Uremic Toxins Induce ET-1 Release by Human Proximal Tubule Cells, which Regulates Organic Cation Uptake Time-Dependently
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Cell Culture
2.3. Enzyme-Linked Immuno Sorbent Assays
2.4. qPCR
2.5. OCT Mediated ASP+ Uptake
2.6. Data Analysis
3. Results and Discussion
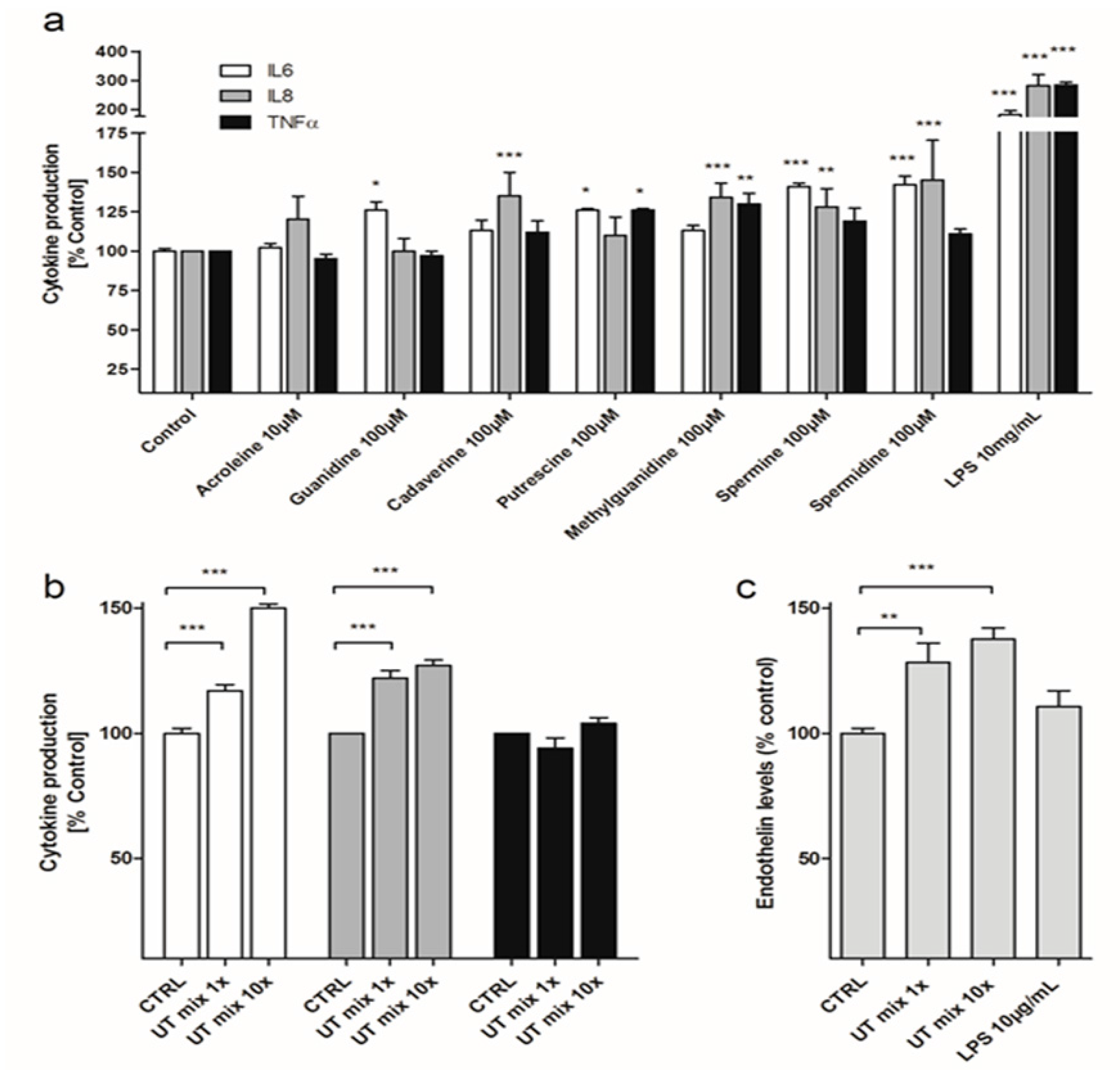
3.1. Various Cationic Uremic Toxins Induce IL-6, IL-8, TNFα and ET-1 Production by ciPTEC
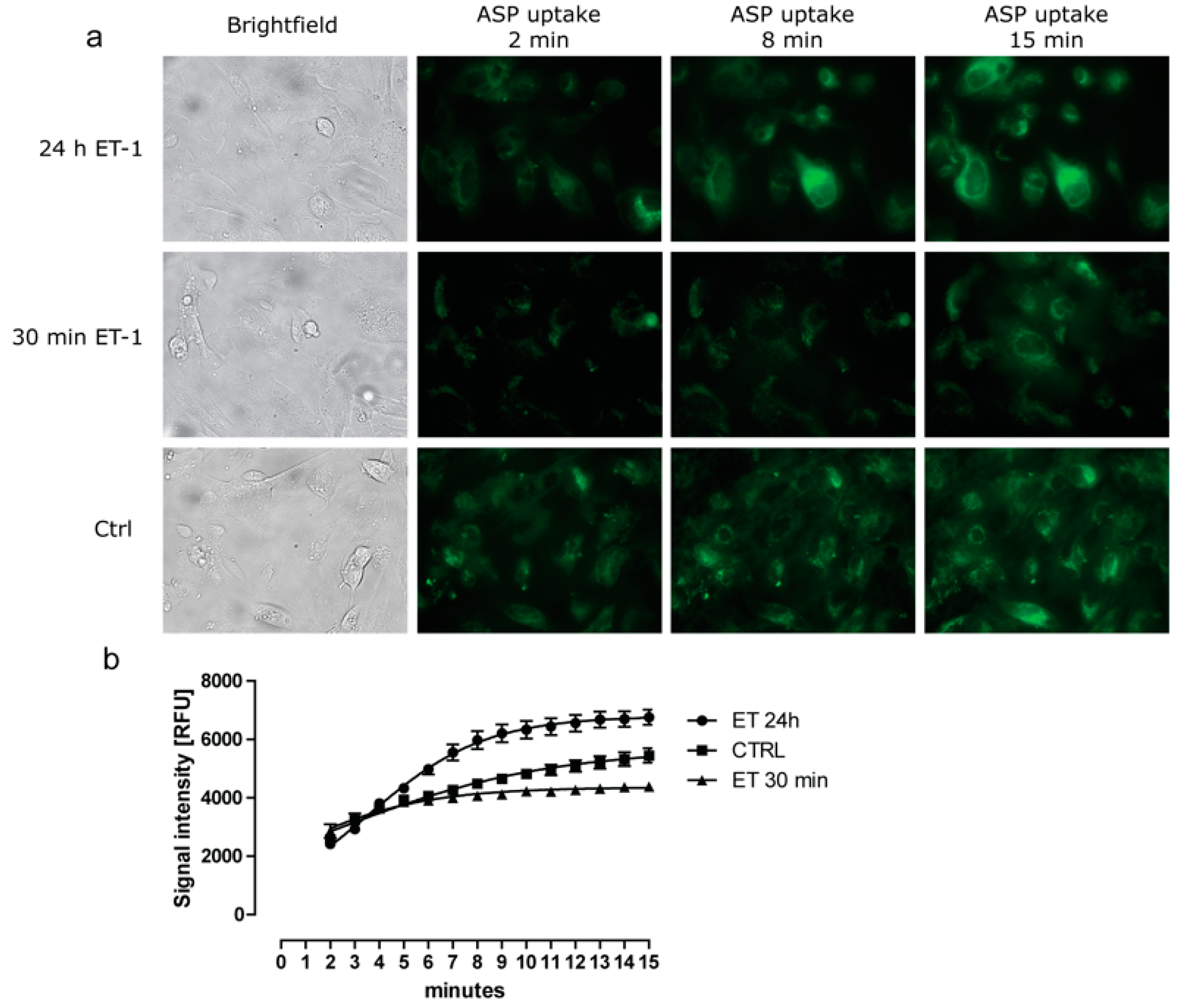
3.2. Differential Effect of ET-1 Exposures on Organic Cation Transport
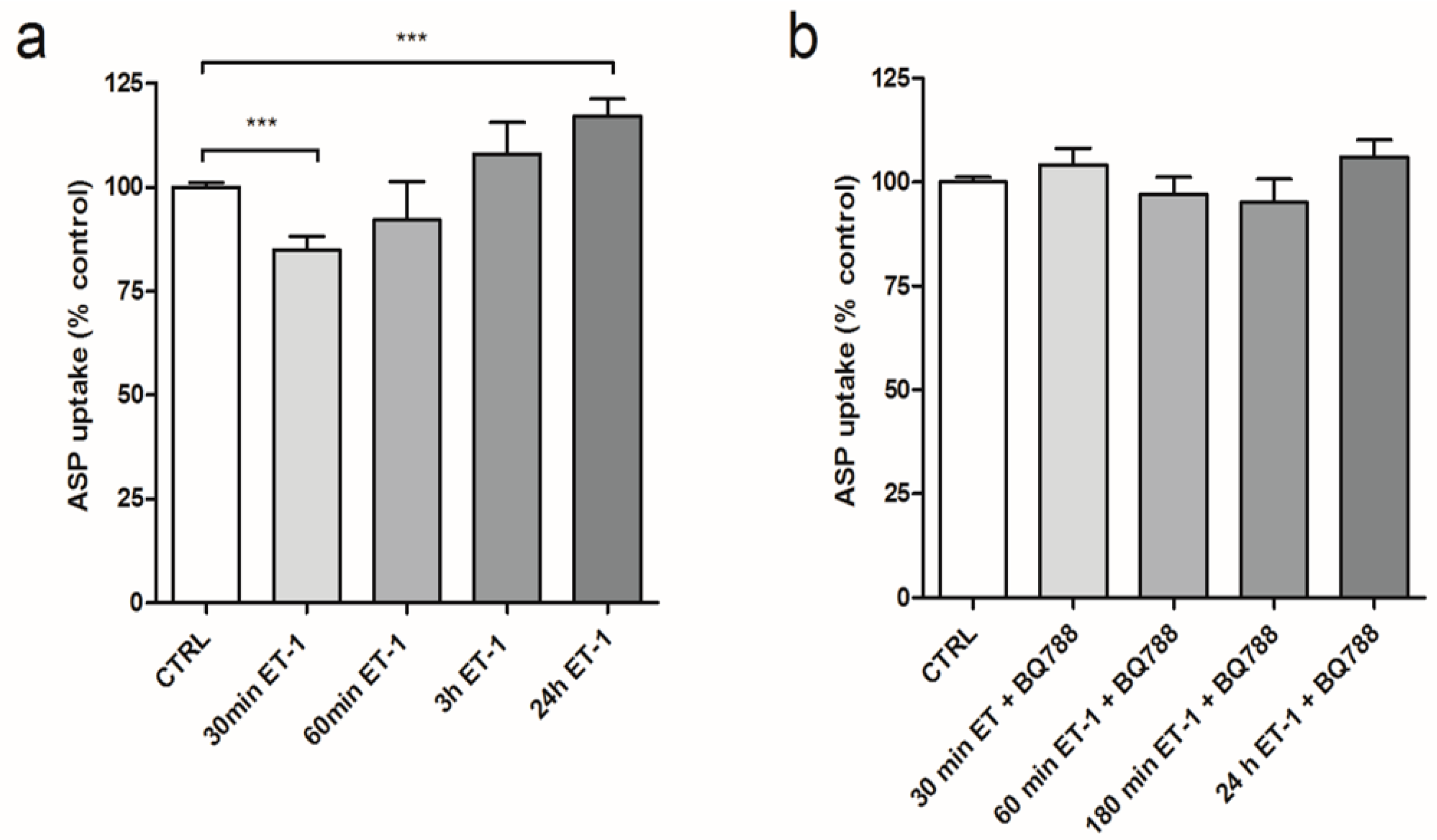
3.3. iNOS Inhibition Attenuates the Inhibitory Effects of ET-1 Exposure on Organic Cation Uptake
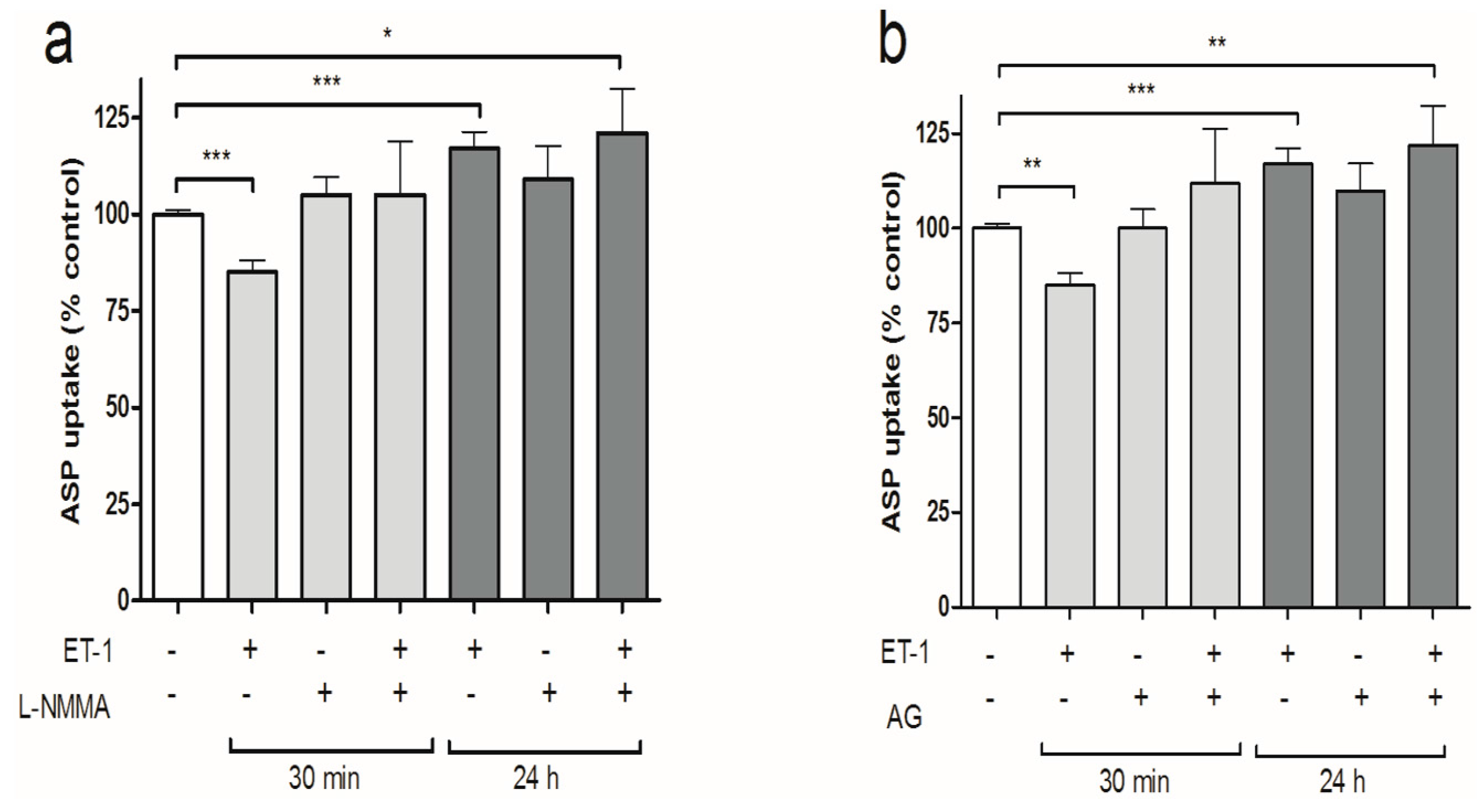
3.4. PKC Activation Restores Short-Term Organic Cation Uptake in ciPTEC after ET-1 Exposure
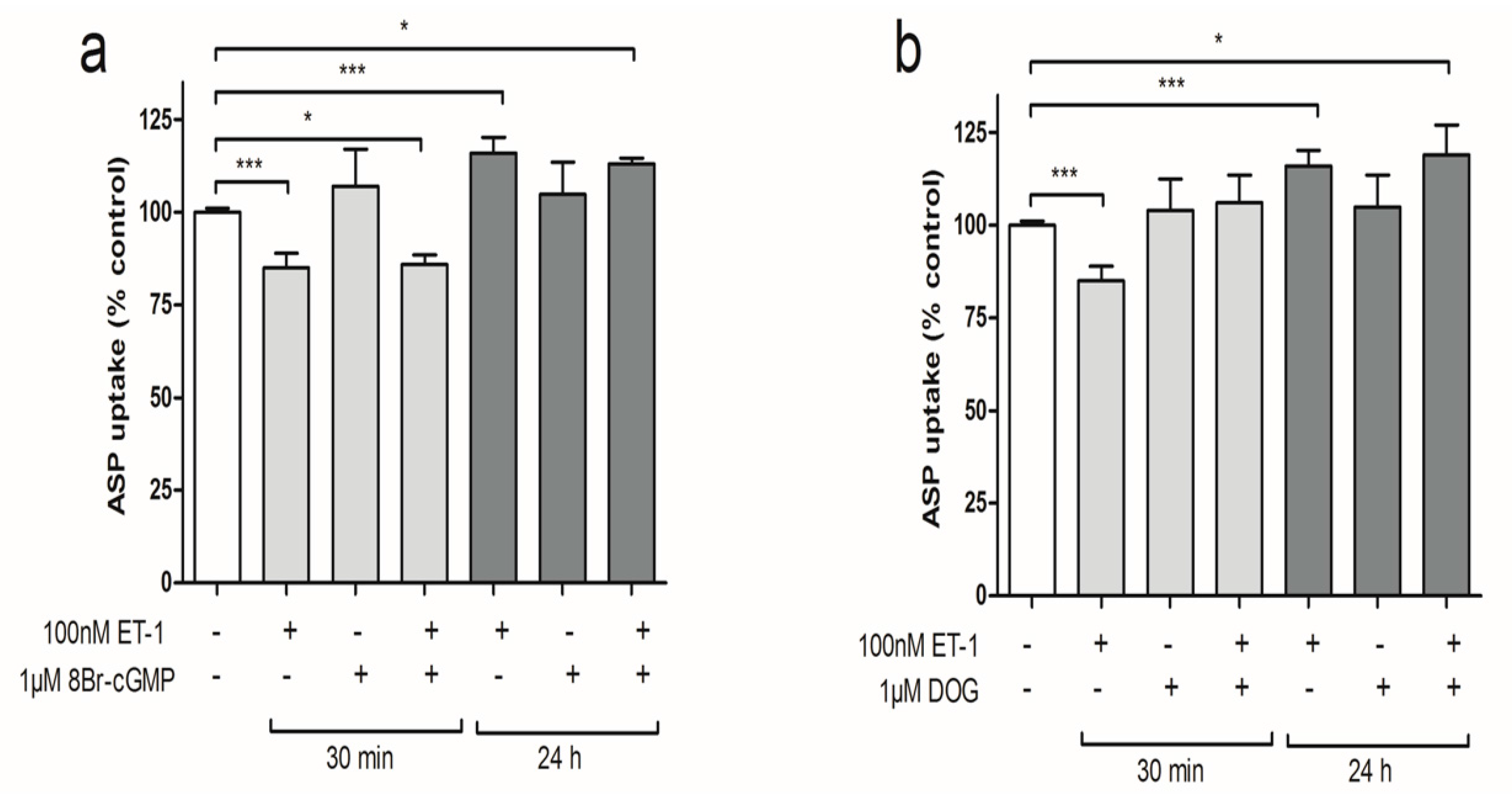
3.5. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duranton, F.; Cohen, G.; de Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Schophuizen, C.M.; Wilmer, M.J.; Jansen, J.; Gustavsson, L.; Hilgendorf, C.; Hoenderop, J.G.; van den Heuvel, L.P.; Masereeuw, R. Cationic uremic toxins affect human renal proximal tubule cell functioning through interaction with the organic cation transporter. Pflugers Arch.Eur. J. Physiol. 2013, 465, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, M.J.; Saleem, M.A.; Masereeuw, R.; Ni, L.; Van Der Velden, T.J.; Russel, F.G.; Mathieson, P.W.; Monnens, L.A.; Van Den Heuvel, L.P.; Levtchenko, E.N. Novel conditionally immortalized human proximal tubule cell line expressing functional influx and efflux transporters. Cell Tissue Res. 2010, 339, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Dhondt, A.; Vanholder, R.; van Biesen, W.; Lameire, N. The removal of uremic toxins. Kidney Int. Suppl. 2000, 58, S47–S59. [Google Scholar] [CrossRef]
- Eloot, S.; Van Biesen, W.; Dhondt, A.; van de Wynkele, H.; Glorieux, G.; Verdonck, P.; Vanholder, R. Impact of hemodialysis duration on the removal of uremic retention solutes. Kidney Int. 2008, 73, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, G.L.; Dhondt, A.W.; Jacobs, P.; van Langeraert, J.; Lameire, N.H.; de Deyn, P.P.; Vanholder, R.C. In vitro study of the potential role of guanidines in leukocyte functions related to atherogenesis and infection. Kidney Int. 2004, 65, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C. Role of uremic toxins in exacerbating anemia in renal failure. Kidney Int. Suppl. 2001, 59, S67–S72. [Google Scholar] [CrossRef]
- Horl, W.H. Neutrophil function and infections in uremia. Am. J. Kidney Dis. 1999, 33, 45–49. [Google Scholar] [CrossRef]
- Cohen, G.; Horl, W.H. Immune dysfunction in uremia—an update. Toxins 2012, 4, 962–990. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, Y.; Sakai, S.; Maeda, S.; Shimojo, N.; Watanabe, S.; Honma, S.; Kuga, K.; Aonuma, K.; Miyauchi, T. Increased plasma levels of big-endothelin-2 and big-endothelin-3 in patients with end-stage renal disease. Life Sci. 2012, 91, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Tbahriti, H.F.; Meknassi, D.; Moussaoui, R.; Messaoudi, A.; Zemour, L.; Kaddous, A.; Bouchenak, M.; Mekki, K. Inflammatory status in chronic renal failure: The role of homocysteinemia and pro-inflammatory cytokines. World J. Nephrol. 2013, 2, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, I.; Moutabarrik, A.; Okada, N.; Kitamura, E.; Hayashi, A.; Syouji, T.; Namiki, M.; Ishibashi, M.; Zaid, D.; Tsubakihara, Y. Interleukin-8 in chronic renal failure and dialysis patients. Nephrol. Dial. Transplant. 1994, 9, 1435–1442. [Google Scholar] [PubMed]
- Panichi, V.; Migliori, M.; de Pietro, S.; Taccola, D.; Bianchi, A.M.; Norpoth, M.; Giovannini, L.; Palla, R.; Tetta, C. C-reactive protein as a marker of chronic inflammation in uremic patients. Blood Purif. 2000, 18, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ottosson-Seeberger, A.; Ahlborg, G.; Hemsen, A.; Lundberg, J.M.; Alvestrand, A. Hemodynamic effects of endothelin-1 and big endothelin-1 in chronic hemodialysis patients. J. Am. Soc. Nephrol. 1999, 10, 1037–1044. [Google Scholar] [PubMed]
- Stenvinkel, P.; Ketteler, M.; Johnson, R.J.; Lindholm, B.; Pecoits-Filho, R.; Riella, M.; Heimburger, O.; Cederholm, T.; Girndt, M. IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. 2005, 67, 1216–1233. [Google Scholar] [CrossRef] [PubMed]
- Therrien, F.J.; Agharazii, M.; Lebel, M.; Lariviere, R. Neutralization of tumor necrosis factor-alpha reduces renal fibrosis and hypertension in rats with renal failure. Am. J. Nephrol. 2012, 36, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Corder, R.; Carrier, M.; Khan, N.; Klemm, P.; Vane, J.R. Cytokine regulation of endothelin-1 release from bovine aortic endothelial cells. J. Cardiovasc. Pharmacol. 1995, 26, S56–S58. [Google Scholar] [CrossRef] [PubMed]
- Marsden, P.A.; Brenner, B.M. Transcriptional regulation of the endothelin-1 gene by TNF-alpha. Am. J. Physiol. 1992, 262, C854–C861. [Google Scholar] [PubMed]
- Kohan, D.E.; Rossi, N.F.; Inscho, E.W.; Pollock, D.M. Regulation of blood pressure and salt homeostasis by endothelin. Physiol. Rev. 2011, 91, 1–77. [Google Scholar] [CrossRef] [PubMed]
- Demuth, K.; Blacher, J.; Guerin, A.P.; Benoit, M.O.; Moatti, N.; Safar, M.E.; London, G.M. Endothelin and cardiovascular remodelling in end-stage renal disease. Nephrol. Dial. Transplant. 1998, 13, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Cottone, S.; Mule, G.; Guarneri, M.; Palermo, A.; Lorito, M.C.; Riccobene, R.; Arsena, R.; Vaccaro, F.; Vadala, A.; Nardi, E.; et al. Endothelin-1 and F2-isoprostane relate to and predict renal dysfunction in hypertensive patients. Nephrol. Dial. Transplant. 2009, 24, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, C.M.; Gerchman, F.; Burttet, L.; Nabinger, G.; Jacques-Silva, M.C.; Canani, L.H.; Gross, J.L. Endothelin-1 levels and albuminuria in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2008, 80, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Notenboom, S.; Miller, D.S.; Smits, P.; Russel, F.G.; Masereeuw, R. Role of NO in endothelin-regulated drug transport in the renal proximal tubule. Am. J. Physiol. Renal Physiol. 2002, 282, F458–F464. [Google Scholar] [CrossRef] [PubMed]
- Masereeuw, R.; Terlouw, S.A.; van Aubel, R.A.; Russel, F.G.; Miller, D.S. Endothelin B receptor-mediated regulation of ATP-driven drug secretion in renal proximal tubule. Mol. Pharmacol. 2000, 57, 59–67. [Google Scholar] [PubMed]
- Heemskerk, S.; Wouterse, A.C.; Russel, F.G.; Masereeuw, R. Nitric oxide down-regulates the expression of organic cation transporters (OCT) 1 and 2 in rat kidney during endotoxemia. Eur. J. Pharmacol. 2008, 584, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Notenboom, S.; Wouterse, A.C.; Peters, B.; Kuik, L.H.; Heemskerk, S.; Russel, F.G.; Masereeuw, R. Increased apical insertion of the multidrug resistance protein 2 (MRP2/ABCC2) in renal proximal tubules following gentamicin exposure. J. Pharmacol. Exp. Ther. 2006, 318, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Notenboom, S.; Miller, D.S.; Kuik, L.H.; Smits, P.; Russel, F.G.; Masereeuw, R. Short-term exposure of renal proximal tubules to gentamicin increases long-term multidrug resistance protein 2 (Abcc2) transport function and reduces nephrotoxicant sensitivity. J. Pharmacol. Exp. Ther. 2005, 315, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Schophuizen, C.M.; Wilmer, M.J.; Lahham, S.H.; Mutsaers, H.A.; Wetzels, J.F.; Bank, R.A.; van den Heuvel, L.P.; Hoenderop, J.G.; Masereeuw, R. A morphological and functional comparison of proximal tubule cell lines established from human urine and kidney tissue. Exp. Cell Res. 2014, 323, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Takagi, T.; Chung, T.G.; Ohta, K. Serum levels of polyamines in patients with chronic renal failure. Kidney Int. Suppl. 1983, 16, S234–S237. [Google Scholar] [PubMed]
- Igarashi, K.; Ueda, S.; Yoshida, K.; Kashiwagi, K. Polyamines in renal failure. Amino Acids 2006, 31, 477–483. [Google Scholar] [CrossRef] [PubMed]
- De Deyn, P.; Marescau, B.; Lornoy, W.; Becaus, I.; Lowenthal, A. Guanidino compounds in uraemic dialysed patients. Clin. Chim. Acta 1986, 157, 143–150. [Google Scholar]
- Marescau, B.; Nagels, G.; Possemiers, I.; De Broe, M.E.; Becaus, I.; Billiouw, J.M.; Lornoy, W.; de Deyn, P.P. Guanidino compounds in serum and urine of nondialyzed patients with chronic renal insufficiency. Metabolism 1997, 46, 1024–1031. [Google Scholar] [CrossRef]
- Motohashi, H.; Sakurai, Y.; Saito, H.; Masuda, S.; Urakami, Y.; Goto, M.; Fukatsu, A.; Ogawa, O.; Inui, K. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J. Am. Soc. Nephrol. 2002, 13, 866–874. [Google Scholar] [PubMed]
- Koepsell, H. The SLC22 family with transporters of organic cations, anions and zwitterions. Mol. Aspects Med. 2013, 34, 413–435. [Google Scholar] [CrossRef] [PubMed]
- Notenboom, S.; Miller, D.S.; Smits, P.; Russel, F.G.; Masereeuw, R. Involvement of guanylyl cyclase and cGMP in the regulation of Mrp2-mediated transport in the proximal tubule. Am. J. Physiol. Renal Physiol. 2004, 287, F33–F38. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, M.; Quiroga, B.; Garcia de Vinuesa, S.; Verdalles, U.; Reque, J.; Panizo, N.; Arroyo, D.; Santos, A.; Macias, N.; Luno, J. Intraindividual interleukin-6 variations on the cardiovascular prognosis of patients with chronic renal disease. Ren. Fail. 2012, 34, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Pecoits-Filho, R.; Heimburger, O.; Barany, P.; Suliman, M.; Fehrman-Ekholm, I.; Lindholm, B.; Stenvinkel, P. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am. J. Kidney Dis. 2003, 41, 1212–1218. [Google Scholar] [CrossRef]
- Hage, D.S.; Jackson, A.; Sobansky, M.R.; Schiel, J.E.; Yoo, M.J.; Joseph, K.S. Characterization of drug-protein interactions in blood using high-performance affinity chromatography. J. Sep. Sci. 2009, 32, 835–853. [Google Scholar] [CrossRef] [PubMed]
- Reidenberg, M.M.; Drayer, D.E. Alteration of drug-protein binding in renal disease. Clin. Pharmacokinet. 1984, 9, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Rowland Yeo, K.; Aarabi, M.; Jamei, M.; Rostami-Hodjegan, A. Modeling and predicting drug pharmacokinetics in patients with renal impairment. Expert Rev. Clin. Pharmacol. 2011, 4, 261–274. [Google Scholar]
- Vanholder, R.; Van Landschoot, N.; De Smet, R.; Schoots, A.; Ringoir, S. Drug protein binding in chronic renal failure: Evaluation of nine drugs. Kidney Int. 1988, 33, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Masereeuw, R.; Russel, F.G. Mechanisms and clinical implications of renal drug excretion. Drug Metab. Rev. 2001, 33, 299–351. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Stewart, D.E.; Pritchard, J.B. Intracellular compartmentation of organic anions within renal cells. Am. J. Physiol. 1993, 264, R882–R890. [Google Scholar] [PubMed]
- Oliver, J.C.; Bland, L.A.; Oettinger, C.W.; Arduino, M.J.; McAllister, S.K.; Aguero, S.M.; Favero, M.S. Cytokine kinetics in an in vitro whole blood model following an endotoxin challenge. Lymphokine Cytokine Res. 1993, 12, 115–120. [Google Scholar] [PubMed]
- Corti, A.; Fassina, G.; Marcucci, F.; Barbanti, E.; Cassani, G. Oligomeric tumour necrosis factor alpha slowly converts into inactive forms at bioactive levels. Biochem. J. 1992, 284, 905–910. [Google Scholar] [PubMed]
- Wollenberg, G.K.; DeForge, L.E.; Bolgos, G.; Remick, D.G. Differential expression of tumor necrosis factor and interleukin-6 by peritoneal macrophages in vivo and in culture. Am. J. Pathol. 1993, 143, 1121–1130. [Google Scholar] [PubMed]
- Terlouw, S.A.; Graeff, C.; Smeets, P.H.; Fricker, G.; Russel, F.G.; Masereeuw, R.; Miller, D.S. Short- and long-term influences of heavy metals on anionic drug efflux from renal proximal tubule. J. Pharmacol. Exp. Ther. 2002, 301, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Terlouw, S.A.; Masereeuw, R.; Russel, F.G.; Miller, D.S. Nephrotoxicants induce endothelin release and signaling in renal proximal tubules: Effect on drug efflux. Mol. Pharmacol. 2001, 59, 1433–1440. [Google Scholar] [PubMed]
- Wever, K.E.; Masereeuw, R.; Miller, D.S.; Hang, X.M.; Flik, G. Endothelin and calciotropic hormones share regulatory pathways in multidrug resistance protein 2-mediated transport. Am. J. Physiol. Renal Physiol. 2007, 292, F38–F46. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I.; Yabuuchi, H.; Nezu, J.; Sai, Y.; Oku, A.; Shimane, M.; Tsuji, A. Cloning and characterization of a novel human ph-dependent organic cation transporter, OCTN1. FEBS Lett. 1997, 419, 107–111. [Google Scholar] [CrossRef]
- Wu, X.; Prasad, P.D.; Leibach, F.H.; Ganapathy, V. cDNA sequence, transport function, and genomic organization of human OCTN2, a new member of the organic cation transporter family. Biochem. Biophys. Res. Commun. 1998, 246, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Lauber, C.; Harrach, S.; Pap, T.; Fischer, M.; Victor, M.; Heitzmann, M.; Hansen, U.; Fobker, M.; Brand, S.M.; Sindic, A.; et al. Transport mechanisms and their pathology-induced regulation govern tyrosine kinase inhibitor delivery in rheumatoid arthritis. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H. Substrate recognition and translocation by polyspecific organic cation transporters. Biol. Chem. 2011, 392, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Urakami, Y.; Akazawa, M.; Saito, H.; Okuda, M.; Inui, K. cDNA cloning, functional characterization, and tissue distribution of an alternatively spliced variant of organic cation transporter hOCT2 predominantly expressed in the human kidney. J. Am. Soc. Nephrol. 2002, 13, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Guckel, D.; Ciarimboli, G.; Pavenstadt, H.; Schlatter, E. Regulation of organic cation transport in isolated mouse proximal tubules involves complex changes in protein trafficking and substrate affinity. Cell. Physiol. Biochem. 2012, 30, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Biermann, J.; Lang, D.; Gorboulev, V.; Koepsell, H.; Sindic, A.; Schroter, R.; Zvirbliene, A.; Pavenstadt, H.; Schlatter, E.; Ciarimboli, G. Characterization of regulatory mechanisms and states of human organic cation transporter 2. Am. J. Physiol. Cell Physiol. 2006, 290, C1521–C1531. [Google Scholar] [CrossRef] [PubMed]
- Bacic, D.; Wagner, C.A.; Hernando, N.; Kaissling, B.; Biber, J.; Murer, H. Novel aspects in regulated expression of the renal type IIa Na/Pi-cotransporter. Kidney Int. Suppl. 2004, 66, S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Murer, H.; Hernando, N.; Forster, I.; Biber, J. Regulation of Na/Pi transporter in the proximal tubule. Annu. Rev. Physiol. 2003, 65, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.H.; Li, G.; Yang, Q.; Suri, V.; Ross, J.J.; Alexander, E.A. Role of SNAREs and H+−ATPase in the targeting of proton pump-coated vesicles to collecting duct cell apical membrane. Kidney Int. 2007, 72, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.A.; Brown, D.; Shih, T.; McKee, M.; Schwartz, J.H. Effect of acidification on the location of H+−ATPase in cultured inner medullary collecting duct cells. Am. J. Physiol. 1999, 276, C758–C763. [Google Scholar] [PubMed]
- Masereeuw, R.; Russel, F.G. Regulatory pathways for ATP-binding cassette transport proteins in kidney proximal tubules. AAPS J. 2012, 14, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Ciarimboli, G.; Schlatter, E. Regulation of organic cation transport. Pflugers Arch. 2005, 449, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Mehrens, T.; Lelleck, S.; Cetinkaya, I.; Knollmann, M.; Hohage, H.; Gorboulev, V.; Boknik, P.; Koepsell, H.; Schlatter, E. The affinity of the organic cation transporter rOCT1 is increased by protein kinase c-dependent phosphorylation. J. Am. Soc. Nephrol. 2000, 11, 1216–1224. [Google Scholar] [PubMed]
- Pietig, G.; Mehrens, T.; Hirsch, J.R.; Cetinkaya, I.; Piechota, H.; Schlatter, E. Properties and regulation of organic cation transport in freshly isolated human proximal tubules. J. Biol. Chem. 2001, 276, 33741–33746. [Google Scholar] [CrossRef] [PubMed]
- Hartz, A.M.; Bauer, B.; Fricker, G.; Miller, D.S. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol. Pharmacol. 2004, 66, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Masereeuw, R.; Karnaky, K.J., Jr. Regulation of MRP2-mediated transport in shark rectal salt gland tubules. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R774–R781. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Knox, F.G. Production and functional roles of nitric oxide in the proximal tubule. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1117–R1124. [Google Scholar] [PubMed]
- McLay, J.S.; Chatterjee, P.; Nicolson, A.G.; Jardine, A.G.; McKay, N.G.; Ralston, S.H.; Grabowski, P.; Haites, N.E.; MacLeod, A.M.; Hawksworth, G.M. Nitric oxide production by human proximal tubular cells: A novel immunomodulatory mechanism? Kidney Int. 1994, 46, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, S.; van Koppen, A.; van den Broek, L.; Poelen, G.J.; Wouterse, A.C.; Dijkman, H.B.; Russel, F.G.; Masereeuw, R. Nitric oxide differentially regulates renal ATP-binding cassette transporters during endotoxemia. Pflugers Arch. 2007, 454, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Hu, N.; Liu, H.Y.; Li, J.; Guo, H.F.; Liu, C.; Liu, L.; Liu, X.D. Biphasic regulation of P-glycoprotein function and expression by NO donors in Caco-2 cells. Acta Pharmacol. Sin. 2012, 33, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, S.; Peters, J.G.; Louisse, J.; Sagar, S.; Russel, F.G.; Masereeuw, R. Regulation of P-glycoprotein in renal proximal tubule epithelial cells by LPS and TNF-alpha. J. Biomed. Biotechnol. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schophuizen, C.M.S.; Hoenderop, J.G.J.; Masereeuw, R.; Heuvel, L.P.v.d. Uremic Toxins Induce ET-1 Release by Human Proximal Tubule Cells, which Regulates Organic Cation Uptake Time-Dependently. Cells 2015, 4, 234-252. https://doi.org/10.3390/cells4030234
Schophuizen CMS, Hoenderop JGJ, Masereeuw R, Heuvel LPvd. Uremic Toxins Induce ET-1 Release by Human Proximal Tubule Cells, which Regulates Organic Cation Uptake Time-Dependently. Cells. 2015; 4(3):234-252. https://doi.org/10.3390/cells4030234
Chicago/Turabian StyleSchophuizen, Carolien M. S., Joost G. J. Hoenderop, Rosalinde Masereeuw, and Lambert P. van den Heuvel. 2015. "Uremic Toxins Induce ET-1 Release by Human Proximal Tubule Cells, which Regulates Organic Cation Uptake Time-Dependently" Cells 4, no. 3: 234-252. https://doi.org/10.3390/cells4030234
APA StyleSchophuizen, C. M. S., Hoenderop, J. G. J., Masereeuw, R., & Heuvel, L. P. v. d. (2015). Uremic Toxins Induce ET-1 Release by Human Proximal Tubule Cells, which Regulates Organic Cation Uptake Time-Dependently. Cells, 4(3), 234-252. https://doi.org/10.3390/cells4030234





