Abstract
Transient receptor potential canonical 6 (TRPC6) is a cation selective, DAG-regulated, Ca2+-permeable channel activated by the agonists of Gq-protein-coupled heptahelical receptors. Dysfunctions of TRPC6 are implicated in the pathogenesis of various cardiovascular and kidney conditions such as vasospasm and glomerulosclerosis. When stimulated by agonists of the histamine H1 receptor (H1R), TRPC6 activity decays to the baseline despite the continuous presence of the agonist. In this study, we examined whether H1R desensitization contributes to regulating the decay rate of TRPC6 activity upon receptor stimulation. We employed the HEK expression system and a biosensor allowing us to simultaneously detect the changes in intracellular diacylglycerol (DAG) and Ca2+ concentrations. We found that the histamine-induced DAG response was biphasic, in which a transient peak was followed by maintained elevated plateau, suggesting that desensitization of H1R takes place in the presence of histamine. The application of PKC inhibitor Gö6983 slowed the decay rate of intracellular DAG concentration. Activation of the mouse H1R mutant lacking a putative PKC phosphorylation site, Ser399, responsible for the receptor desensitization, resulted in a prolonged intracellular DAG increase and greater Mn2+ influx through the TRPC6 channel. Thus, our data support the hypothesis that PKC-dependent H1R phosphorylation leads to a reduced production of intracellular DAG that contributes to TRPC6 activity regulation.
1. Introduction
Transient Receptor Potential Canonical (TRPC) proteins form plasma membrane cation channels that are Ca2+-permeable. TRPCs are activated by the agonists of G-protein coupled receptors (GPCRs) in a phospholipase C (PLC) dependent manner. There are seven members in the TRPC subfamily (TRPC1-7). The TRPC6 channel is widely expressed in vascular smooth muscle, where it contributes to regulating vascular tone [1], and the kidney, where it is localized to the glomerular podocyte “split diaphragm” complex [1,2]. Increased expression of TRPC6 was implicated in the pathogenesis of idiopathic pulmonary arterial hypertension (IPAH) [3,4]. The gain-of-function mutants of TRPC6 are strongly associated with the progression of focal segmental glomerulosclerosis (FSGS) [5]. Besides TRPC6 roles in the cardiovascular systems and the kidney, the channel upregulation was reported in some cancer cell models, such as liver cancer cells [6].
It has been demonstrated that diacylglycerol (DAG) is important for gating the TRPC6 channel. DAG is produced by PLC that breaks down phosphatidylinositol diphosphate (PIP2) [7]. Two signaling molecules, DAG and inositol triphosphate, are formed upon PIP2 break down by PLC. IP3 binds to its receptor, IP3R, on the endoplasmic reticulum (ER) and releases Ca2+ from the ER, an intracellular Ca2+ store. This results in a transient intracellular [Ca2+] increase in the stimulated cells. DAG acting in concert with intracellular Ca2+-activates protein kinase C (PKC), which then translocates to the plasma membrane, where the kinase phosphorylates various protein including channels and membrane receptors.
The activation of non-selective Ca2+-permeable channels, such as TRPCs, contributes to the second sustained phase of receptor-induced intracellular Ca2+ increases. When activated by histamine via the H1 receptor (H1R), TRPC6 exhibits robust activity that decays over time despite the continuous presence of the agonist. Several seminal studies implicated the DAG-dependent activation of PKC followed by TRPC6 phosphorylation at the residues Serine448 [8] and Ser768/714 (Ser768 in TRPC6A isoforms and Ser714 in TRPC6B) [9] as a cause of TRPC6 activity decay. However, the role of histamine receptor phosphorylation and inactivation has not been considered so far. Indeed, DAG can regulate the functional activity of a plethora of G-protein coupled receptors. There have been several studies that indicated that serine residues (Ser396, Ser398) in the intracellular domain of H1R are potential phosphorylation sites of PKC and site-directed mutagenesis studies suggested that Ser398 residue was primarily involved in PKC-mediate receptor desensitization [10,11,12,13]. Since phosphorylation of GPCR by various kinases is suggested to be an important step in initiating receptor desensitization [14], we hypothesized that TRPC6 channel inactivation may involve the desensitization of the H1 receptor.
In the present study, we used the human embryonic kidney (HEK) cell expression model to investigate the relationship between the intracellular DAG concentration and TRPC6 activity. We found that the histamine-induced DAG production, assessed by using a recently developed fluorescent biosensor [15], results in negative feedback on H1R itself via a PKC-dependent pathway, indicating that desensitization of H1R takes place during prolonged application of histamine. Our results indicate that the inactivation of TRPC6 channel activity in the presence of histamine might be attributed in part to H1R desensitization.
2. Experimental Section
2.1. Cell Culture and Transfection
HEK cells (American Type Culture Collection, Manassas, VA, USA) were cultured in the Eagle’s minimum essential medium supplemented with 10% fetal bovine serum. HEK cells were transfected using the Lipofectamine LTX reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s recommendations. The Downward DAG+R-GECO sensor plasmid and the red fluorescent PIP2 sensor plasmid were a gift from Montana Molecular (Bozeman, MT, USA). The mouse H1 receptor plasmid was a gift from Dr. Jean-Charles Schwartz (Centre de recherche, Bioprojet-Biotech, Saint Gregoire Cedex, France) and the pig TRPC6 plasmid was cloned by us previously [16]. We used the following cDNAs mixtures during the transfection procedures: (1) 0.5 µg DAG+R-GECO sensor plasmid, 0.5 µg H1R, and 2 µg TRPC6 plasmid; (2) 0.5 µg DAG+R-GECO sensor plasmid, 0.5 µg H1R, and 2 µg pcDNA3; (3) 0.5 µg PIP2 sensor plasmid [17] and 0.5 µg H1R. The cells were cultured for 24–50 h before the fluorescence imaging experiments were performed.
2.2. Fluorescence Imaging
Transfected HEK cells were imaged either directly 30–50 h post-transfection time or loaded with Fura-2AM (4 µM) in PBS containing Ca2+ and Mg2+ for an hour, followed by a 30 min additional incubation in PBS containing no fluorescence dye. A Till Photonics single-cell fluorescence imaging system equipped with an Andor DU885 charge-coupled device camera (Andor Technology PLC, South Windsor, CT, USA) was used to monitor intracellular Ca2+ changes in single Fura-2-loaded HEK cells and sensor-expressing HEK cells. The cells were perfused continuously with the test solutions at a rate of 1.5 mL/min.
2.3. Molecular Biology
The single-point mutation in the mouse H1 histamine receptor was introduced using a QuikChange Lightning site-directed mutagenesis kit from Agilent Technologies (Santa Clara, CA, USA) as described in the manufacturer’s instructions. The following primer set was used to construct the mutant: GAGGCTCCGCTCACATGCCAGACAGTATGTG (forward primer) and CACATACTGTCTGGC-ATGTGAGCGGAGCCTC (reverse primer). The mutation was verified by sequencing.
2.4. Materials
Eagle’s minimum essential medium and fetal bovine serum for HEK cell culture were purchased from Life Technology (Carlsbad, CA, USA); Histamine, Gö6983 and other chemicals were brought from Sigma-Aldrich (St. Louis, MO, USA). The stock solutions were made in water or DMSO and were diluted to the indicated concentrations with the standard extracellular solutions.
2.5. Statistics
The statistical analysis was performed using the SigmaPlot 12 software package. The t-test followed by the Mann Whitney Rank Sum Test was used to determine whether there is a statistically significant difference between the tested groups. The significance level was set to <0.05. All of the data were presented as mean ± SEM.
3. Results and Discussion
3.1. Histamine-induced TRPC6 Activity Decays in the Present of Histamine
The TRPC6 channel mediates Ca2+ influx in various physiological cellular systems. It was demonstrated that DAG production is a key event for activating TRPC6 [7]. In this work, we employed a dual fluorescent downward DAG and Ca2+ biosensor (downward DAG+R-GECO sensor, DDRG) [15] to examine whether the kinetics of TRPC6-mediated Ca2+ influx correlates with DAG intracellular concentration changes. We also used a PIP2 sensor to confirm that DAG changes reflect the changes in PIP2 hydrolysis as was described elsewhere [17]. Since TRPC6 also allows Mn2+ influx, we assessed both Ca2+ intracellular transients and Mn2+ influx in HEK cells expressing pig TRPC6 using the Mn2+ quench approach [7,18].
Fura-2 Mn2+ quench experiments demonstrated that histamine (40 µM)-induced Mn2+ influx was significantly greater in HEK expressing H1R (the percent of Fura-2 quenching was 0.28 ± 0.021, n = 26) and TRPC6 than in cells expressing H1R-pcDNA3 (empty plasmid, 0.14 ± 0.017, n = 21, Figure 1A). Since Mn2+ quench assays the cumulative Mn2+ influx through TRPC6, we did not use the approach for kinetics studies. Instead, we investigated the kinetics of intracellular Ca2+ transients that were consistently larger in TRPC6 expressing cells as compared to pcDNA3-expressing control HEK cells. As noted by many previous studies [7], we observed that during the continuous presence of histamine, the intracellular [Ca2+] increased to a peak at about 30 s after histamine application and subsequently declined slowly with a time constant of 43.89 ± 5.265 s (n = 18; Figure 1B). To avoid potential interference due to TRPC6-mediated intracellular Ca2+ changes, we next examined intracellular DAG/Ca2+ changes in HEK cells expressing H1R and a novel fluorescent dual downward DAG/Ca2+ sensor. The downward DAG/Ca2+ sensor is a fusion of a PKCδ fragment with circular permuted enhanced green fluorescent protein (GFP) combined by a 2A peptide sequence with a red fluorescent Ca2+ sensor R-GECO in one plasmid for stoichiometric co-expression of the sensors ([15], Figure 1C, D). The downward DAG+R-GECO sensor (DAG/Ca2+R) was excited at 570 nm for detecting intracellular Ca2+ transients and at 480 nm for detecting DAG. Figure 1C, D showed Ca2+ changes (Figure 1C, excited at 570 nm) and DAG transients (Figure 1D, excited at 480 nm). Using this approach, we found that histamine-induced [Ca2+]i changes were transient in H1R-expressing HEK cells, reflecting the depletion of the intracellular Ca2+ stores. We also determined that the production of DAG was not constant over time and exponentially decayed with a close time constant of 48.61 ± 7.07 s (n = 23) as intracellular [Ca2+] declined (43.89 ± 5.265 s, n = 18), suggesting that H1R desensitization may take place. This result was consistent with the data, which we obtained using the red fluorescent PIP2 sensor, a fusion of the pleckstrin homology (PH) domain of PLCδ and dimerization-dependent red fluorescent proteins [17] that showed a slowing of intracellular PIP2 hydrolysis despite the continuous presence of histamine in the bath (Figure 1E). Importantly, since DAG activates the TRPC6 channel, decreased production of DAG would result in a reduced TRPC6 activity.
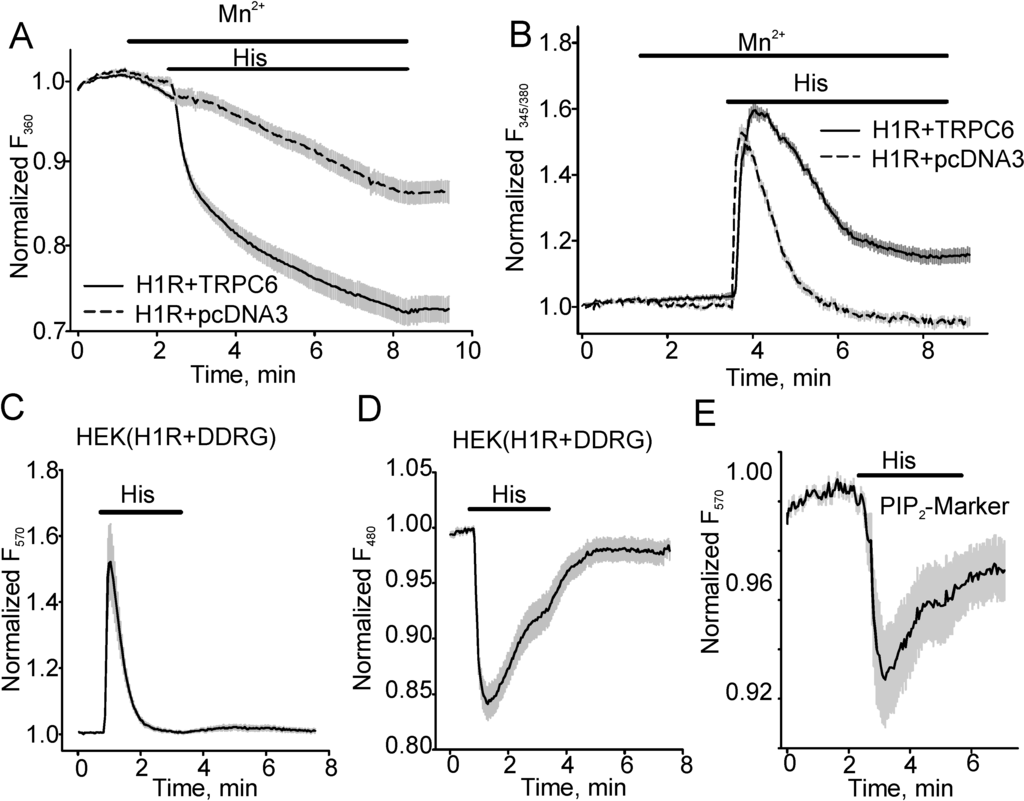
Figure 1.
Histamine induces transient intracellular diacylglycerol (DAG), phosphatidylinositol diphosphate (PIP2) and Ca2+ increases in histamine H1 receptor (H1R)-expressing human embryonic kidney (HEK) cells. (A, B) The traces show fluorescence changes in HEK cells expressing H1R + pcDNA3 or H1R + TRPC6. Mn2+ (0.5 mM) influx was determined in the control group and cells expressing TRPC6 channel using the Mn2+ quench approach. Fura-2 fluorescence was excited at 360 nm and the percent of fura-2 fluorescence decrease was shown in (A). (B) shows fluorescence intensity ratios (F345/F380) acquired during the same experiment. The fluorescence was normalized to the baseline fluorescence. To detect intracellular Ca2+ transients, the downward DAG+R-GECO sensor (DAG/Ca2+R) was excited at 570 nm, whereas DAG transients were assessed when DAG+R-GECO sensor fluorescence was excited at 480 nm (C, D). (E) shows the averaged trace (n = 12) of PIP2 level changes in the presence of histamine in HEK cells co-expressing the red fluorescent PIP2 sensor and H1R.
3.2. The Effect of PKC Inhibition on TRPC6 Inactivation Rate and Decay of DAG
It was demonstrated that PKC phosphorylation inactivates TRPC6. Therefore, we first used Gö6983 to confirm whether PKC activity is important for regulating the kinetics of TRPC6 responses. Indeed, we observed that TRPC6 responses decayed much slower in the presence of the PKC inhibitor (48.56 ± 14.77 s, n = 41 to 136.30 ± 29.36 s, n = 39, p < 0.05, Figure 2A, B). Since DAG production also decayed with time, as was determined using the dual DAG/Ca2+ sensor, we next investigated whether PKC phosphorylation regulates H1R activity that is known to exhibit PKC-dependent inactivation in other species [19]. We observed that 500 nM Gö6983 also slowed the decay of DAG (48.61 ± 7.07 s, n = 17 vs. 132.84 ± 23.97 s, n = 27, p < 0.05, Figure 2C, D).
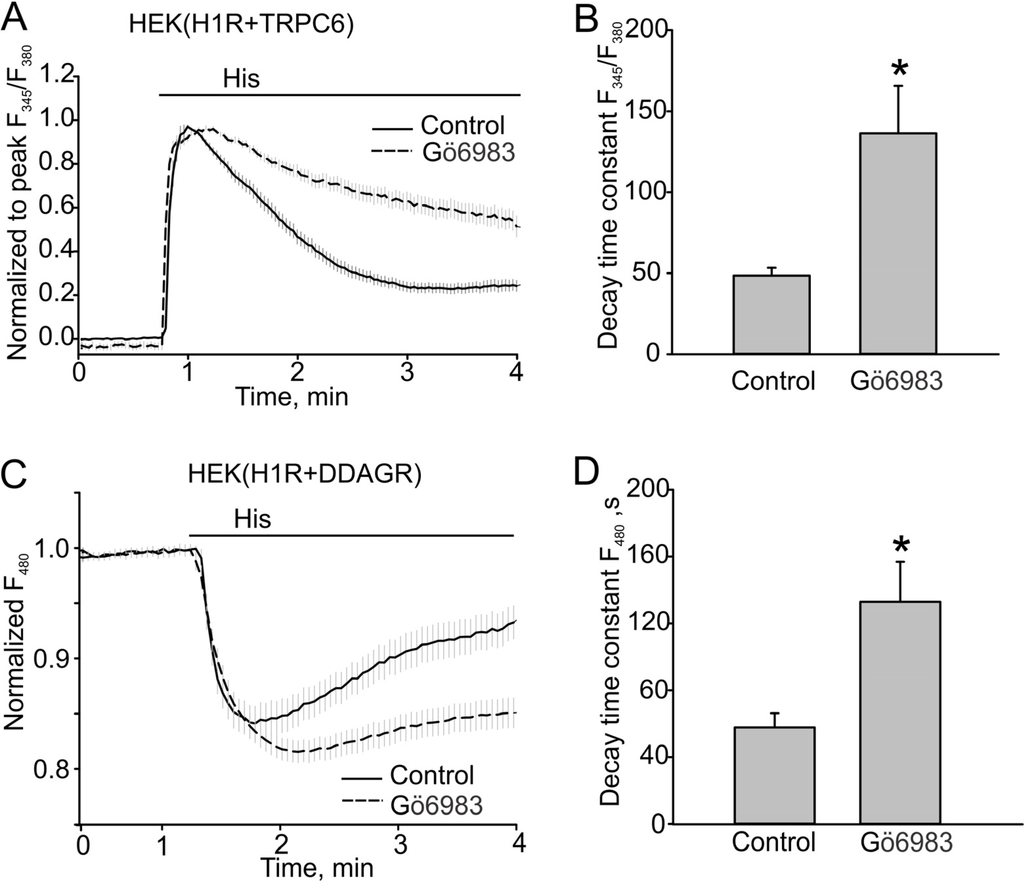
Figure 2.
PKC inhibitor Gö6983 slowed the decay of Ca2+ and DAG signals in the HEK model. (A, B) Histamine-induced Ca2+ transients in HEK cells expressing H1R + TRPC6 in the absence or presence of PKC inhibitor Gö6983 (500 nM). Fura-2 fluorescence ratios (F345/F380) are shown. (A) Traces of averaged Ca2+ transients in the control group and cells treated with Gö6983 (control, n = 41 vs. Gö6983, n = 39). The ratio data were normalized to the peak amplitude following the baseline fluorescence ratio at time zero was subtracted. (B) Summary data comparing the decay time constants for the Ca2+ transients shown in (A). (C, D) DAG transients assessed with the downwards DAG+R-GECO sensor excited at 480 nm. The fluorescence was normalized to the baseline fluorescence. The bold and broken lines represent the averaged traces, and the vertical gray lines are the SEM values. Data in (B, D) are presented as mean ± S.E. Asterisk = p < 0.05.
3.3. H1 Receptor Mutant (H1RS399A) Maintained A Prolonged DAG Production
In 1998, Fukui’s study provided evidence that ser398 in human H1 receptor was primarily involved in the PKC-mediated desensitization of the receptor [19]. Therefore, we constructed the mouse H1R S399A mutant, H1RS398A, lacking a homologous PKC-phosphorylation site (Figure 3A), to investigate whether the mutated receptor would exhibit a slower inactivation rate and more prolonged production of DAG. Figure 3B, C show that the H1R mutant potentiated the Ca2+ and DAG responses induced by 40 µM histamine. The normalized DAG response traces and summary data recorded in HEK cells expressing either H1R or its S399A mutant (H1R: 44.31 ± 6.54 s vs. S399A: 68.78 ± 11.21 s) are shown in Figure 3D, E.
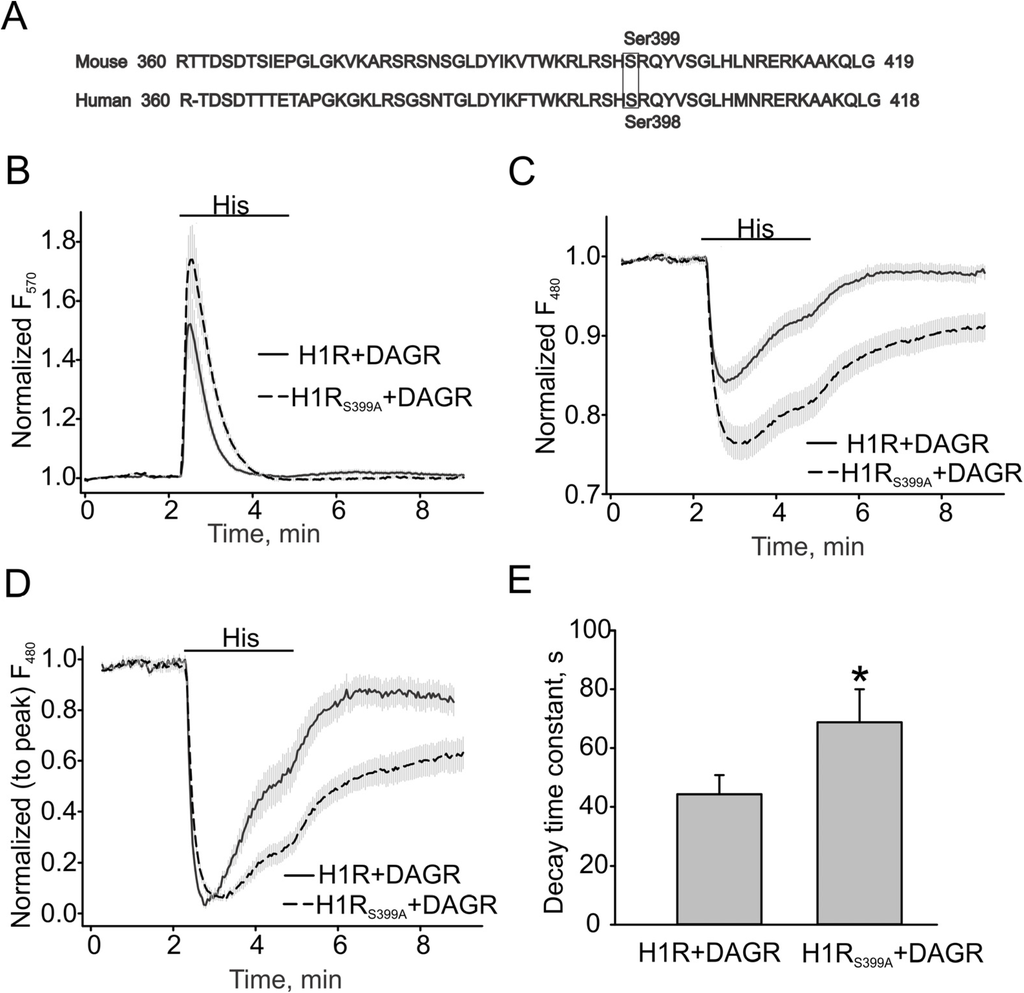
Figure 3.
DAG production is more sustained in H1RS399A-expressing HEK cells. (A) Amino acid sequences and PKC phosphorylation sites in human and mouse H1 receptors. (B) Ca2+ changes in H1R and H1RS399A expressing HEK cells. The downward DAG+R-GECO sensor (DAG/Ca2+R) was excited at 570 nm for detecting intracellular Ca2+ transients. (C) DAG transients assessed with the downward DAG + R-GECO sensor excited at 480 nm. (D, E) Summary data on the decay time constants for DAG transients. The bold and broken lines represent the averaged traces, and the vertical gray lines are the SEM values. Averaged traces in H1R and H1RS399A expressing HEK cells are shown as solid and broken lines. Data in (E) are presented as mean ± S.E. Asterisk = p < 0.05.
We next compared Mn2+ influx in HEK cells expressing either H1R and TRPC6 or H1RS399A and TRPC6 to assess the effect of increased DAG production on TRPC6 activity. Indeed, we observed a significantly greater Mn2+ influx in the H1R mutant expressing cells, indicating that H1R desensitization results in reduced production of DAG and smaller TRPC6 responses. The summary data are shown in Figure 4B. The percent of fura-2 quenching in H1R and TRPC6 expressing cells was 0.27 ± 0.026, n = 17, while that in H1R mutant cells co-expressing the TRPC6 channel was 0.35 ± 0.025, n = 18. The influx of Mn2+ was significantly increased in cells expressing PKC-phosphorylated deficient H1R mutant (Figure 4, p < 0.05).
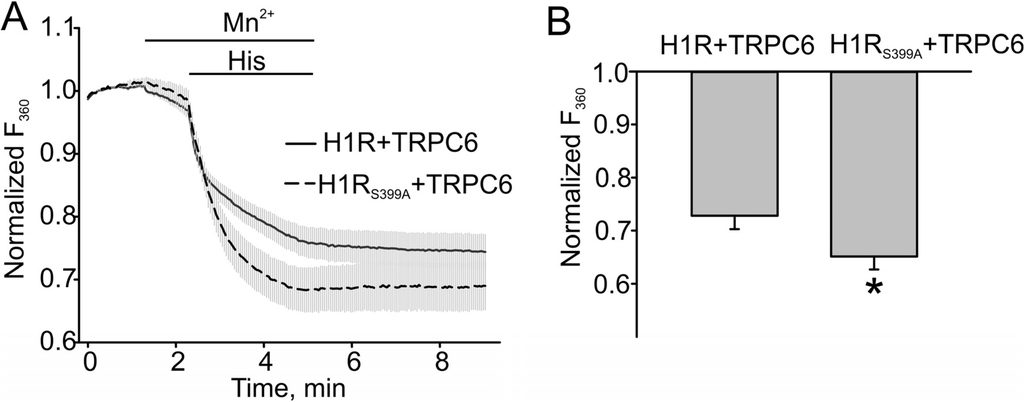
Figure 4.
The H1 receptor mutant potentiated the TRPC6 channel activity in HEK model. (A) Mn2+ (0.5 mM) influx in H1R/TRPC6 expressing HEK cells versus H1RS399A/TRPC6 expressing HEK cells, which were measured by the percent of Fura-2 quenching (Fura-2 was excited at 360 nm) at the time point of 5 min. (B) The summary data comparing the percent of Fura-2 quenching are shown. The bold and broken lines represent the averaged traces, and the vertical gray lines are the SEM values. Data in (B) are presented as mean ± S.E. Asterisk = p < 0.05.
Thus, we investigated the role of H1R desensitization in regulating TRPC6 activity using the pharmacological and molecular tools. PKC inhibitor Gö6983 slowed the decay of DAG and prolonged TRPC6 activity. The mouse H1 receptor mutant, S399A, which is lacking a critical PKC-phosphorylation site, also potentiated Mn2+ influx through TRPC6. However, we also noticed that the effect of Gö6983 was more pronounced on the decay of DAG production and TRPC6 activity as compared to the data obtained with H1RS399A. Comparing with Gö6983, the mutant effect only reached a half of the PKC inhibitor efficacy: Gö6983 prolonged the decay time constant from 48.61 ± 7.07 s to 132.84 ± 23.97 s, while the mutant did from 44.31 ± 6.54 s to 68.78 ± 11.21 s. This indicates that PKC phosphorylation of the TRPC6 channel is also a key step for regulating TRPC6 activity. Indeed, it was shown that TRPC6 ser448 is the substrate of PKC [8]. Therefore, Gö6983 not only inhibited receptor phosphorylation but also channel phosphorylation, resulting in a more powerful effect on TRPC6 activity. Our data also suggest that the termination of DAG signaling may be driven, at least in part, by receptor desensitization in addition to the activation of such DAG regulating enzymes as diacylglycerol kinase.
4. Conclusions
Here, for the first time, we present evidence that PKC activation negatively regulates TRPC6 activity in part due to H1R desensitization. Our data also support the conclusions of other groups [8,9] that PKC phosphorylation of the TRPC6 protein is important for regulating TRPC6 inactivation rate (see graphic abstract). Increased understanding of the molecular mechanisms underlying TRPC6 channel inactivation may be important for developing novel therapeutic approaches for treating TRPC6 associated dysfunctions. This study also demonstrates that the novel dual DAG/Ca2+ and the red fluorescent PIP2 sensors are useful tools for studying PLC dependent pathways in native cells.
Acknowledgments
We thank the members of Obukhov lab for useful discussions and technical assistance and Jean-Charles Schwartz for the histamine H1 receptor cDNA. This work was supported by grants from the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01HL083381 and R01HL115140 to AGO).
Author Contributions
XC, CLE, and WL performed the fluorescence imaging experiments. AMR generated the H1 receptor mutant. XC analyzed the data. PT, TEH and AMQ developed the downward DAG+R-GECO and PIP2 sensors and contributed to manuscript preparation. AGO and XC designed the experiments and prepared the manuscript.
Conflicts of Interest
Paul Tewson, Thomas E. Hughes and Anne Marie Quinn are employed by Montana Molecular (Bozeman, Montana) with funding from the National Institute of Neurologic Disease and Stroke (NINDS) 9R44NS082222-02A1, the National Science Foundation 1248138 and Montana State Matching Funds #13–50 RCSBIR-003.
References
- Loga, F.; Domes, K.; Freichel, M.; Flockerzi, V.; Dietrich, A.; Birnbaumer, L.; Hofmann, F.; Wegener, J.W. The role of cGMP/cGKI signalling and trpc channels in regulation of vascular tone. Cardiovasc. Res. 2013, 100, 280–287. [Google Scholar] [CrossRef]
- Kistler, A.D.; Singh, G.; Altintas, M.M.; Yu, H.; Fernandez, I.C.; Gu, C.; Wilson, C.; Srivastava, S.K.; Dietrich, A.; Walz, K.; et al. Transient receptor potential channel 6 (TRPC6) protects podocytes during complement-mediated glomerular disease. J. Biol. Chem. 2013, 288, 36598–36609. [Google Scholar] [CrossRef]
- Ding, Y.; Winters, A.; Ding, M.; Graham, S.; Akopova, I.; Muallem, S.; Wang, Y.; Hong, J.H.; Gryczynski, Z.; Yang, S.H.; et al. Reactive oxygen species-mediated TRPC6 protein activation in vascular myocytes, a mechanism for vasoconstrictor-regulated vascular tone. J. Biol. Chem. 2011, 286, 31799–31809. [Google Scholar] [CrossRef]
- Yu, Y.; Keller, S.H.; Remillard, C.V.; Safrina, O.; Nicholson, A.; Zhang, S.L.; Jiang, W.; Vangala, N.; Landsberg, J.W.; Wang, J.Y.; et al. A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation 2009, 119, 2313–2322. [Google Scholar] [CrossRef]
- Krall, P.; Canales, C.P.; Kairath, P.; Carmona-Mora, P.; Molina, J.; Carpio, J.D.; Ruiz, P.; Mezzano, S.A.; Li, J.; Wei, C.; et al. Podocyte-specific overexpression of wild type or mutant trpc6 in mice is sufficient to cause glomerular disease. PLoS One 2010, 5, e12859. [Google Scholar] [CrossRef]
- El, B.C.; Bidaux, G.; Enfissi, A.; Delcourt, P.; Prevarskaya, N.; Capiod, T. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology 2008, 47, 2068–2077. [Google Scholar] [CrossRef]
- Hofmann, T.; Obukhov, A.G.; Schaefer, M.; Harteneck, C.; Gudermann, T.; Schultz, G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 1999, 397, 259–263. [Google Scholar] [CrossRef]
- Bousquet, S.M.; Monet, M.; Boulay, G. Protein kinase C-dependent phosphorylation of transient receptor potential canonical 6 (TRPC6) on serine 448 causes channel inhibition. J. Biol. Chem. 2010, 285, 40534–40543. [Google Scholar] [CrossRef]
- Kim, J.Y.; Saffen, D. Activation of M1 muscarinic acetylcholine receptors stimulates the formation of a multiprotein complex centered on TRPC6 channels. J. Biol. Chem. 2005, 280, 32035–32047. [Google Scholar] [CrossRef]
- Miyoshi, K.; Das, A.K.; Fujimoto, K.; Horio, S.; Fukui, H. Recent advances in molecular pharmacology of the histamine systems: Regulation of histamine H1 receptor signaling by changing its expression level. J. Pharmacol. Sci. 2006, 101, 3–6. [Google Scholar] [CrossRef]
- Horio, S.; Kato, T.; Ogawa, M.; Fujimoto, K.; Fukui, H. Two threonine residues and two serine residues in the second and third intracellular loops are both involved in histamine H1 receptor downregulation. FEBS Lett. 2004, 573, 226–230. [Google Scholar] [CrossRef]
- Horio, S.; Ogawa, M.; Kawakami, N.; Fujimoto, K.; Fukui, H. Identification of amino acid residues responsible for agonist-induced down-regulation of histamine H(1) receptors. J. Pharmacol. Sci. 2004, 94, 410–419. [Google Scholar] [CrossRef]
- Kawakami, N.; Miyoshi, K.; Horio, S.; Yoshimura, Y.; Yamauchi, T.; Fukui, H. Direct phosphorylation of histamine H1 receptor by various protein kinases in vitro. Methods Find. Exp. Clin. Pharmacol. 2003, 25, 685–693. [Google Scholar] [CrossRef]
- Liggett, S.B. Phosphorylation barcoding as a mechanism of directing GPCR signaling. Sci. Signal. 2011, 4, e36. [Google Scholar] [CrossRef]
- Tewson, P.; Westenberg, M.; Zhao, Y.; Campbell, R.E.; Quinn, A.M.; Hughes, T.E. Simultaneous detection of Ca2+ and diacylglycerol signaling in living cells. PLoS One 2012, 7, e42791. [Google Scholar]
- Hu, G.; Oboukhova, E.A.; Kumar, S.; Sturek, M.; Obukhov, A.G. Canonical transient receptor potential channels expression is elevated in a porcine model of metabolic syndrome. Mol. Endocrinol. 2009, 23, 689–699. [Google Scholar] [CrossRef]
- Tewson, P.H.; Quinn, A.M.; Hughes, T.E. A multiplexed fluorescent assay for independent second-messenger systems: Decoding GPCR activation in living cells. J. Biomol. Screen. 2013, 18, 797–806. [Google Scholar] [CrossRef]
- Merritt, J.E.; Jacob, R.; Hallam, T.J. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J. Biol. Chem. 1989, 264, 1522–1527. [Google Scholar]
- Fujimoto, K.; Ohta, K.; Kangawa, K.; Kikkawa, U.; Ogino, S.; Fukui, H. Identification of protein kinase C phosphorylation sites involved in phorbol ester-induced desensitization of the histamine H1 receptor. Mol. Pharmacol. 1999, 55, 735–742. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).