Role of Intermediate Filaments in Vesicular Traffic
Abstract
:1. Introduction
2. Novel Roles of IFs: Involvement in Vesicular Trafficking
2.1. Endocytosis and IFs
2.2. Exocytosis and IFs
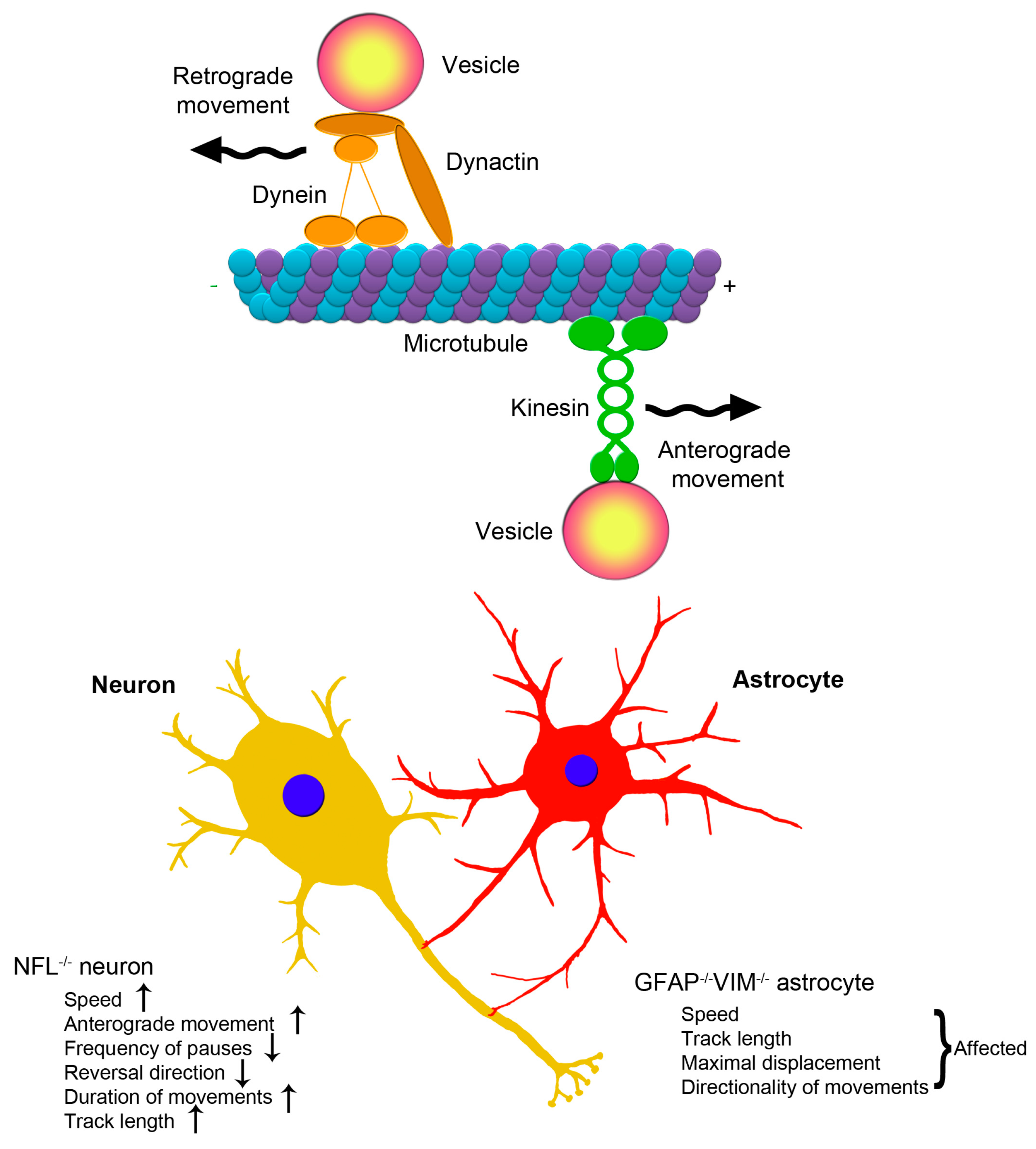
2.3. Clinical Aspects of the Association between Intermediate Filaments and Vesicular Trafficking in Neurons and Astrocytes
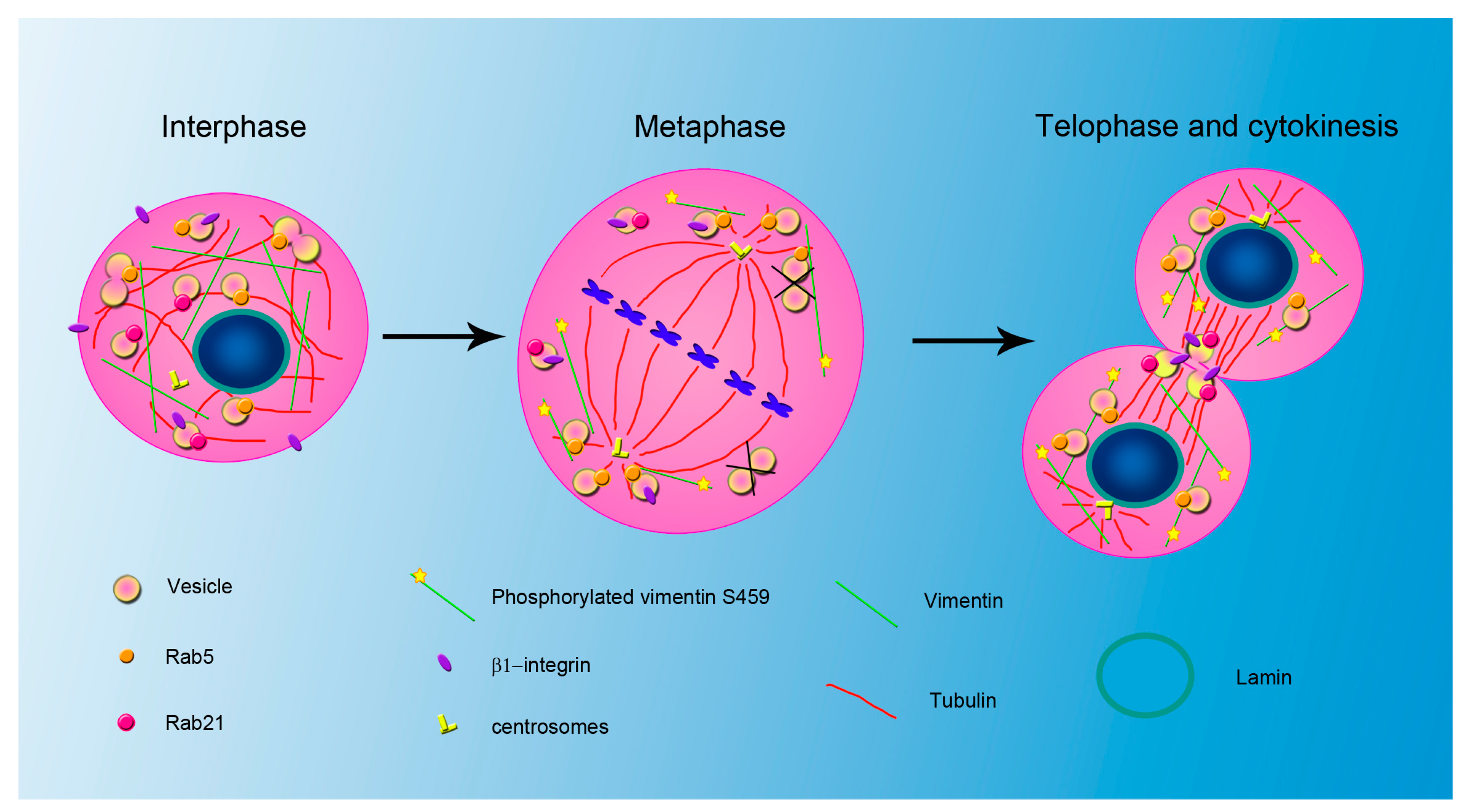
3. Relationship between IFs and Endocytosis during Mitosis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALS | amyotrophic lateral sclerosis |
| ANP | atrial natriuretic peptide |
| BLOC-1 | biogenesis of lysosome-related organelles complex 1 |
| Cdk1 | cyclin-dependent kinase 1 |
| CMT | Charcot-Marie-Tooth |
| CMT2B | Charcot-Marie-Tooth type 2B |
| CNS | central nervous system |
| DRG | dorsal root ganglion |
| ERK | extracellular-signal-regulated kinase |
| GCP6 | γ-tubulin complex protein 6 |
| GFAP | glial fibrillary acidic protein |
| IFs | intermediate filament proteins |
| LAMP-1 | Lysosomal-associated membrane protein 1 |
| LAMP-2 | Lysosomal-associated membrane protein 2 |
| MDC | Monodansylcadaverine |
| NF | Neurofilament |
| NF-H | neurofilament heavy |
| NF-L | neurofilament light |
| NF-M | neurofilament medium |
| NPC1 | Niemann-Pick type C1 |
| Plk1 | Polo-like kinase 1 |
| PtdIns3P | phosphatidylinositol-3-phosphate |
| SNARE | SNAP (Soluble NSF Attachment Protein) REceptor |
References
- Hesse, M.; Magin, T.M.; Weber, K. Genes for intermediate filament proteins and the draft sequence of the human genome: Novel keratin genes and a surprisingly high number of pseudogenes related to keratin genes 8 and 18. J. Cell Sci. 2001, 114, 2569–2575. [Google Scholar] [PubMed]
- Rogers, M.A.; Edler, L.; Winter, H.; Langbein, L.; Beckmann, I.; Schweizer, J. Characterization of new members of the human type II keratin gene family and a general evaluation of the keratin gene domain on chromosome 12q13.13. J. Investig. Dermatol. 2005, 124, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Winter, H.; Langbein, L.; Bleiler, R.; Schweizer, J. The human type I keratin gene family: Characterization of new hair follicle specific members and evaluation of the chromosome 17q21.2 gene domain. Differentiation 2004, 75, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Szeverenyi, I.; Cassidy, A.J.; Chung, C.W.; Lee, B.T.; Common, J.E.; Ogg, S.C.; Chen, H.; Sim, S.Y.; Goh, W.L.; Ng, K.W.; et al. The human intermediate filament database: Comprehensive information on a gene family involved in many human diseases. Hum. Mutat. 2008, 29, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Bischoff, R.; Holtzer, H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J. Cell Biol. 1968, 38, 538–555. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.D.; Follett, E.A. The structure of the major cell processes of isolated BHK21 fibroblasts. Exp. Cell. Res. 1969, 57, 263–276. [Google Scholar] [CrossRef]
- Goldman, R.D.; Follett, E.A. Birefringent filamentous organelle in BHK-21 cells and its possible role in cell spreading and motility. Science 1970, 169, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Franke, W.W.; Schmid, E.; Osborn, M.; Weber, K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc. Natl. Acad. Sci. USA 1978, 75, 5034–5038. [Google Scholar] [CrossRef] [PubMed]
- Geisler, N.; Weber, K. Comparison of the proteins of two immunologically distinct intermediate-sized filaments by amino acid sequence analysis: Desmin and vimentin. Proc. Natl. Acad. Sci. USA 1981, 78, 4120–4123. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Weber, K. Intermediate filaments: Structure, dynamics, function, and disease. Annu. Rev. Biochem. 1994, 63, 345–382. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Strelkov, S.V.; Burkhard, P.; Aebi, U. Intermediate filaments: Primary determinants of cell architecture and plasticity. J. Clin. Investig. 2009, 119, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.A.; Strelkov, S.V.; Burkard, P.; Aebi, U.; Herrmann, H. Towards a molecular description of intermediate filament structure and assembly. Exp. Cell Res. 2007, 313, 2204–2216. [Google Scholar] [CrossRef] [PubMed]
- Geisler, N.; Weber, K. The amino acid sequence of chicken muscle desmin provides a common structural model for intermediate filaments proteins. EMBO J. 1982, 1, 1649–1656. [Google Scholar] [PubMed]
- Quax-Jeuken, Y.E.; Quax, W.J.; Bloemendal, H. Primary and secondary structure of hamster vimentin predicted from the nucleotide sequence. Proc. Natl. Acad. Sci. USA 1983, 80, 3548–3552. [Google Scholar] [CrossRef] [PubMed]
- Strelkov, S.V.; Hermann, H.; Aebi, U. Molecular architecture of intermediate filaments. Bioessays 2003, 25, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Izawa, I.; Inagaki, M. Regulatory mechanisms and functions of intermediate filaments: A study using site- and phosphorylation state-specific antibodies. Cancer Sci. 2006, 97, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Kornreich, M.; Avinery, R.; Malka-Gibor, E.; Laser-Azogui, A.; Beck, R. Order and disorder in intermediate filament proteins. FEBS Lett. 2015, 589, 2464–2476. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.K.; Khuon, S.; Goldman, R.D. Dynamics of keratin assembly: Exogenous type I keratin rapidly associates with type II keratin in vivo. J. Cell Biol. 1993, 122, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, R.A.; Franke, W.W. Heteropolymer filaments of vimentin and desmin in vascular smooth muscle tissue and cultured Baby Hamster Kidney cells demonstrated by chemical crosslinking. Proc. Natl. Acad. Sci. USA 1982, 79, 3452–3456. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, R.A.; Franke, W.W. Molecular interactions in intermediate-sized filaments revealed by chemical cross-linking. Heteropolymers of vimentin and glial filament protein in cultured human glioma cells. Eur. J. Biochem. 1983, 132, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.J.; Cleveland, D.W. Expression of NF-L and NF-M in fibroblasts reveals coassembly of neurofilament and vimentin subunits. J. Cell Biol. 1989, 108, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Lilienbaum, A.; Legagneux, V.; Portier, M.M.; Dellagi, K.; Paulin, D. Vimentin gene: Expression in human lymphocytes and in Burkitt’s lymphoma cells. EMBO J. 1986, 5, 2809–2814. [Google Scholar] [PubMed]
- Franke, W.W.; Schmid, E.; Osborn, M.; Weber, K. Intermediate-sized filaments of human endothelial cells. J. Cell Biol. 1979, 81, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Schmid, E.; Tapscott, S.; Bennett, G.S.; Croop, J.; Fellini, S.A.; Holtzer, H.; Franke, W.W. Differential location of different types of intermediate-sized filaments in various tissues of the chicken embryo. Differentiation 1979, 15, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Shea, T.B.; Beermann, M.L.; Fischer, I. Transient requirement for vimentin in neuritogenesis: Intracellular delivery of anti-vimentin antibodies and antisense oligonucleotides inhibit neurite initiation but not elongation of existing neurites in neuroblastoma. J. Neurosci. Res. 1993, 36, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Cochard, P.; Paulin, D. Initial expression of neurofilaments and vimentin in the central and peripheral nervous system of the mouse embryo in vivo. J. Neurosci. 1984, 4, 2080–2094. [Google Scholar] [PubMed]
- Lazarides, E. Intermediate filaments: A chemically heterogeneous, developmentally regulated class of proteins. Annu. Rev. Biochem. 1982, 51, 219–250. [Google Scholar] [CrossRef] [PubMed]
- Chiu, F.C.; Norton, W.T.; Fields, K.L. The cytoskeleton of primary astrocytes in culture contains actin, glial fibrillary acidic protein, and the fibroblast-type filament protein, vimentin. J. Neurochem. 1981, 37, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature 1980, 286, 736–737. [Google Scholar] [CrossRef] [PubMed]
- Portier, M.M.; de Néchaud, B.; Gros, F. Peripherin, a new member of the intermediate filament protein family. Dev. Neurosci. 1984, 6, 335–344. [Google Scholar] [CrossRef]
- Portier, M.M.; Escurat, M.; Landon, F.; Djabali, K.; Bousquet, O. Peripherin and neurofilaments: Expression and role during neural development. C. R. Acad. Sci. III 1993, 316, 1124–1140. [Google Scholar] [PubMed]
- Errante, L.; Tang, D.; Gardon, M.; Sekerkova, G.; Mugnaini, E.; Shaw, G. The intermediate filament protein peripherin is a marker for cerebellar climbing fibres. J. Neurocytol. 1998, 27, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Pannese, E. Detection of neurofilaments in the perikaryon of hypertrophic nerve cells. J. Cell Biol. 1962, 13, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Toivola, D.M.; Boor, P.; Alam, C.; Strnad, P. Keratins in health and disease. Curr. Opin. Cell Biol. 2015, 32, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Norgren, N.; Rosengren, L.; Stigbrand, T. Elevated neurofilament levels in neurological diseases. Brain Res. 2003, 987, 25–31. [Google Scholar] [CrossRef]
- Gaiottino, J.; Norgren, N.; Dobson, R.; Topping, J.; Nissim, A.; Malaspina, A.; Bestwick, J.P.; Monsch, A.U.; Regeniter, A.; Lindberg, R.L.; et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE 2013, 8, e75091. [Google Scholar]
- Hol, E.M.; Pekny, M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; McLean, J.; Robertson, J. Neuronal intermediate filaments and als: A new look at an old question. Biochim. Biophys. Acta 2006, 1762, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Stamenovic, D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am. J. Physiol. 2000, 279, C188–C194. [Google Scholar]
- Haudenschild, D.; Chen, J.; Pang, N.; Steklov, N.; Grogan, S.P.; Lotz, M.K.; D’Lima, D.D. Vimentin contributes to changes in chondrocyte stiffness in osteoarthritis. J. Orthop. Res. 2011, 29, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Ehrlicher, A.J.; Mahammad, S.; Fabich, H.; Jensen, M.H.; Moore, J.R.; Fredberg, J.J.; Goldman, R.D.; Weitz, D.A. The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys. J. 2013, 105, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.G.; Restle, D.; Janmey, P.A. Vimentin enhances cell elastic behaviour and protects against compressive stress. Biophys. J. 2014, 107, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Bordeleau, F.; Myrand Lapierre, M.-E.; Sheng, Y.; Marceau, N. Keratin 8/18 regulation of cell stiffness-extracellular matrix interplay through modulation of rho-mediated actin cytoskeleton dynamics. PLoS ONE 2012, 6, e38780. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, C.J. Lamins: Building blocks or regulators of gene expression? Nat. Rev. Mol. Cell Biol. 2002, 3, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Bloom, S.; Lockard, V.G.; Bloom, M. Intermediate filament-mediated stretch-induced changes in chromatin: A hypothesis for growth initiation in cardiac myocytes. J. Mol. Cell. Cardiol. 1996, 28, 2123–2127. [Google Scholar] [CrossRef] [PubMed]
- Tolstonog, G.V.; Sabasch, M.; Traub, P. Cytoplasmatic intermediate filaments are stably associated with nuclear matrices and potentially modulate their DNA-binding function. DNA Cell Biol. 2002, 21, 213–239. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, E.; Allen, T.D.; Rutherford, S.; Bucci, M.; Wente, S.R.; Goldberg, M.W. Yeast nuclear pore complexes have a cytoplasmic ring and internal filaments. J. Struct. Biol. 2004, 145, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Stoffler, D.; Feja, B.; Fahrenkrog, B.; Walz, J.; Typke, D.; Aebi, U. Cryo-electron tomography provides novel insights into nuclear pore architecture: Implications for nucleocytoplasmic transport. J. Mol. Biol. 2003, 328, 243–251. [Google Scholar] [CrossRef]
- Ellis, D.J.; Jenkins, H.; Whitfield, W.G.; Hutchinson, C.J. GST-lamin fusion proteins act as dominant negative mutants in xenopus egg extract and reveal the function of the lamina in DNA replication. J. Cell Sci. 1997, 110, 2507–2518. [Google Scholar] [PubMed]
- Spann, T.P.; Moir, R.D.; Goldman, A.E.; Stick, R.; Goldman, R.D. Disruption of nuclear lamin organization alters the distribution of replication factors and inhibits DNA synthesis. J. Cell Biol. 1997, 136, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Solovei, I.; Wang, A.S.; Thanisch, K.; Schmidt, C.S.; Krebs, S.; Zwerger, M.; Cohen, T.V.; Devys, D.; Foisner, R.; Peichl, L.; et al. LBR and lamina A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 2013, 152, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Parnaik, V.K. Lamin A rod domain mutants target heterochromatin protein 1alpha and beta for proteasomal degradation by activation of F-box protein, fbxw10. PLoS ONE 2010, 5, e10620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Schones, D.E.; Malicet, C.; Rochman, M.; Zhou, M.; Foisner, R.; Bustin, M. High mobility group protein N5 (HMGN5) and lamina-associated polypeptide 2α (LAP2α) interact and reciprocally affect their genome-wide chromatin organization. J. Biol. Chem. 2013, 288, 18104–18109. [Google Scholar] [CrossRef] [PubMed]
- Rochman, M.; Malicet, C.; Bustin, M. Hmgn5/nsbp1: A new member of the HMGN protein family that affects chromatin structure and function. Biochim. Biophys. Acta 2010, 1799, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Montes de Oca, R.; Andreassen, P.R.; Wilson, K.L. Barrier-to-autointegration factor influences specific histone modifications. Nucleus 2011, 2, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Spann, T.P.; Goldman, A.E.; Wang, C.; Huang, S.; Goldman, R.D. Alteration of nuclear lamin organization inhibits rna polymerase ii-dependent transcription. J. Cell Biol. 2002, 156, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Manjiu, K.; Muralikrishna, B.; Parnaik, V.K. Expression of disease-causing lamin A mutants impairs the formation of DNA repair foci. J. Cell Sci. 2006, 199, 2704–2714. [Google Scholar] [CrossRef] [PubMed]
- Gibbs-Seymour, I.; Markiewicz, E.; Bekker-Jensen, S.; Mailand, N.; Hutchison, C.J. Lamin A/C-dependent interaction with 53BP1 promotes cellular responses to DNA damage. Aging Cell 2015, 14, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Liu, B.; Wang, Y.; Hao, Q.; Zhou, Z. Lamin a is an endogenous SIRT6 activator and promotes SIRT6-mediated DNA repair. Cell Rep. 2015, 13, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Suarez, I.; Redwood, A.B.; Perkins, S.M.; Vermolen, B.; Lichtensztejin, D.; Grotsky, D.A.; Morgado-Palacin, L.; Gapud, E.J.; Sleckman, B.P.; Sullivan, T.; et al. Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. EMBO J. 2009, 28, 2414–2427. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, J.; Gerlich, D.; Daigle, N.; Eils, R.; Ellenberg, J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell 2002, 108, 83–96. [Google Scholar] [CrossRef]
- Ward, G.E.; Kirschner, M.W. Identification of cell-cycle regulated phosphorylation sites on nuclear lamin C. Cell 1990, 61, 561–577. [Google Scholar] [CrossRef]
- Peter, M.; Nakagawa, J.; Dorée, M.; Labbé, J.C.; Nigg, E.A. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell 1990, 61, 591–602. [Google Scholar] [CrossRef]
- Kochin, V.; Shimi, T.; Torvaldson, E.; Adam, S.A.; Goldman, A.; Pack, C.G.; Melo-Cardenas, J.; Imanishi, S.Y.; Goldman, R.D.; Eriksson, J.E. Interphase phosphorylation of lamin A. J. Cell Sci. 2014, 127, 2683–2696. [Google Scholar] [CrossRef] [PubMed]
- Coffinier, C.; Jung, H.J.; Nobumori, C.; Chang, S.; Tu, Y.; Barnes, R.H.N.; Yoshinaga, Y.; de Jong, P.J.; Vergnes, L.; Reue, K.; et al. Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol. Biol. Cell 2011, 22, 4683–4693. [Google Scholar] [CrossRef] [PubMed]
- Sarria, A.J.; Lieber, J.G.; Nordeen, S.K.; Evans, R.M. The presence or absence of a vimentin-type intermediate filament network affects the shape of the nucleus in human SW-13 cells. J. Cell Sci. 1994, 107, 1593–1607. [Google Scholar] [PubMed]
- Oriolo, A.S.; Wald, F.A.; Canessa, G.; Salas, P.J. GCP6 binds to intermediate filaments: A novel function of keratins in the organization of microtubules in epithelial cells. Mol. Biol. Cell 2006, 18, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Conover, G.M.; Gregorio, C.C. The desmin coil 1b mutation K190A impairs nebulin Z-disc assembly and destabilizes actin thin filaments. J. Cell Sci. 2011, 124, 3464–3476. [Google Scholar] [CrossRef] [PubMed]
- Cary, R.B.; Klymkowsky, M.W.; Evans, R.M.; Domingo, A.; Dent, J.A.; Backhus, L.E. Vimentin’s tail interacts with actin-containing structures in vivo. J. Cell Sci. 1994, 107, 1609–1622. [Google Scholar] [PubMed]
- Esue, O.; Carson, A.A.; Tseng, Y.; Wirtz, D. A direct interaction between actin and vimentin filaments mediated by the tail domain of vimentin. J. Biol. Chem. 2006, 281, 30393–30399. [Google Scholar] [CrossRef] [PubMed]
- Virtakoivu, R.; Mai, A.; Mattila, E.; De Franceschi, N.; Imanishi, S.Y.; Corthals, G.; Kaukonen, R.; Saari, M.; Cheng, F.; Torvaldson, E.; et al. Vimentin-ERK signaling uncouples Slug gene regulatory function. Cancer Res. 2015, 75, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Perlson, E.; Hanz, S.; Ben-Yaakov, K.; Segal-Ruder, Y.; Seger, R.; Fainzilber, M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron 2005, 45, 715–726. [Google Scholar] [PubMed]
- Perlson, E.; Michaelevski, I.; Kowalsman, N.; Ben-Yaakov, K.; Shaked, M.; Seger, R.; Eisenstein, M.; Fainzilber, M. Vimentin binding to phosphorylated ERK sterically hinders enzymatic dephosphorylation of the kinase. J. Mol. Biol. 2006, 364, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Robidoux, J.; Daniel, K.W.; Guzman, G.; Floering, L.M.; Collins, S. Requirement of vimentin filament assembly for beta3-adrenergic receptor activation of ERK MAP kinase and lipolysis. J. Biol. Chem. 2007, 282, 9244–9250. [Google Scholar] [CrossRef] [PubMed]
- Toivola, D.M.; Nieminen, M.I.; Hesse, M.; He, T.; Baribault, H.; Magin, T.M.; Omary, M.B.; Eriksson, J.E. Disturbances in hepatic cell-cycle regulation in mice with assembly-deficient keratins 8/18. Hepatology 2001, 34, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Caulin, C.; Ware, C.F.; Magin, T.M.; Oshima, R.G. Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis. J. Cell Biol. 2000, 149, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Perez, D.; White, E. Lamin proteolysis facilitates nuclear events during apoptosis. J. Cell Biol. 1996, 135, 1441–1455. [Google Scholar] [CrossRef] [PubMed]
- Gilles, C.; Polette, M.; Zahm, J.M.; Turnier, J.M.; Volders, L.; Foidart, J.M.; Birembaut, P. Vimentin contributes to human mammary epithelial cell migration. J. Cell Sci. 1999, 112, 4615–4625. [Google Scholar] [PubMed]
- Sutoh Yoneyama, M.; Hatakeyama, S.; Habuchi, T.; Inoue, T.; Nakamura, T.; Funyu, T.; Wiche, G.; Ohyama, C.; Tsuboi, S. Vimentin intermediate filament and plectin provide a scaffold for invadopodia, facilitating cancer cell invasion and extravasation for metastasis. Eur. J. Cell Biol. 2014, 93, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.H.; Yang, W.; Lee, K.M.; Oh, S.; Nam, K.; Shim, S.; Shin, S.Y.; Gye, M.C.; Chu, I.S.; Shin, I. Regulation of cell proliferation and migration by keratin 19-induced nuclear import of early growth response-1 in breast cancer cells. Clin. Cancer Res. 2013, 19, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- Fortier, A.M.; Asselin, E.; Cadrin, M. Keratin 8 and 18 loss in epithelial cancer cells increases collective cell migration and cisplatin sensitivity through claudin1 up-regulation. J. Biol. Chem. 2013, 288, 11555–11571. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Matsuda, Y.; Seike, M.; Naito, Z.; Gemma, A.; Ishiwata, T. Nestin regulates proliferation, migration, invasion and stemness of lung adenocarcinoma. Int. J. Oncol. 2014, 44, 1118–1130. [Google Scholar] [PubMed]
- Jahn, R. Principles of exocytosis and membrane fusion. Ann. N. Y. Acad. Sci. 2004, 1014, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.A. The endosomal network. Int. J. Clin. Pharmacol. Ther. 2009, 47, S9–S17. [Google Scholar] [CrossRef] [PubMed]
- Bhuin, T.; Roy, J.K. Rab proteins: The key regulators of intracellular vesicle transport. Exp. Cell Res. 2014, 328, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bucci, C.; Parton, R.G.; Mather, I.H.; Stunnenberg, H.; Simons, K.; Hoflack, B.; Zerial, M. The small GTPase Rab5 functions as a regulatory factor in the early endocytic pathway. Cell 1992, 70, 715–728. [Google Scholar] [CrossRef]
- Nielsen, E.; Severin, F.; Backer, J.M.; Hyman, A.A.; Zerial, M. Rab5 regulates motility of early endosomes on microtubules. Nat. Cell Biol. 1999, 1, 376–382. [Google Scholar] [PubMed]
- Zeigerer, A.; Gilleron, J.; Bogorad, R.L.; Marsico, G.; Nonaka, H.; Seifert, S.; Epstein-Barash, H.; Kuchimanchi, S.; Peng, C.G.; Ruda, V.M.; et al. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature 2012, 485, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Gorvel, J.P.; Chavrier, P.; Zerial, M.; Gruenberg, J. Rab5 controls early endosome fusion in vitro. Cell 1991, 64, 915–925. [Google Scholar] [CrossRef]
- McCaffrey, M.W.; Bielli, A.; Cantalupo, G.; Mora, S.; Roberti, V.; Santillo, M.; Drummond, F.; Bucci, C. Rab4 affects both recycling and degradative endosomal trafficking. FEBS Lett. 2001, 495, 21–30. [Google Scholar] [CrossRef]
- Ullrich, O.; Reinsch, S.; Urbé, S.; Zerial, M.; Parton, R.G. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996, 135, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.E.; Wang, X.; Kumar, R.; Bhartur, S.G.; Navarre, J.; Woodrum, J.E.; Altschuler, Y.; Ray, G.S.; Goldenring, J.R. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 1999, 10, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Blankson, H.; Holen, I.; Seglen, P.O. Disruption of the cytokeratin cytoskeleton and inhibition of hepatocytic autophagy by okadaic acid. Exp. Cell Res. 1995, 218, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Ameen, N.A.; Figueroa, Y.; Salas, P.J. Anomalous apical plasma membrane phenotype in CK8-deficient mice indicates a novel role for intermediate filaments in the polarization of simple epithelia. J. Cell Sci. 2001, 114, 563–575. [Google Scholar] [PubMed]
- Cogli, L.; Progida, C.; Bramato, R.; Bucci, C. Vimentin phosphorylation and assembly are regulated by the small GTPase Rb7a. Biochim. Biophys. Acta 2013, 1833, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, U.; Faiz, M.; de Pablo, Y.; Sjöqvist, M.; Andersson, D.; Widestrand, A.; Potokar, M.; Stenovec, M.; Smith, P.L.; Shinjyo, N.; et al. Astrocytes negatively regulate neurogenesis through the Jagged1-mediated Notch pathway. Stem Cells 2012, 30, 2320–2329. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, K.; Satou, A.; Fukuhara, M.; Matsumura, S.; Sugiyama, N.; Goto, H.; Fukuda, M.; Inagaki, M.; Ishihama, Y.; Toyoshima, F. Inhibition of endocytic vesicle fusion by Plk1-mediated phosphorylation of vimentin during mitosis. Cell Cycle 2014, 13, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Styers, M.L.; Salazar, G.; Love, R.; Peden, A.A.; Kowalczyk, A.P.; Faundez, V. The endo-lysosomal sorting machinery interacts with the intermediate filament cytoskeleton. Mol. Biol. Cell 2004, 15, 5369–5382. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Chen, F.W.; Tamari, F.; Wang, R.; Ioannou, Y.A. Endosomal lipid accumulation in NPC1 leads to inhibition of PKC, hypophosphorylation of vimentin and Rab9 entrapment. Biol. Cell 2009, 101, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Kurzchalia, T.V.; Gorvel, J.P.; Dupree, P.; Parton, R.; Kellner, R.; Houthaeve, T.; Gruenberg, J.; Simons, K. Interactions of Rab5 with cytosolic proteins. J. Biol. Chem. 1992, 267, 18419–18423. [Google Scholar] [PubMed]
- Potokar, M.; Kreft, M.; Li, L.; Daniel Andersson, J.; Pangrsic, T.; Chowdhury, H.H.; Pekny, M.; Zorec, R. Cytoskeleton and vesicle mobility in astrocytes. Traffic 2007, 8, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Potokar, M.; Stenovec, M.; Gabrijel, M.; Li, L.; Kreft, M.; Grilc, S.; Pekny, M.; Zorec, R. Intermediate filaments attenuate stimulation-dependent mobility of endosomes/lysosomes in astrocytes. Glia 2010, 58, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Vardjan, N.; Gabrijel, M.; Potokar, M.; Svajger, U.; Kreft, M.; Jeras, M.; de Pablo, Y.; Faiz, M.; Pekny, M.; Zorec, R. IFN-γ-induced increase in the mobility of MHC class II compartments in astrocytes depends on intermediate filaments. J. Neuroinflamm. 2012, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, J.; Vuoriluoto, K.; Huovinen, T.; Izawa, I.; Inagaki, M.; Parker, P.J. PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J. 2005, 24, 3834–3845. [Google Scholar] [CrossRef] [PubMed]
- Faigle, W.; Colucci-Guyon, E.; Louvard, D.; Amigorena, S.; Galli, T. Vimentin filaments in fibroblasts are a reservoir for SNAP23, a component of the membrane fusion machinery. Mol. Biol. Cell 2000, 11, 3485–3494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mruk, D.D.; Lau, A.S.; Sarkar, O.; Xia, W. Rab4a GTPase catenin interactions are involved in cell junction dynamics in the testis. J. Androl. 2007, 28, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Gillard, B.K.; Clement, R.; Colucci-Guvon, E.; Babinet, C.; Schwarzmann, G.; Taki, T.; Kasama, T.; Marcus, D.M. Decreased synthesis of glycosphingolipids in cells lacking vimentin intermediate filaments. Exp. Cell Res. 1998, 242, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Cogli, L.; Progida, C.; Thomas, C.L.; Spencer-Dene, B.; Donno, C.; Schiavo, G.; Bucci, C. Charcot-Marie-Tooth type 2b disease-causing Rab7a mutant proteins show altered interaction with the neuronal intermediate filament peripherin. Acta Neuropathol. 2013, 25, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Perrot, R.; Julien, J.P. Real-time imaging reveals defects of fast axonal transport induced by disorganization of intermediate filaments. FASEB J. 2009, 23, 3213–3225. [Google Scholar] [CrossRef] [PubMed]
- Gentil, B.J.; McLean, J.R.; Xiao, S.; Zhao, B.; Durham, H.D.; Robertson, J. A two-hybrid screen identifies an unconventional role for the intermediate filament peripherin in regulating the subcellular distribution of the SNAP25-interacting protein, SIP30. J. Neurochem. 2014, 131, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Kouloumenta, A.; Mayroidis, M.; Capetanaki, Y. Proper perinuclear localization of the TRIM-like protein myospryn requires its binding partner desmin. J. Biol. Chem. 2007, 282, 35211–35221. [Google Scholar] [CrossRef] [PubMed]
- Capalbo, L.; D’Avino, P.P.; Archambault, V.; Glover, D.M. Rab5 GTPase controls chromosome alignment through Lamin disassembly and relocation of the NuMA-like protein Mud to the poles during mitosis. Proc. Natl. Acad. Sci. USA 2011, 108, 17343–17348. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, J.; Whelan, R.D.; Watson, R.; Parker, P.J. PKC epsilon controls the traffic of beta1 integrins in motile cells. EMBO J. 2002, 21, 3608–3619. [Google Scholar] [CrossRef] [PubMed]
- Bucci, C.; Frunzio, R.; Chiariotti, L.; Brown, A.L.; Rechler, M.M.; Bruni, C.B. A new member of the ras gene superfamily identified in a rat liver cell line. Nucleic Acids Res. 1988, 16, 9979–9993. [Google Scholar] [CrossRef] [PubMed]
- Bucci, C.; Thomsen, P.; Nicoziani, P.; McCarthy, J.; van Deurs, B. Rab7: A key to lysosome biogenesis. Mol. Biol. Cell 2000, 11, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.; Bucci, C.; Vieira, O.; Schroer, T.; Grinstein, S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: Role of Rab7 and RILP. Mol. Cell. Biol. 2003, 23, 6494–6506. [Google Scholar] [CrossRef] [PubMed]
- Jager, S.; Bucci, C.; Tanida, I.; Ueno, T.; Kominami, E.; Saftig, P.; Eskelinen, E.L. Role for Rab7 in maturation of late autophagic vacuoles. J. Cell Sci. 2004, 117, 4837–4848. [Google Scholar] [CrossRef] [PubMed]
- Riederer, M.A.; Soldati, T.; Shapiro, A.D.; Lin, J.; Pfeffer, S.R. Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-golgi network. J. Cell Biol. 1994, 125, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Ganley, I.G.; Carroll, K.; Bittova, L.; Pfeffer, S. Rab9 GTPase regulates late endosome size and requires effector interaction for its stability. Mol. Biol. Cell 2004, 15, 5420–5430. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.E.; Dell’Angelica, E.C.; Bonifacino, J.S. ADP-Ribosylation Factor 1 (ARF1) regulates recrutiment of the AP-3 adaptor complex to membranes. J. Cell Biol. 1998, 142, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Zhu, Y.; Kornfeld, S. The assembly of AP-3 adaptor complex-containing clathrin-coated vesicles on synthetic liposomes. Mol. Biol. Cell 2000, 11, 3723–3736. [Google Scholar] [CrossRef] [PubMed]
- Styers, M.L.; Kowalczyk, A.P.; Faundez, V. Architecture of the vimentin cytoskeleton is modified by perturbation of the GTPase ARF1. J. Cell Sci. 2006, 119, 3643–3654. [Google Scholar] [CrossRef] [PubMed]
- Mihai, C.; Chrisler, W.B.; Xie, Y.; Hu, D.; Szymanski, C.J.; Tolic, A.; Klein, J.A.; Smith, J.N.; Tarasevich, B.J.; Orr, G. Intracellular accumulation dynamics and fate of zinc ions in alveolar epithelial cells exposed to airborne ZNO nanoparticles at the air-liquid interface. Nanotoxicology 2015, 9, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Beuchat, M.H.; Lindsay, M.; Frias, S.; Palmiter, R.D.; Sakuraba, H.; Parton, R.G.; Gruenberg, J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat. Cell Biol. 1999, 1, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Salazar, G.; Love, R.; Styers, M.L.; Werner, E.; Peden, A.; Rodriguez, S.; Gearing, M.; Wainer, B.H.; Faundez, V. AP-3-dependent mechanisms control the targeting of a chloride channel (ClC-3) in neuronal and non-neuronal cells. J. Biol. Chem. 2004, 279, 25430–25439. [Google Scholar] [CrossRef] [PubMed]
- Holen, I.; Gordon, P.B.; Seglen, P.O. Protein kinase-dependent effects of okadaic acid on hepatocytic autophagy and cytoskeletal integrity. Biochem. J. 1992, 284, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Milner, D.J.; Mayroidis, M.; Weisleder, N.; Capetanaki, Y. Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J. Cell Biol. 2000, 150, 1283–1298. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, E.L.; Illert, A.L.; Tanaka, Y.; Schwarzmann, G.; Blanz, J.; Von Figura, K.; Saftig, P. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol. Biol. Cell 2002, 13, 3355–3368. [Google Scholar] [CrossRef] [PubMed]
- Salas, P.J.; Rodriguez, M.L.; Viciana, A.L.; Vega-Salas, D.E.; Hauri, H.P. The apical submembrane cytoskeleton participates in the organization of the apical pole in epithelial cells. J. Cell Biol. 1997, 137, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Grafstein, B.; Forman, D.S. Intracellular transport in neurons. Physiol. Rev. 1980, 60, 1167–1283. [Google Scholar] [PubMed]
- Fliegner, K.H.; Ching, G.Y.; Liem, R.K. The predicted amino acid sequence of alpha-internexin is that of a novel neuronal intermediate filament protein. EMBO J. 1990, 9, 749–755. [Google Scholar] [PubMed]
- Brownlees, J.; Ackerley, S.; Grierson, A.J.; Jacobsen, N.J.; Shea, K.; Anderton, B.H.; Leigh, P.N.; Shaw, C.E.; Miller, C.C. Charcot-Marie-Tooth disease neurofilament mutations disrupt neurofilament assembly and axonal transport. Hum. Mol. Genet. 2002, 11, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Kimelberg, H.K. Active accumulation and exchange transport of chloride in astroglial cells in culture. Biochim. Biophys. Acta 1981, 646, 179–184. [Google Scholar] [CrossRef]
- Kimelberg, H.K.; Katz, D.M. Regional differences in 5-hydroxytryptamine and catecholamine uptake in primary astrocyte cultures. J. Neurochem. 1986, 47, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Kimelberg, H.K.; Pelton, E.W.N. High-affinity uptake of [3H]norepinephrine by primary astrocyte cultures and its inhibition by tricyclic antidepressants. Neurochem. Int. 1983, 40, 1265–1270. [Google Scholar] [CrossRef]
- Walz, W.; Wuttke, W.; Hertz, L. Astrocytes in primary cultures: Membrane potential characteristics reveal exclusive potassium conductance and potassium accumulator properties. Brain Res. 1984, 292, 367–374. [Google Scholar] [CrossRef]
- Syková, E.; Chvátal, A. Extracellular ionic and volume changes: The role in glia-neuron interaction. J. Chem. Neuroanat 1993, 6, 247–260. [Google Scholar] [CrossRef]
- Parpura, V.; Basarsky, T.A.; Liu, F.; Jeftinija, K.; Jeftinija, S.; Haydon, P.G. Glutamate-mediated astrocyte-neuron signalling. Nature 1994, 369, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Bezzi, P.; Gundersen, V.; Galbete, J.L.; Seifert, G.; Steinhäuser, C.; Pilati, E.; Volterra, A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat. Neurosci. 2004, 7, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Pangrsic, T.; Kreft, M.; Krzan, M.; Li, N.; Sul, J.Y.; Halassa, M.; Van Bockstaele, E.; Zorec, R.; Haydon, P.G. Fusion-related release of glutamate from astrocytes. J. Biol. Chem. 2004, 279, 12724–12733. [Google Scholar] [CrossRef] [PubMed]
- Baertschi, A.J.; Monnier, D.; Schmidt, U.; Levitan, E.S.; Fakan, S.; Roatti, A. Acid prohormone sequence determines size, shape, and docking of secretory vesicles in atrial myocytes. Circ. Res. 2001, 89, E23–E29. [Google Scholar] [CrossRef] [PubMed]
- Krzan, M.; Stenovec, M.; Kreft, M.; Pangrsic, T.; Grilc, S.; Haydon, P.G.; Zorec, R. Calcium-dependent exocytosis of atrial natriuretic peptide from astrocytes. J. Neurosci. 2003, 23, 1580–1583. [Google Scholar] [PubMed]
- Kreft, M.; Stenovec, M.; Rupnik, M.; Grilc, S.; Krzan, M.; Potokar, M.; Pangrsic, T.; Haydon, P.G.; Zorec, R. Properties of Ca(2+)-dependent exocytosis in cultured astrocytes. Glia 2004, 46, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Coco, S.; Calegari, F.; Pravettoni, E.; Pozzi, D.; Taverna, E.; Rosa, P.; Matteoli, M.; Verderio, C. Storage and release of ATP from astrocytes in culture. J. Biol. Chem. 2003, 278, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Martineau, M.; Galli, T.; Baux, G.; Mothet, J.P. Confocal imaging and tracking of the exocytotic routes for D-serine-mediated gliotransmission. Glia 2008, 56, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Taraska, J.W.; Perrais, D.; Ohara-Imaizumi, M.; Nagamatsu, S.; Almers, W. Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc. Natl. Acad. Sci. USA 2003, 100, 2070–2075. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, J.; Franke, W.W.; Schachner, M. Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. J. Cell Biol. 1981, 90, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Bignami, A.; Raju, T.; Dahl, D. Localization of vimentin, the nonspecific intermediate filament protein, in embryonal glia and in early differentiating neurons. In vivo and in vitro immunofluorescence study of the rat embryo with vimentin and neurofilament antisera. Dev. Biol. 1982, 91, 286–295. [Google Scholar] [CrossRef]
- Lendahl, U.; Zimmerman, L.B.; McKay, R.D. CNS stem cells express a new class of intermediate filament protein. Cell 1990, 60, 585–595. [Google Scholar] [CrossRef]
- Bovolenta, P.; Liem, R.K.; Mason, C.A. Development of cerebellar astroglia: Transitions in form and cytoskeletal content. Dev. Biol. 1984, 102, 248–259. [Google Scholar] [CrossRef]
- Pixley, S.K.; de Vellis, J. Transition between immature radial glia and mature astrocytes studied with a monoclonal antibody to vimentin. Brain Res. 1984, 317, 201–209. [Google Scholar] [CrossRef]
- Migheli, A.; Pezzulo, T.; Attanasio, A.; Schiffer, D. Peripherin immunoreactive structures in amyotrophic lateral sclerosis. Lab. Investig. 1993, 68, 185–191. [Google Scholar] [PubMed]
- Pappolla, M.A. Lewy bodies of parkinson’s disease. Immune electron microscopic demonstration of neurofilament antigens in constituent filaments. Arch. Pathol. Lab. Med. 1986, 110, 1160–1163. [Google Scholar] [PubMed]
- Côté, F.; Collard, J.F.; Julien, J.P. Progressive neuronopathy in transgenic mice expressing the human neurofilament heavy gene: A mouse model of amyotrophic lateral sclerosis. Cell 1993, 73, 35–46. [Google Scholar] [CrossRef]
- Lee, M.K.; Marszalek, J.R.; Cleveland, D.W. A mutant neurofilament subunit causes massive, selective motor neuron death: Implications for the pathogenesis of human motor neuron disease. Neuron 1994, 13, 975–988. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Nguyen, M.D.; Julien, J.P. Late onset of motor neurons in mice overexpressing wild-type peripherin. J. Cell Biol. 1999, 147, 531–544. [Google Scholar] [CrossRef] [PubMed]
- De Vos, K.J.; Chapman, A.L.; Tennant, M.E.; Manser, C.; Tudor, E.L.; Lau, K.F.; Brownlees, J.; Ackerley, S.; Shaw, P.J.; McLoughlin, D.M.; et al. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum. Mol. Genet. 2007, 16, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Kieran, D.; Hafezparast, M.; Bohnert, S.; Dick, J.R.; Martin, J.; Schiavo, G.; Fisher, E.M.; Greensmith, L. A mutation in dynein rescues axonal transport defects and extends the life span of ALS mice. J. Cell Biol. 2005, 169, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Hafezparast, M.; Klocke, R.; Ruhrberg, C.; Marquardt, A.; Ahmad-Annuar, A.; Bowen, S.; Lalli, G.; Witherden, A.S.; Hummerich, H.; Nicholson, S.; et al. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science 2003, 300, 808–812. [Google Scholar] [CrossRef] [PubMed]
- LaMonte, B.H.; Wallace, K.E.; Holloway, B.A.; Shelly, S.S.; Ascaño, J.; Tokito, M.; Van Winkle, T.; Howland, D.S.; Holzbaur, E.L. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron 2002, 34, 715–727. [Google Scholar] [CrossRef]
- Corbo, M.; Hays, A.P. Peripherin and neurofilament protein coexist in spinal spheroids of motor neuron disease. J. Neuropathol. Exp. Neurol. 1992, 51, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.K.; He, B.P.; Strong, M.J. Characterization of neuronal intermediate filament protein expression in cervical spinal motor neurons in sporadic Amyotrophic Lateral Sclerosis (ALS). J. Neuropathol. Exp. Neurol. 2000, 59, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, C.; Beric-Maskarel, K.; Muntasser, S.; Weyer, L.; Somerville, M.J.; Percy, M.E. Neurofilament light and polyadenylated mRNA levels are decreased in Amyotrophic Lateral Sclerosis motor neurons. J. Neuropathol. Exp. Neurol. 1994, 53, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Oguri, T.; Inoko, A.; Shima, H.; Izawa, I.; Arimura, N.; Yamaguchi, T.; Inagaki, N.; Kaibuchi, K.; Kikuchi, K.; Inagaki, M. Vimentin-Ser82 as a memory phosphorylation site in astrocytes. Genes Cells 2006, 11, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Laronne, A.; Rotkopf, S.; Hellman, A.; Gruenbaum, Y.; Porter, A.C.; Brandeis, M. Synchronization of interphase events depends neither on mitosis nor on cdk1. Mol. Biol. Cell 2003, 14, 3730–3740. [Google Scholar] [CrossRef] [PubMed]
- Broers, J.L.; Machiels, B.M.; Kuijpers, H.J.; Smedts, F.; van den Kieboom, R.; Raymond, Y.; Ramaekers, F.C. A- and B-type lamins are differentially expressed in normal human tissues. Histochem. Cell Biol. 1997, 107, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Gruenbaum, Y.; Foisner, R. Lamins: Nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu. Rev. Biochem. 2015, 84, 134–164. [Google Scholar] [CrossRef] [PubMed]
- Stuurman, N.; Heins, S.; Aebi, U. Nuclear lamins: Their structure, assembly, and inteactions. J. Struct. Biol. 1998, 122, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.J.; Bollen, M.; Fields, A.P. Identification of protein phosphatase 1 as a mitotic lamin phosphatase. J. Biol. Chem. 1997, 272, 29693–29697. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.E.; Osborn, M.; Franke, W.W.; Weber, K. Intermediate filaments of the vimentin-type and the cytokeratin-type are distributed differently during mitosis. Exp. Cell Res. 1980, 129, 149–165. [Google Scholar] [CrossRef]
- Jones, J.C.; Goldman, A.E.; Yang, H.Y.; Goldman, R.D. The organizational fate of intermediate filament networks in two epithelial cell types during mitosis. J. Cell Biol. 1985, 100, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Rosevear, E.R.; McReynolds, M.; Goldman, R.D. Dynamic properties of intermediate filaments: Disassembly and reassembly during mitosis in Baby Hamster Kidney cells. Cell Motil. Cytoskelet. 1990, 17, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Berlin, R.D.; Oliver, J.M. Surface functions during mitosis. II. Quantitation of pinocytosis and kinetic characterization of the mitotic cycle with a new fluorescence technique. J. Cell Biol. 1980, 85, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Berlin, R.D.; Oliver, J.M.; Walter, R.J. Surface functions during mitosis I: Phagocytosis, pinocytosis and mobility of surface-bound con A. Cell 1978, 15, 327–341. [Google Scholar] [CrossRef]
- Tuomikoski, T.; Felix, M.A.; Dorée, M.; Gruenberg, J. Inhibition of endocytic vesicle fusion in vitro by the cell-cycle control protein kinase cdc2. Nature 1989, 342, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Boucrot, E.; Kirchhausen, T. Endosomal recycling controls plasma membrane area during mitosis. Proc. Natl. Acad. Sci. USA 2007, 104, 7939–7944. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, J.K.; Burke, E.E.; Goodson, H.V.; D’Souza-Schorey, C. Endocytosis resumes during late mitosis and is required for cytokinesis. J. Biol. Chem. 2005, 280, 41628–41635. [Google Scholar] [CrossRef] [PubMed]
- Lanzetti, L.; Margaria, V.; Melander, F.; Virgili, L.; Lee, M.H.; Bartek, J.; Jensen, S. Regulation of the Rab5 GTPase-activating protein RN-tre by the dual specificity phosphatase Cdc14A in human cells. J. Biol. Chem. 2007, 282, 15258–15270. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Kim, M.; Lipinski, M.; Li, J.; Kim, D.; Lu, T.; Shen, Y.; Rameh, L.; Yankner, B.; Tsai, L.H.; et al. Negative regulation of VPS34 by Cdk mediated phosphorylation. Mol. Cell 2010, 38, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Schu, P.V.; Takegawa, K.; Fry, M.J.; Stack, J.H.; Waterfield, M.D.; Emr, S.D. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science 1993, 260, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, A.; Lippé, R.; Christoforidis, S.; Gaullier, J.M.; Brech, A.; Callaghan, J.; Toh, B.H.; Murphy, C.; Zerial, M.; Stenmark, H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 1998, 394, 494–498. [Google Scholar] [PubMed]
- Bergeland, T.; Haugen, L.; Landsverk, O.J.; Stenmark, H.; Bakke, O. Cell-cycle-dependent binding kinetics for the early endosomal tethering factor EEA1. EMBO Rep. 2008, 9, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Dyer, N.; Rebollo, E.; Dominguez, P.; Elkhatib, N.; Chavrier, P.; Daviet, L.; Gonzalez, C.; Gonzalez-Gaitan, M. Spermatocyte cytokinesis requires rapid membrane addition mediated by ARF6 on central spindle recycling endosomes. Development 2007, 134, 4437–4447. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, J.K.; D’Souza-Schorey, C. Localization and activation of the ARF6 GTPase during cleavage furrow ingression and cytokinesis. J. Biol. Chem. 2002, 277, 27210–27216. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.M.; Fieling, A.B.; Simon, G.C.; Yu, X.; Andrews, P.D.; Hames, R.S.; Frey, A.M.; Peden, A.A.; Gould, G.W.; Prekeris, R. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol. Biol. Cell 2005, 16, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Morita, E.; Sandrin, V.; Chung, H.Y.; Morham, S.G.; Gygi, S.P.; Rodesch, C.K.; Sundquist, W.I. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007, 26, 4215–4227. [Google Scholar] [CrossRef] [PubMed]
- Miserey-Lenkei, S.; Couedel-Courteille, A.; Del Nerv, E.; Bardin, S.; Piel, M.; Racine, V.; Sibarita, J.B.; Perez, F.; Bornens, M.; Goud, B. A role for the Rab6a′ GTPase in the inactivation of the Mad2-spindle checkpoint. EMBO J. 2006, 25, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Kouranti, I.; Sachse, M.; Arounche, N.; Goud, B.; Echard, A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr. Biol. 2006, 16, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Audhya, A.; Desai, A.; Oegema, K. A role for Rab5 in structuring the endoplasmic reticulum. J. Cell Biol. 2007, 178, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Pellinen, T.; Tuomi, S.; Arjonen, A.; Wolf, M.; Edgren, H.; Meyer, H.; Grosse, R.; Kitzing, T.; Rantala, J.K.; Kallioniemi, O.; et al. Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev. Cell 2008, 15, 371–385. [Google Scholar] [CrossRef] [PubMed]
| IF | Interaction | Function in Vesicular Trafficking |
|---|---|---|
| Keratin 8 | Formation of autophagosomes [93] | |
| Syntaxin 3 targeting [94] | ||
| Vimentin | Rab7a regulates vimentin phosphorylation state and assembly [95] | |
| Endocytosis of Jagged-1 [96] | ||
| Inhibition of endocytic vesicles fusion in mitosis [97] | ||
| Rab21-regulated β1-integrin trafficking to the cleavage furrow [97] | ||
| AP-3 [98] | Positioning of late endosomal-lysosomal compartments, luminal ionic composition of endocytic organelles and content of autophagosomes [98] | |
| Rab9 [99] | ||
| Rab5 [100] | ||
| Directional mobility of vesicles [101] | ||
| Activity-dependent mobility of endosomes/lysosomes [102,103] | ||
| Integrin recycling [104] | ||
| Reservoir for SNAP23 [105] | ||
| Rab4A [106] | ||
| Intracellular transport of glicolipids [107] | ||
| GFAP | Directional mobility of vesicles [101] | |
| Endocytosis of Jagged-1 [96] | ||
| Activity-dependent mobility of endosomes/lysosomes [102,103] | ||
| Peripherin | Rab7a regulates peripherin assembly [108] | |
| AP-3 [98] | ||
| Lysosomal transport [109] | ||
| SIP30 affects peripherin assembly [110] | Subcellular distribution of SIP30 and SNAP25 [110] | |
| Desmin | Lysosomal distribution [111] | |
| Rab5 [100] | ||
| NF-L | Lysosomal transport [109] | |
| α-Internexin | AP-3 [98] | |
| Drosophila lamin | Rab5 regulates lamin disassembly [112] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margiotta, A.; Bucci, C. Role of Intermediate Filaments in Vesicular Traffic. Cells 2016, 5, 20. https://doi.org/10.3390/cells5020020
Margiotta A, Bucci C. Role of Intermediate Filaments in Vesicular Traffic. Cells. 2016; 5(2):20. https://doi.org/10.3390/cells5020020
Chicago/Turabian StyleMargiotta, Azzurra, and Cecilia Bucci. 2016. "Role of Intermediate Filaments in Vesicular Traffic" Cells 5, no. 2: 20. https://doi.org/10.3390/cells5020020
APA StyleMargiotta, A., & Bucci, C. (2016). Role of Intermediate Filaments in Vesicular Traffic. Cells, 5(2), 20. https://doi.org/10.3390/cells5020020







