A Drosophila Model to Image Phagosome Maturation
Abstract
:Abbreviations
| IMD | immune deficiency |
| Rab | Ras-associated binding proteins |
| GFP | green fluorescent protein |
| Lamp1 | lysosomal-associated membrane protein 1 |
1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Establishing a Model of Phagosome Maturation

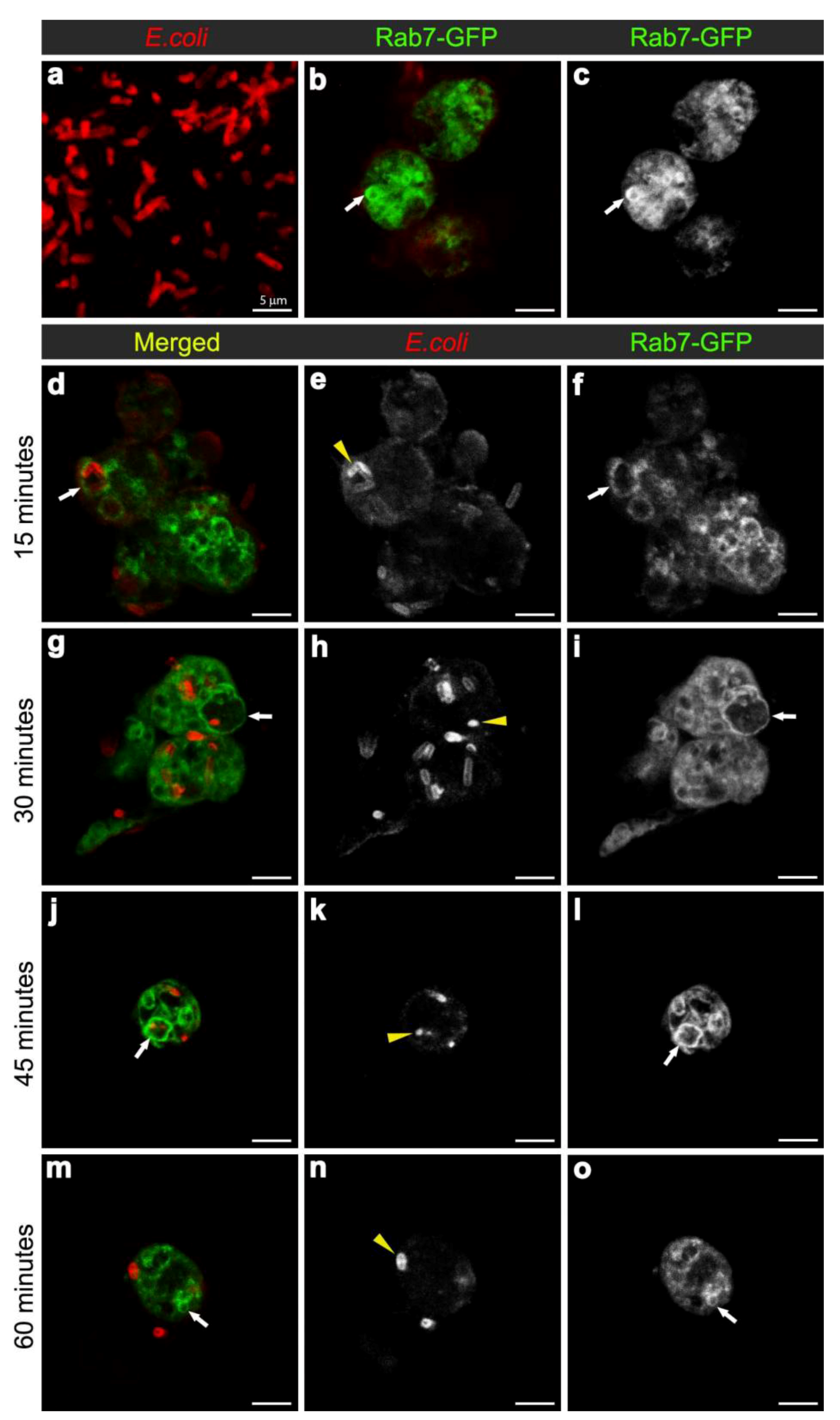
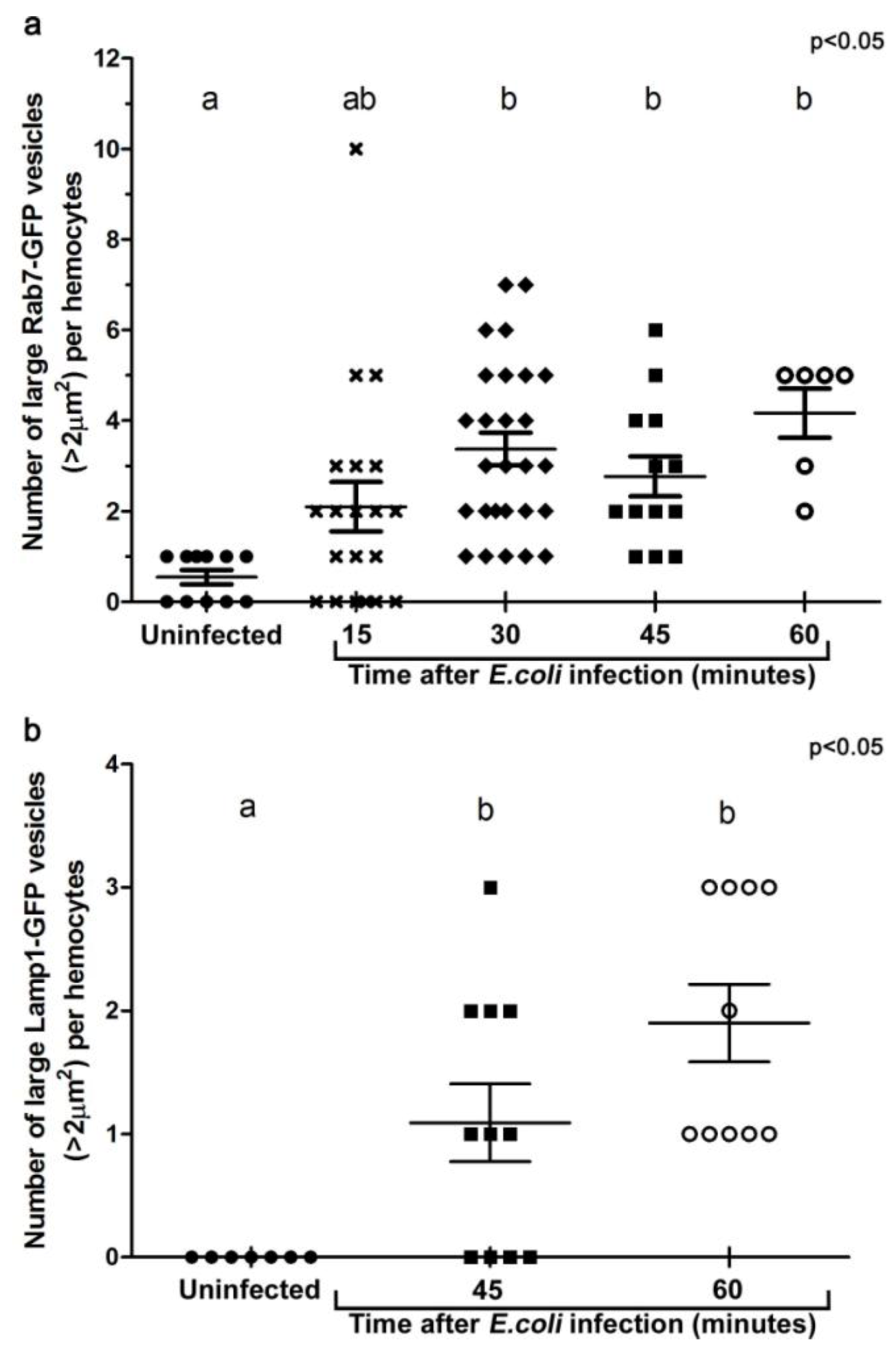
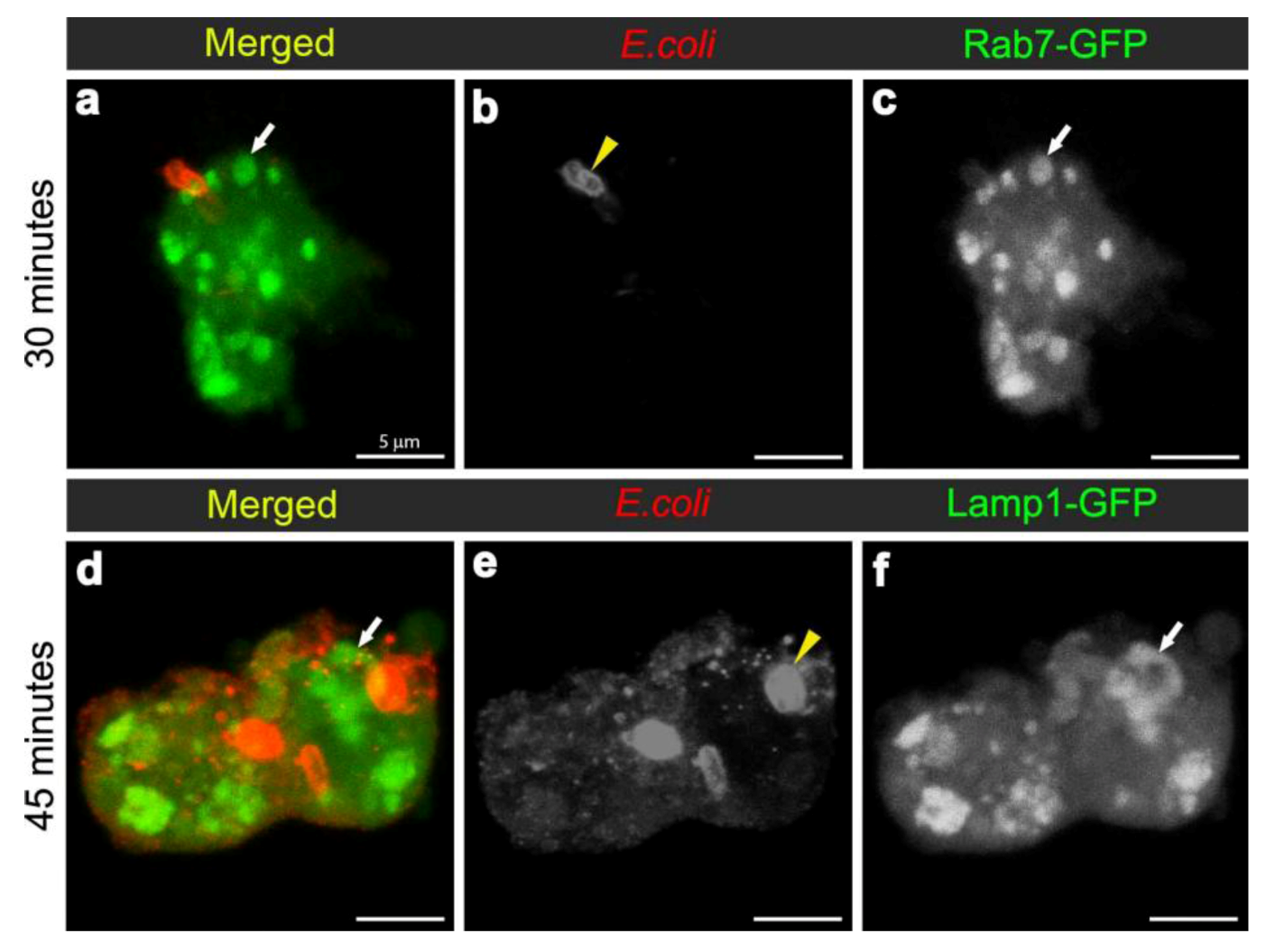
3.2. E. coli was Localised to Rab7-Positive Phagosomes at 15-45 Minutes after Infection
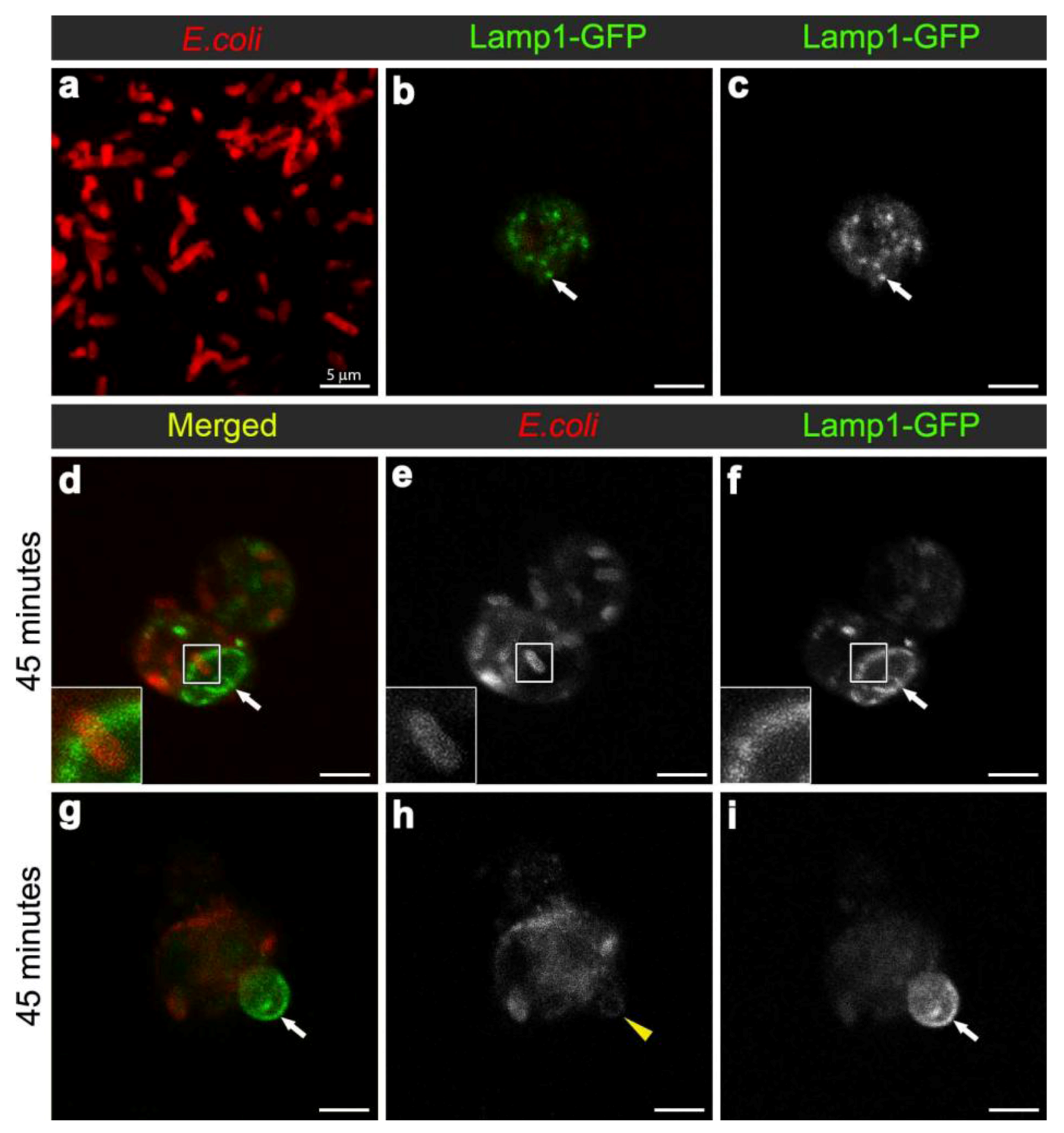
3.3. E. coli was Localised to Lamp1-Positive Phagosomes 45 Minutes after Infection
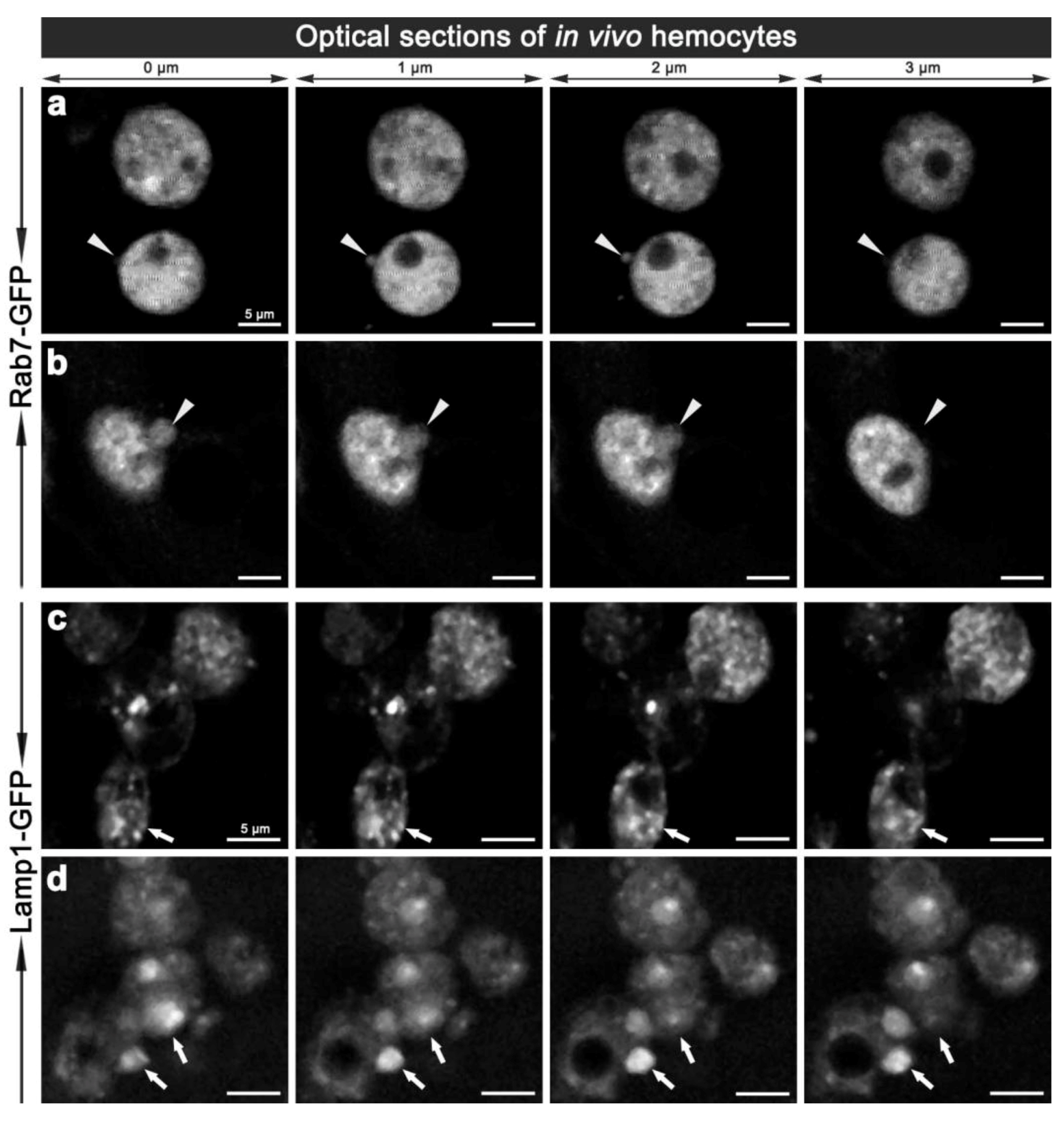
3.4. Towards Dissecting the Genetic Control of Phagosome Maturation
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Mechnikov, I. On the Present State of the Question of Immunity in Infectious Diseases. Scandinavian J. Immunol. 1989, 4, 387–398. [Google Scholar] [CrossRef]
- Desjardins, M. Biogenesis of phagolysosomes: The 'kiss and run' hypothesis. Trends Cell. Biol. 1995, 5, 183–186. [Google Scholar] [CrossRef]
- Desjardins, M.; Nzala, N.N.; Corsini, R.; Rondeau, C. Maturation of phagosomes is accompanied by changes in their fusion properties and size-selective acquisition of solute materials from endosomes. J. Cell. Sci. 1997, 110, 2303–2314. [Google Scholar]
- Savina, A.; Amigorena, S. Phagocytosis and antigen presentation in dendritic cells. Immunol. Rev. 2007, 219, 143–156. [Google Scholar] [CrossRef]
- Braun, A.; Hoffmann, J.A.; Meister, M. Analysis of the Drosophila host defense in domino mutant larvae, which are devoid of hemocytes. Proc. Natl. Acad. Sci. USA 1998, 95, 14337–14342. [Google Scholar]
- Elrod-Erickson, M.; Mishra, S.; Schneider, D. Interactions between the cellular and humoral immune responses in Drosophila. Curr. Biol. 2000, 10, 781–784. [Google Scholar] [CrossRef]
- Fang, J.; Brzostowski, J.A.; Ou, S.; Isik, N.; Nair, V.; Jin, T. A vesicle surface tyrosine kinase regulates phagosome maturation. J. Cell Biol. 2007, 178, 411. [Google Scholar] [CrossRef]
- Vieira, O.V.; Botelho, R.J.; Grinstein, S. Phagosome maturation: Aging gracefully. Biochemical. J. 2002, 366, 689–704. [Google Scholar]
- Kinchen, J.M.; Ravichandran, K.S. Phagosome maturation: Going through the acid test. Nat. Rev. Mol. Cell Biol. 2008, 9, 781–795. [Google Scholar] [CrossRef]
- Rupper, A.; Grove, B.; Cardelli, J. Rab7 regulates phagosome maturation in Dictyostelium. J. Cell Sci. 2001, 114, 2449. [Google Scholar]
- Garin, J.; Diez, R.; Kieffer, S.; Dermine, J.F.; Duclos, S.; Gagnon, E.; Sadoul, R.; Rondeau, C.; Desjardins, M. The phagosome proteome: insight into phagosome functions. J. Cell Sci. 2001, 152, 165. [Google Scholar] [CrossRef]
- Stow, J.L.; Manderson, A.P.; Murray, R.Z. SNAREing immunity: the role of SNAREs in the immune system. Nat. Rev. Immunol. 2006, 6, 919–929. [Google Scholar] [CrossRef]
- Huynh, K.K.; Eskelinen, E.L.; Scott, C.C.; Malevanets, A.; Saftig, P.; Grinstein, S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 2007, 26, 313–324. [Google Scholar] [CrossRef]
- Stuart, L.M.; Ezekowitz, R.A.B. Phagocytosis elegant complexity. Immunity 2005, 22, 539–550. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Ramet, M.; Manfruelli, P.; Pearson, A.; Mathey-Prevot, B.; Ezekowitz, R.A. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 2002, 416, 644–648. [Google Scholar]
- Matskevich, A.A.; Quintin, J.; Ferrandon, D. The Drosophila PRR GNBP3 assembles effector complexes involved in antifungal defenses independently of its Toll-pathway activation function. Eur. J. Immunol. 2010, 40, 1244–1254. [Google Scholar] [CrossRef]
- Kim, T.; Kim, Y. Overview of innate immunity in Drosophila. J. Biochem. Mol. Biol. 2005, 38, 121. [Google Scholar] [CrossRef]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401. [Google Scholar]
- Asha, H.; Nagy, I.; Kovacs, G.; Stetson, D.; Ando, I.; Dearolf, C.R. Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics 2003, 163, 203–215. [Google Scholar]
- Shandala, T.; Woodcock, J.M.; Ng, Y.; Biggs, L.; Skoulakis, E.M.C.; Brooks, D.A.; Lopez, A.F. Drosophila 14–3-3ε has a crucial role in anti-microbial peptide secretion and innate immunity. J. Cell Sci. 2011, 124, 2165–2174. [Google Scholar] [CrossRef]
- Rothstein, E.C.; Nauman, M.; Chesnick, S.; Balaban, R.S. Multi-photon excitation microscopy in intact animals. J. Microsc. 2006, 222, 58–64. [Google Scholar] [CrossRef]
- Agaisse, H.; Burrack, L.S.; Philips, J.A.; Rubin, E.J.; Perrimon, N.; Higgins, D.E. Genome-wide RNAi screen for host factors required for intracellular bacterial infection. Science 2005, 309, 1248–1251. [Google Scholar] [CrossRef]
- Cheng, L.W.; Viala, J.P.; Stuurman, N.; Wiedemann, U.; Vale, R.D.; Portnoy, D.A. Use of RNA interference in Drosophila S2 cells to identify host pathways controlling compartmentalization of an intracellular pathogen. Proc. Natl. Acad. Sci. USA 2005, 102, 13646–13651. [Google Scholar]
- Ayres, J.S.; Schneider, D.S. Genomic dissection of microbial pathogenesis in cultured Drosophila cells. Trends Microbiol. 2006, 14, 101–104. [Google Scholar] [CrossRef]
- Brennan, C.A.; Anderson, K.V. Drosophila: The genetics of innate immune recognition and response. Annu. Rev. Immunol. 2004, 22, 457–483. [Google Scholar] [CrossRef]
- Skarstad, K.; Steen, H.B.; Boye, E. Escherichia coli DNA distributions measured by flow cytometry and compared with theoretical computer simulations. J. Bacteriol. 1985, 163, 661. [Google Scholar]
- Stinchcombe, J.; Bossi, G.; Griffiths, G.M. Linking albinism and immunity: The secrets of secretory lysosomes. Science 2004, 305, 55. [Google Scholar] [CrossRef]
- Griffiths, G.M. Secretory lysosomes--a special mechanism of regulated secretion in haemopoietic cells. Trends Cell Biol. 1996, 6, 329–332. [Google Scholar] [CrossRef]
- Hess, C.; Sadallah, S.; Hefti, A.; Landmann, R.; Schifferli, J.A. Ectosomes released by human neutrophils are specialized functional units. J. Immunol. 1999, 163, 4564–4573. [Google Scholar]
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Chaput, N.; Thery, C. Exosomes: Immune properties and potential clinical implementations. Semin. Immunopathol. 2011, 33, 419–440. [Google Scholar] [CrossRef]
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.; Stoorvogel, W.; Geuze, H.J. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell. Sci. 2000, 113, 3365–3374. [Google Scholar]
- Dobrowolski, R.; De Robertis, E.M. Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles. Nat. Rev. Mol. Cell Biol. 2012, 13, 53–60. [Google Scholar]
- Philips, J.A.; Rubin, E.J.; Perrimon, N. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science 2005, 309, 1251–1253. [Google Scholar] [CrossRef]
- Ulvila, J.; Vanha-aho, L.M.; Kleino, A.; Vaha-Makila, M.; Vuoksio, M.; Eskelinen, S.; Hultmark, D.; Kocks, C.; Hallman, M.; Parikka, M.; Ramet, M. Cofilin regulator 14–3-3zeta is an evolutionarily conserved protein required for phagocytosis and microbial resistance. J. Leukoc Biol. 2011, 89, 649–659. [Google Scholar] [CrossRef]
- Govind, S. Innate immunity in Drosophila: Pathogens and pathways. Insect Sci. 2008, 15, 29–43. [Google Scholar] [CrossRef]
- Stuart, L.M.; Boulais, J.; Charriere, G.M.; Hennessy, E.J.; Brunet, S.; Jutras, I.; Goyette, G.; Rondeau, C.; Letarte, S.; Huang, H.; Ye, P.; Morales, F.; Kocks, C.; Bader, J.S.; Desjardins, M.; Ezekowitz, R.A.B. A systems biology analysis of the Drosophila phagosome. Nature 2007, 445, 95–101. [Google Scholar]
- Gilbert, L.I. Drosophila is an inclusive model for human diseases, growth and development. Mol. Cell. Endocrinol. 2008, 293, 25–31. [Google Scholar] [CrossRef]
Electronic Supplementary Information (ESI)
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shandala, T.; Lim, C.; Sorvina, A.; Brooks, D.A. A Drosophila Model to Image Phagosome Maturation. Cells 2013, 2, 188-201. https://doi.org/10.3390/cells2020188
Shandala T, Lim C, Sorvina A, Brooks DA. A Drosophila Model to Image Phagosome Maturation. Cells. 2013; 2(2):188-201. https://doi.org/10.3390/cells2020188
Chicago/Turabian StyleShandala, Tetyana, Chiaoxin Lim, Alexandra Sorvina, and Douglas A. Brooks. 2013. "A Drosophila Model to Image Phagosome Maturation" Cells 2, no. 2: 188-201. https://doi.org/10.3390/cells2020188
APA StyleShandala, T., Lim, C., Sorvina, A., & Brooks, D. A. (2013). A Drosophila Model to Image Phagosome Maturation. Cells, 2(2), 188-201. https://doi.org/10.3390/cells2020188




