Dalle, S.; Wojtusciszyn, A. Proteasome dysfunction mediates high glucose-induced apoptosis in rodent β cells and human islets. PLoS ONE 2014, 9, e92066. [Google Scholar]
- Maedler, K.; Spinas, G.A.; Lehmann, R.; Sergeev, P.; Weber, M.; Fontana, A.; Kaiser, N.; Donath, M.Y. Glucose induces β-cell apoptosis via upregulation of the Fas receptor in human islets. Diabetes 2001, 50, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Imagawa, A.; Hanafusa, T.; Waguri, M.; Yamamoto, K.; Iwahashi, H.; Moriwaki, M.; Nakajima, H.; Miyagawa, J.; Namba, M.; et al. Requirement of Fas for the development of autoimmune diabetes in nonobese diabetic mice. J. Exp. Med. 1997, 186, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Chervonsky, A.V.; Wang, Y.; Wong, F.S.; Visintin, I.; Flavell, R.A.; Janeway, C.A., Jr.; Matis, L.A. The role of Fas in autoimmune diabetes. Cell 1997, 89, 17–24. [Google Scholar] [CrossRef]
- Allison, J.; Strasser, A. Mechanisms of β cell death in diabetes: A minor role for CD95. Proc. Natl. Acad. Sci. USA 1998, 95, 13818–13822. [Google Scholar] [CrossRef] [PubMed]
- Maier, B.; Ogihara, T.; Trace, A.P.; Tersey, S.A.; Robbins, R.D.; Chakrabarti, S.K.; Nunemaker, C.S.; Stull, N.D.; Taylor, C.A.; Thompson, J.E.; et al. The unique hypusine modification of eIF5A promotes islet β cell inflammation and dysfunction in mice. J. Clin. Investig. 2010, 20, 2156–2170. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, T.; Fujimura, T.; Murayama, K.; Okumura, K.; Seko, Y. Oxidative Stress-Responsive Apoptosis Inducing Protein (ORAIP) Plays a Critical Role in High Glucose-Induced Apoptosis in Rat Cardiac Myocytes and Murine Pancreatic β-Cells. Cells 2017, 6, 35. https://doi.org/10.3390/cells6040035
Yao T, Fujimura T, Murayama K, Okumura K, Seko Y. Oxidative Stress-Responsive Apoptosis Inducing Protein (ORAIP) Plays a Critical Role in High Glucose-Induced Apoptosis in Rat Cardiac Myocytes and Murine Pancreatic β-Cells. Cells. 2017; 6(4):35. https://doi.org/10.3390/cells6040035
Chicago/Turabian StyleYao, Takako, Tsutomu Fujimura, Kimie Murayama, Ko Okumura, and Yoshinori Seko. 2017. "Oxidative Stress-Responsive Apoptosis Inducing Protein (ORAIP) Plays a Critical Role in High Glucose-Induced Apoptosis in Rat Cardiac Myocytes and Murine Pancreatic β-Cells" Cells 6, no. 4: 35. https://doi.org/10.3390/cells6040035
APA StyleYao, T., Fujimura, T., Murayama, K., Okumura, K., & Seko, Y. (2017). Oxidative Stress-Responsive Apoptosis Inducing Protein (ORAIP) Plays a Critical Role in High Glucose-Induced Apoptosis in Rat Cardiac Myocytes and Murine Pancreatic β-Cells. Cells, 6(4), 35. https://doi.org/10.3390/cells6040035



