Behavioral Cooperation or Conflict of Human Intestinal Roundworms and Microbiomes: A Bio-Activity Perspective
Abstract
1. Introduction
2. Interaction Between Roundworm and Bacteria (Overlooking the Significance of the Iron Uptake System)
2.1. Some Bacteria Can Kill the Roundworm
2.2. Gram-Positive Bacteria
2.3. Gram-Negative Bacteria
2.4. Effect of Roundworm on Bacterial Life
2.5. Antimicrobial Peptides Secreted by Pathogenic Nematodes Kill Bacteria
2.6. A Brief Attention to the Interaction Between Probiotics and Roundworms
3. Interaction Between Roundworm and Bacteria (Direct Attention Towards the Iron Uptake System)
3.1. Effect of Iron Uptake System in Bacteria and Microbiome
3.2. Effects of the Iron Uptake System on Nematodes and the Microbiome
3.3. Iron Uptake System and Nematodes Compete with Bacteria
3.3.1. Secretion of Iron-Chelating Compounds
3.3.2. Physical Disruption of Bacterial Biofilms
3.3.3. Predation on Bacteria
3.3.4. Induction of Host Immune Response
3.3.5. Nutritional Symbiosis with Bacteria
3.3.6. Exploitation of Bacterial Iron Acquisition Systems (BIAS)
3.3.7. Competition for Host Resources
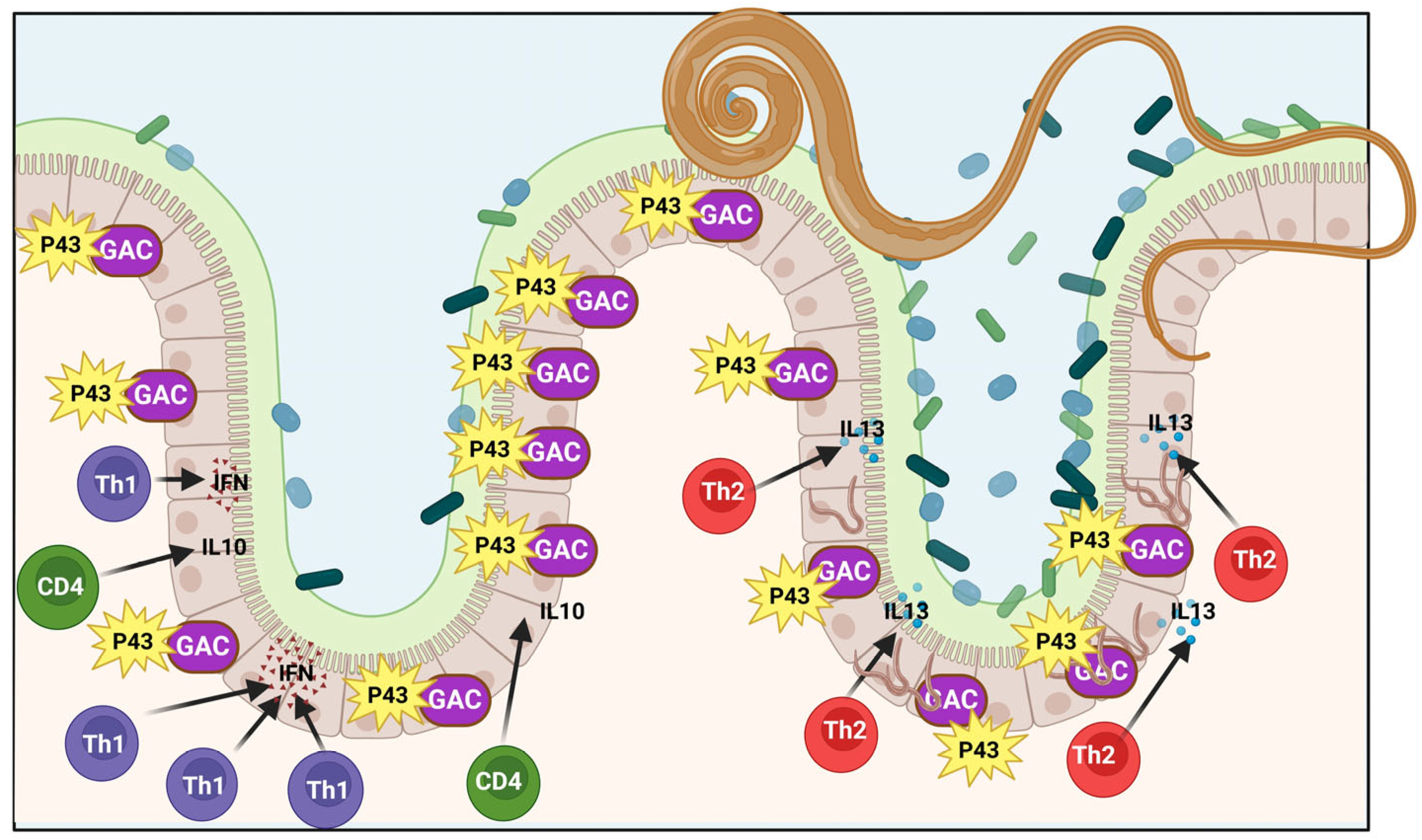
3.4. The P43 Protein and Killing Bacteria Role in High Levels of Iron
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPNs | Entomopathogenic nematodes |
| IUS | Iron uptake system |
| GI | Gastrointestinal tract |
| LD | Linear dichroism |
| PO | Pellicula Ovi |
| PBM | Permeability barrier membrane |
| EdPC | Electron-dense parietal coating |
| ESP | Excretory/secretory products |
| BF | Body fluid |
| ASABF | A. suum antibacterial factor |
| ROS | Reactive oxygen species |
| FAC | Ferric ammonium citrate |
| mtROS | Mitochondrial reactive oxygen species |
| SOD | Superoxide dismutase |
| NOX | tissue-specific NADPH oxidase |
| BIAS | bacterial iron acquisition systems |
| ESRE | Ethanol and Stress Response Element |
| IL-13 | Interleukin-13 |
| GAC | Glycosaminoglycans |
References
- Bhat, A.H.; Malik, I.M.; Tak, H.; Ganai, B.A.; Bharti, P. Host, parasite, and microbiome interaction: Trichuris ovis and its effect on sheep gut microbiota. Vet. Parasitol. 2025, 333, 110356. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.S.; Ozawa, S.; Hasegawa, K. Composition of the Cockroach Gut Microbiome in the Presence of Parasitic Nematodes. Microbes Environ. 2016, 31, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Midha, A.; Schlosser, J.; Hartmann, S. Reciprocal Interactions between Nematodes and Their Microbial Environments. Front. Cell. Infect. Microbiol. 2017, 7, 144. [Google Scholar] [CrossRef]
- Yilmaz, L.S.; Walhout, A.J. Worms, bacteria, and micronutrients: An elegant model of our diet. Trends Genet. 2014, 30, 496–503. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, D.; Lei, Y.; Lozano-Torres, J.L.; Deng, Y.; Xu, J.; Hu, L. Cover crop rotation suppresses root-knot nematode infection by shaping soil microbiota. New Phytol. 2025, 245, 363–377. [Google Scholar] [CrossRef]
- Grondin, J.A.; Jamal, A.; Mowna, S.; Seto, T.; Khan, W.I. Interaction between Intestinal Parasites and the Gut Microbiota: Implications for the Intestinal Immune Response and Host Defence. Pathogens 2024, 13, 608. [Google Scholar] [CrossRef]
- Shears, R.K.; Grencis, R.K. Whipworm secretions and their roles in host-parasite interactions. Parasites Vectors 2022, 15, 348. [Google Scholar] [CrossRef]
- Mate, L.; Alvarez, L.I.; Lloberas, M.; Imperiale, F.; Lanusse, C.E.; Liron, J.P. Interaction between bacterial microbiota and nematode parasite communities in sheep’s gastrointestinal tract. PLoS ONE 2024, 19, e0306390. [Google Scholar] [CrossRef]
- Kennedy, M.W.; Bancroft, A.J.; Grencis, R.K. The immunomodulatory p43 secreted protein of Trichuris whipworm parasites is a lipid carrier that binds signalling lipids and precursors. bioRxiv 2025. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, Y.; Wang, Z.; Ma, R.; Jin, H.; Zhang, J.; Liu, F.; Ding, Y. Causal relationship between gut microbiota and ageing: A multi-omics Mendelian randomization study. Arch. Gerontol. Geriatr. 2025, 131, 105765. [Google Scholar] [CrossRef]
- Will, I.; Stevens, E.J.; Belcher, T.; King, K.C. ’Re-Wilding’ an Animal Model With Microbiota Shifts Immunity and Stress Gene Expression During Infection. Mol. Ecol. 2025, 34, e17586. [Google Scholar] [CrossRef] [PubMed]
- Midha, A.; Jarquín-Díaz, V.H.; Ebner, F.; Hayani, R.; Kundik, A.; Löber, U.; Cardilli, A.; Heitlinger, E.; Forslund, S.K.; Hartmann, S. Guts within Guts: The Microbiome of the Intestinal Helminth Parasite Ascaris suum Is Derived but Distinct from Its Host. Microbiome 2022, 10, 229. [Google Scholar]
- Harnett, W.; Harnett, M.M. Epigenetic changes induced by parasitic worms and their excretory-secretory products. Biochem. Soc. Trans. 2024, 52, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, A.J.; Grencis, R.K. Immunoregulatory molecules secreted by Trichuris muris. Parasitology 2021, 148, 1757–1763. [Google Scholar] [CrossRef]
- Tahmasebi, H.; Dehbashi, S.; Nasaj, M.; Arabestani, M.R. Molecular epidemiology and collaboration of siderophore-based iron acquisition with surface adhesion in hypervirulent Pseudomonas aeruginosa isolates from wound infections. Sci. Rep. 2022, 12, 7791. [Google Scholar] [CrossRef]
- Zahedani, S.S.; Tahmasebi, H.; Jahantigh, M. Coexistence of Virulence Factors and Efflux Pump Genes in Clinical Isolates of Pseudomonas aeruginosa: Analysis of Biofilm-Forming Strains from Iran. Int. J. Microbiol. 2021, 2021, 5557361. [Google Scholar] [CrossRef]
- Heydari, N.; Alikhani, M.Y.; Tahmasebi, H.; Asghari, B.; Arabestani, M.R. Design of Melting Curve Analysis (MCA) by Real-Time Polymerase Chain Reaction Assay for Rapid Distinction of Staphylococci and Antibiotic Resistance. Arch. Clin. Infect. Dis. 2019, 14, e81604. [Google Scholar] [CrossRef]
- Papaiakovou, M.; Littlewood, D.T.J.; Doyle, S.R.; Gasser, R.B.; Cantacessi, C. Worms and bugs of the gut: The search for diagnostic signatures using barcoding, and metagenomics-metabolomics. Parasites Vectors 2022, 15, 118. [Google Scholar] [CrossRef]
- Porbaran, M.; Tahmasebi, H.; Arabestani, M. A Comprehensive Study of the Relationship between the Production of β-Lactamase Enzymes and Iron/Siderophore Uptake Regulatory Genes in Clinical Isolates of Acinetobacter baumannii. Int. J. Microbiol. 2021, 2021, 5565537. [Google Scholar] [CrossRef]
- Hu, B.; Yue, K.; Zhang, D.; Feng, S.; Zhao, N.; Li, G.; Gao, S.; Xing, Y.; Han, S.; He, H. Association between Capillaria hepatica infection-induced alterations in gut microbiota and estrogen expression in Brandt’s voles (Lasiopodomys brandtii). BMC Vet. Res. 2025, 21, 126. [Google Scholar] [CrossRef]
- Tahmasebi, H.; Dehbashi, S.; Arabestani, M.R. Antibiotic resistance alters through iron-regulating Sigma factors during the interaction of Staphylococcus aureus and Pseudomonas aeruginosa. Sci. Rep. 2021, 11, 18509. [Google Scholar] [CrossRef] [PubMed]
- Cabreiro, F.; Gems, D. Worms need microbes too: Microbiota, health and aging in Caenorhabditis elegans. EMBO Mol. Med. 2013, 5, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.; Pedrinho, A.; Mendes, L.W.; Freitas, C.C.G.; Andreote, F.D. Interactions between Soil Bacterial Diversity and Plant-Parasitic Nematodes in Soybean Plants. Appl. Environ. Microbiol. 2022, 88, e0096322. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.R.; Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Siderophores in Iron Metabolism: From Mechanism to Therapy Potential. Trends Mol. Med. 2016, 22, 1077–1090. [Google Scholar] [CrossRef]
- Deng, X.; Li, S.; Wu, Y.; Yao, J.; Hou, W.; Zheng, J.; Liang, B.; Liang, X.; Hu, Q.; Wu, Z.; et al. Correlation analysis of the impact of Clonorchis sinensis juvenile on gut microbiota and transcriptome in mice. Microbiol. Spectr. 2025, 13, e0155024. [Google Scholar] [CrossRef]
- Nguélé, A.T.; Mozzicafreddo, M.; Chen, H.; Piersanti, A.; Salum, S.S.; Ali, S.M.; Zhang, J.; Miceli, C. Trichuris trichiura infection is associated with changes in gut microbiome composition and function among women of reproductive age from Pemba, Tanzania. Front. Trop. Dis. 2024, 5, 1276210. [Google Scholar] [CrossRef]
- Rosa, B.A.; Snowden, C.; Martin, J.; Fischer, K.; Kupritz, J.; Beshah, E.; Supali, T.; Gankpala, L.; Fischer, P.U.; Urban, J.F., Jr.; et al. Whipworm-Associated Intestinal Microbiome Members Consistent Across Both Human and Mouse Hosts. Front. Cell. Infect. Microbiol. 2021, 11, 637570. [Google Scholar] [CrossRef]
- Irazoqui, J.; Ausubel, F. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Caenorhabditis elegans as a model to study tissues involved in host immunity and microbial pathogenesis. Clin. Exp. Immunol. 2010, 160, 48–57. [Google Scholar] [CrossRef]
- Grad, Y.; Aach, J.; Hayes, G.D.; Reinhart, B.J.; Church, G.M.; Ruvkun, G.; Kim, J. Computational and experimental identification of C. elegans microRNAs. Mol. Cell 2003, 11, 1253–1263. [Google Scholar] [CrossRef]
- Aballay, A.; Yorgey, P.; Ausubel, F.M. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr. Biol. 2000, 10, 1539–1542. [Google Scholar] [CrossRef]
- Garsin, D.A.; Sifri, C.D.; Mylonakis, E.; Qin, X.; Singh, K.V.; Murray, B.E.; Calderwood, S.B.; Ausubel, F.M. A simple model host for identifying Gram-positive virulence factors. Proc. Natl. Acad. Sci. USA 2001, 98, 10892–10897. [Google Scholar] [PubMed]
- Jansen, W.; Bolm, M.; Balling, R.; Chhatwal, G.; Schnabel, R. Hydrogen peroxide-mediated killing of Caenorhabditis elegans by Streptococcus pyogenes. Infect. Immun. 2002, 70, 5202–5207. [Google Scholar] [PubMed]
- Labrousse, A.; Chauvet, S.; Couillault, C.; Kurz, C.L.; Ewbank, J.J. Caenorhabditis elegans is a model host for Salmonella typhimurium. Curr. Biol. 2000, 10, 1543–1545. [Google Scholar]
- Sifri, C.D.; Begun, J.; Ausubel, F.M.; Calderwood, S.B. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect. Immun. 2003, 71, 2208–2217. [Google Scholar] [CrossRef]
- Sifri, C.D.; Mylonakis, E.; Singh, K.V.; Qin, X.; Garsin, D.A.; Murray, B.E.; Ausubel, F.M.; Calderwood, S.B. Virulence effect of Enterococcus faecalis protease genes and the quorum-sensing locus fsr in Caenorhabditis elegans and mice. Infect. Immun. 2002, 70, 5647–5650. [Google Scholar]
- Zhou, X.; Liang, L.; Sun, B.; Li, K.; Guo, H.; Zhang, Y. The Effects of Yeast Protein on Gut Microbiota in Mice When Compared with Soybean Protein and Whey Protein Isolates. Nutrients 2024, 16, 458. [Google Scholar] [CrossRef]
- Schiffer, J.A.; Stumbur, S.V.; Seyedolmohadesin, M.; Xu, Y.; Serkin, W.T.; McGowan, N.G.; Banjo, O.; Torkashvand, M.; Lin, A.; Hosea, C.N.; et al. Modulation of sensory perception by hydrogen peroxide enables Caenorhabditis elegans to find a niche that provides both food and protection from hydrogen peroxide. PLoS Pathog. 2021, 17, e1010112. [Google Scholar] [CrossRef]
- Servello, F.A.; Fernandes, R.; Eder, M.; Harris, N.; Martin, O.M.F.; Oswal, N.; Lindberg, A.; Derosiers, N.; Sengupta, P.; Stroustrup, N.; et al. Neuronal temperature perception induces specific defenses that enable C. elegans to cope with the enhanced reactivity of hydrogen peroxide at high temperature. eLife 2022, 11, 78941. [Google Scholar] [CrossRef]
- Romanelli-Cedrez, L.; Vairoletti, F.; Salinas, G. Rhodoquinone-dependent electron transport chain is essential for Caenorhabditis elegans survival in hydrogen sulfide environments. J. Biol. Chem. 2024, 300, 107708. [Google Scholar] [CrossRef]
- Gabaldon, C.; Karakuzu, O.; Garsin, D.A. SKN-1 activation during infection of Caenorhabditis elegans requires CDC-48 and endoplasmic reticulum proteostasis. Genetics 2024, 228, iyae131. [Google Scholar] [CrossRef]
- Tahmasebi, H.; Dehbashi, S.; Jahantigh, M.; Arabestani, M.R. Relationship between biofilm gene expression with antimicrobial resistance pattern and clinical specimen type based on sequence types (STs) of methicillin-resistant S. aureus. Mol. Biol. Rep. 2020, 47, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Bae, T.; Banger, A.K.; Wallace, A.; Glass, E.M.; Åslund, F.; Schneewind, O.; Missiakas, D.M. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc. Natl. Acad. Sci. USA 2004, 101, 12312–12317. [Google Scholar] [PubMed]
- Tan, M.-W.; Mahajan-Miklos, S.; Ausubel, F.M. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 715–720. [Google Scholar] [PubMed]
- Xiao, Y.; Li, L.; Han, C.; Huang, T.; Ren, S.; Wang, X.; Xing, Q.; Liu, F. Chlorogenic acid inhibits Pseudomonas toxin pyocyanin and activates mitochondrial UPR to protect host against pathogen infection. Sci. Rep. 2025, 15, 5508. [Google Scholar] [CrossRef]
- Goulding, D.; Tolley, C.; Mkandawire, T.T.; Doyle, S.R.; Hart, E.; Airs, P.M.; Grencis, R.K.; Berriman, M.; Duque-Correa, M.A. Hatching of whipworm eggs induced by bacterial contact is serine-protease dependent. PLoS Pathog. 2025, 21, e1012502. [Google Scholar] [CrossRef]
- Filipowicz, A.; Allard, P. Caenorhabditis elegans as a Model for Environmental Epigenetics. Curr. Environ. Health Rep. 2025, 12, 6. [Google Scholar] [CrossRef]
- Robertson, A.; Sall, J.; Venzon, M.; Olivas, J.J.; Zheng, X.; Cammer, M.; Antao, N.; Zhou, C.; Devlin, J.C.; Saes Thur, R.; et al. Bacterial contact induces polar plug disintegration to mediate whipworm egg hatching. PLoS Pathog. 2023, 19, e1011647. [Google Scholar] [CrossRef]
- Vejzagić, N.; Adelfio, R.; Keiser, J.; Kringel, H.; Thamsborg, S.M.; Kapel, C.M.O. Bacteria-induced egg hatching differs for Trichuris muris and Trichuris suis. Parasites Vectors 2015, 8, 371. [Google Scholar]
- Vassallo, B.G.; Scheidel, N.; Fischer, S.E.J.; Kim, D.H. Bacteria are a major determinant of Orsay virus transmission and infection in Caenorhabditis elegans. eLife 2024, 12, 92534. [Google Scholar] [CrossRef]
- Kopper, J.J.; Theis, K.R.; Barbu, N.I.; Patterson, J.S.; Bell, J.A.; Gettings, J.R.; Mansfield, L.S. Comparison of Effects of Trichuris muris and Spontaneous colitis on the Proximal Colon Microbiota in C3H/HeJ and C3Bir IL10−/− Mice. Comp. Med. 2021, 71, 46–65. [Google Scholar] [CrossRef]
- Houlden, A.; Hayes, K.S.; Bancroft, A.J.; Worthington, J.J.; Wang, P.; Grencis, R.K.; Roberts, I.S. Chronic Trichuris muris Infection in C57BL/6 Mice Causes Significant Changes in Host Microbiota and Metabolome: Effects Reversed by Pathogen Clearance. PLoS ONE 2015, 10, e0125945. [Google Scholar] [CrossRef]
- Braga, B.V.; Lima, L.R.; Belem, L.F.; Oliveira, D.A.; de Miranda, K.R.; Lopes-Torres, E.J. Structural insights into Trichuris muris eggs through 3D modeling, Cryo-SEM, and TEM of samples prepared with HPF-FS. Exp. Parasitol. 2025, 271, 108924. [Google Scholar] [CrossRef] [PubMed]
- Scharer, A.; Biendl, S.; Keiser, J. Trichuris muris egg-hatching assay for anthelminthic drug discovery and characterization. Int. J. Parasitol. Drugs Drug Resist. 2023, 23, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.B.; Sorobetea, D.; Kiilerich, P.; Ramayo-Caldas, Y.; Estelle, J.; Ma, T.; Madsen, L.; Kristiansen, K.; Svensson-Frej, M. Chronic Trichuris muris Infection Decreases Diversity of the Intestinal Microbiota and Concomitantly Increases the Abundance of Lactobacilli. PLoS ONE 2015, 10, e0125495. [Google Scholar] [CrossRef]
- Schytz Andersen-Civil, A.I.; Arora, P.; Zhu, L.; Myhill, L.J.; Budeyri Gokgoz, N.; Castro-Mejia, J.L.; Leppa, M.M.; Hansen, L.H.; Lessard-Lord, J.; Salminen, J.P.; et al. Gut microbiota-mediated polyphenol metabolism is restrained by parasitic whipworm infection and associated with altered immune function in mice. Gut Microbes 2024, 16, 2370917. [Google Scholar] [CrossRef]
- Lawson, M.A.E.; Roberts, I.S.; Grencis, R.K. The interplay between Trichuris and the microbiota. Parasitology 2021, 148, 1806–1813. [Google Scholar] [CrossRef]
- Beyhan, Y.E.; Yildiz, M.R. Microbiota and parasite relationship. Diagn. Microbiol. Infect. Dis. 2023, 106, 115954. [Google Scholar] [CrossRef]
- Hoang, D.; Flanagan, K.; Ding, Q.; Cazeault, N.R.; Li, H.; Diaz-Valerio, S.; Rus, F.; Darfour, E.A.; Kass, E.; Petersson, K.H.; et al. Bacillus thuringiensis Cry14A family proteins as novel anthelmintics against gastrointestinal nematode parasites. PLoS Negl. Trop. Dis. 2024, 18, e0012611. [Google Scholar] [CrossRef]
- Castañeda, S.; Adeniyi-Ipadeola, G.; Wu, Y.; Suarez-Reyes, C.; Jain, A.; Ramírez, J.D.; Weatherhead, J.E. Characterizing Excretory-Secretory Products Proteome Across Larval Development Stages in Ascaris suum. bioRxiv 2024. [Google Scholar] [CrossRef]
- de Almeida Lopes, C.; Wang, J.; Liffner, B.; Absalon, S.; Gazzinelli-Guimaraes, P.H. Ascaris Mouse Model Protocols: Advancing Research on Larval Ascariasis Biology. Curr. Protoc. 2024, 4, e1074. [Google Scholar] [CrossRef]
- Kato, Y. Humoral defense of the nematode Ascaris suum: Antibacterial, bacteriolytic and agglutinating activities in the body fluid. Zool. Sci. 1995, 12, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, S.; Poveda, C.; Suarez-Reyes, C.; Wu, Y.; Haugen, N.; Patiño, L.H.; Weatherhead, J.E.; Ramírez, J.D. Microbiota dynamics during Ascaris suum larval migration: Implications for host microbial communities in a murine model. Microb. Pathog. 2025, 198, 107122. [Google Scholar] [CrossRef] [PubMed]
- Kam, A.; Loo, S.; Qiu, Y.; Liu, C.-F.; Tam, J.P. Ultrafast Biomimetic Oxidative Folding of Cysteine-rich Peptides and Microproteins in Organic Solvents. Angew. Chem. Int. Ed. 2024, 63, e202317789. [Google Scholar] [CrossRef]
- Midha, A.; Ebner, F.; Schlosser-Brandenburg, J.; Rausch, S.; Hartmann, S. Trilateral Relationship: Ascaris, Microbiota, and Host Cells. Trends Parasitol. 2021, 37, 251–262. [Google Scholar] [CrossRef]
- Peng, H.; Bai, H.; Pan, Y.; Li, J.; Pei, Z.; Liao, Y.; Wu, C.; Li, C.; Tao, L.; Zhong, S.; et al. Immunological pathogenesis of Bovine E. coli infection in a model of C. elegans. BMC Microbiol. 2022, 22, 311. [Google Scholar] [CrossRef]
- Midha, A.; Janek, K.; Niewienda, A.; Henklein, P.; Guenther, S.; Serra, D.O.; Schlosser, J.; Hengge, R.; Hartmann, S. The intestinal roundworm Ascaris suum releases antimicrobial factors which interfere with bacterial growth and biofilm formation. Front. Cell. Infect. Microbiol. 2018, 8, 271. [Google Scholar]
- Ciccarelli, E.J.; Bendelstein, M.; Yamamoto, K.K.; Reich, H.; Savage-Dunn, C. BMP signaling to pharyngeal muscle in the C. elegans response to a bacterial pathogen regulates anti-microbial peptide expression and pharyngeal pumping. Mol. Biol. Cell 2024, 35, ar52. [Google Scholar] [CrossRef]
- Goyache, I.; Yavorov-Dayliev, D.; Milagro, F.I.; Aranaz, P. Caenorhabditis elegans as a Screening Model for Probiotics with Properties against Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 1321. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Nutraceuticals Prepared with Specific Strains of Probiotics for Supplementing Gut Microbiota in Hosts Allergic to Certain Foods or Their Additives. Nutrients 2023, 15, 2979. [Google Scholar] [CrossRef]
- Zeyni, B.; Arabestani, M.R.; Yousefi Mashuf, R.; Tahmasebi, H. Evaluation of real-time PCR-based DNA melting method for detection of enterococcus faecalis and Enterococcus faecium in clinical isolates. J. Babol Univ. Med. Sci. 2017, 19, 26–33. [Google Scholar] [CrossRef]
- Loke, P.n.; Harris, N.L. Networking between helminths, microbes, and mammals. Cell Host Microbe 2023, 31, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Olmedo, M.; Holdorf, A.D.; Shang, Y.; Artal-Sanz, M.; Yilmaz, L.S.; Walhout, A.J.M. A Delicate Balance between Bacterial Iron and Reactive Oxygen Species Supports Optimal C elegans Development. Cell Host Microbe 2019, 26, 400–411.e403. [Google Scholar] [CrossRef] [PubMed]
- Klebba, P.E.; Newton, S.M.C.; Six, D.A.; Kumar, A.; Yang, T.; Nairn, B.L.; Munger, C.; Chakravorty, S. Iron Acquisition Systems of Gram-negative Bacterial Pathogens Define TonB-Dependent Pathways to Novel Antibiotics. Chem. Rev. 2021, 121, 5193–5239. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ma, Y.; Chua, S.L. Bacterivorous nematodes decipher microbial iron siderophores as prey cue in predator–prey interactions. Proc. Natl. Acad. Sci. USA 2024, 121, e2314077121. [Google Scholar] [CrossRef]
- Page, M.G.P. The Role of Iron and Siderophores in Infection, and the Development of Siderophore Antibiotics. Clin. Infect. Dis. 2019, 69, S529–S537. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-Based Iron Acquisition and Pathogen Control. Microbiol. Mol. Biol. Rev. 2007, 71, 413. [Google Scholar] [CrossRef]
- Longshaw, C.; Manissero, D.; Tsuji, M.; Echols, R.; Yamano, Y. In vitro activity of the siderophore cephalosporin, cefiderocol, against molecularly characterized, carbapenem-non-susceptible Gram-negative bacteria from Europe. JAC Antimicrob. Resist. 2020, 2, dlaa060. [Google Scholar] [CrossRef]
- Chen, T.; Dong, G.; Zhang, S.; Zhang, X.; Zhao, Y.; Cao, J.; Zhou, T.; Wu, Q. Effects of iron on the growth, biofilm formation and virulence of Klebsiella pneumoniae causing liver abscess. BMC Microbiol. 2020, 20, 36. [Google Scholar] [CrossRef]
- Vasantha-Srinivasan, P.; Park, K.B.; Kim, K.Y.; Jung, W.-J.; Han, Y.S. The role of Bacillus species in the management of plant-parasitic nematodes. Front. Microbiol. 2025, 15, 1510036. [Google Scholar] [CrossRef]
- Semprucci, F.; Grassi, E.; Cocozza di Montanara, A.; Sandulli, R.; Baldrighi, E. Emerging Marine Nematodes as Model Organisms: Which Species for Which Question? Diversity 2025, 17, 59. [Google Scholar] [CrossRef]
- Yadav, S.P.; Sharma, C.; Pathak, P.; Kanaujia, A.; Saxena, M.J.; Kalra, A. Management of phyto-parasitic nematodes using bacteria and fungi and their consortia as biocontrol agents. Environ. Sci. Adv. 2025, 4, 335–354. [Google Scholar] [CrossRef]
- Zhang, L.; Gade, V.; Kirienko, N.V. Pathogen-induced dormancy in liquid limits gastrointestinal colonization of Caenorhabditis elegans. Virulence 2023, 14, 2204004. [Google Scholar] [CrossRef] [PubMed]
- Sewell, A.K.; Cui, M.; Zhu, M.; Host, M.R.; Han, M. Enterobactin carries iron into Caenorhabditis elegans and mammalian intestinal cells by a mechanism independent of divalent metal transporter DMT1. J. Biol. Chem. 2025, 301, 108158. [Google Scholar] [CrossRef]
- Hajdu, G.; Szathmari, C.; Soti, C. Modeling Host-Pathogen Interactions in C. elegans: Lessons Learned from Pseudomonas aeruginosa Infection. Int. J. Mol. Sci. 2024, 25, 7034. [Google Scholar] [CrossRef]
- Das, P.; Ravi; Singh, J. Iron-deplete diet enhances Caenorhabditis elegans lifespan via oxidative stress response pathways. bioRxiv 2025. [Google Scholar] [CrossRef]
- Chan, S.Y.; Liu, S.Y.; Seng, Z.; Chua, S.L. Biofilm matrix disrupts nematode motility and predatory behavior. ISME J. 2021, 15, 260–269. [Google Scholar]
- Jeong, G.-J.; Khan, F.; Khan, S.; Tabassum, N.; Mehta, S.; Kim, Y.-M. Pseudomonas aeruginosa virulence attenuation by inhibiting siderophore functions. Appl. Microbiol. Biotechnol. 2023, 107, 1019–1038. [Google Scholar]
- Jahantigh, M.; Tahmasebi, H. Correlation between efflux pumps activity and frequency of pslABD genes in clinical isolates of Pseudomonas aeruginosa collected from diabetic foot infections. J. Knowl. Health Basic. Med. Sci. 2024, 19, 22–40. [Google Scholar] [CrossRef]
- Proença, D.N.; Heine, T.; Senges, C.H.; Bandow, J.E.; Morais, P.V.; Tischler, D. Bacterial metabolites produced under iron limitation kill pinewood nematode and attract Caenorhabditis elegans. Front. Microbiol. 2019, 10, 2166. [Google Scholar]
- de Oliveira, D.A.; Oliveira, R.; Braga, B.V.; Straker, L.C.; Rodrigues, L.S.; Bueno, L.L.; Fujiwara, R.T.; Lopes-Torres, E.J. Experimental trichuriasis: Changes in the immune response and bacterial translocation during acute phase development illustrated with 3D model animation. PLoS Negl. Trop. Dis. 2025, 19, e0012841. [Google Scholar] [CrossRef]
- Corrêa, P.S.; Fernandes, M.A.; Jimenez, C.R.; Mendes, L.W.; Lima, P.d.M.T.; Abdalla, A.L.; Louvandini, H. Interaction between methanotrophy and gastrointestinal nematodes infection on the rumen microbiome of lambs. FEMS Microbiol. Ecol. 2024, 100, fiae083. [Google Scholar] [CrossRef]
- Mirza, Z.; Walhout, A.J.M.; Ambros, V. A bacterial pathogen induces developmental slowing by high reactive oxygen species and mitochondrial dysfunction in Caenorhabditis elegans. Cell Rep. 2023, 42, 113189. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, C.; Zhang, K.; Li, K.; Xie, J.; Peng, Y.; Quan, M.; Sun, Y.; Hu, Y.; Xia, L.; et al. Function and Global Regulation of Type III Secretion System and Flagella in Entomopathogenic Nematode Symbiotic Bacteria. Int. J. Mol. Sci. 2024, 25, 7579. [Google Scholar] [CrossRef] [PubMed]
- Habteweld, A.; Kantor, M.; Kantor, C.; Handoo, Z. Understanding the dynamic interactions of root-knot nematodes and their host: Role of plant growth promoting bacteria and abiotic factors. Front. Plant Sci. 2024, 15, 1377453. [Google Scholar] [CrossRef]
- Sheehy, L.; Cutler, J.; Weedall, G.D.; Rae, R. Microbiome Analysis of Malacopathogenic Nematodes Suggests No Evidence of a Single Bacterial Symbiont Responsible for Gastropod Mortality. Front. Immunol. 2022, 13, 878783. [Google Scholar] [CrossRef]
- Llinás-Caballero, K.; Caraballo, L. Helminths and bacterial microbiota: The interactions of two of humans’ “old friends”. Int. J. Mol. Sci. 2022, 23, 13358. [Google Scholar] [CrossRef]
- Buret, A.G.; Motta, J.-P.; Allain, T.; Ferraz, J.; Wallace, J.L. Pathobiont release from dysbiotic gut microbiota biofilms in intestinal inflammatory diseases: A role for iron? J. Biomed. Sci. 2019, 26, 1. [Google Scholar]
- Rosa, B.A.; Supali, T.; Gankpala, L.; Djuardi, Y.; Sartono, E.; Zhou, Y.; Liu, Y.; Fischer, K.; Martin, J.; Tyagi, R.; et al. Differential human gut microbiome assemblages during soil-transmitted helminth infections in Indonesia and Liberia. Microbiome 2018, 6, 33. [Google Scholar]
- Ayaz, M.; Zhao, J.-T.; Zhao, W.; Chi, Y.-K.; Ali, Q.; Ali, F.; Khan, A.R.; Yu, Q.; Yu, J.-W.; Wu, W.-C.; et al. Biocontrol of plant parasitic nematodes by bacteria and fungi: A multi-omics approach for the exploration of novel nematicides in sustainable agriculture. Front. Microbiol. 2024, 15, 1433716. [Google Scholar] [CrossRef]
- Thiers, I.; Lissens, M.; Langie, H.; Lories, B.; Steenackers, H. Salmonella biofilm formation diminishes bacterial proliferation in the C. elegans intestine. Biofilm 2024, 8, 100225. [Google Scholar] [CrossRef]
- Deng, P.; Uma Naresh, N.; Du, Y.; Lamech, L.T.; Yu, J.; Zhu, L.J.; Pukkila-Worley, R.; Haynes, C.M. Mitochondrial UPR repression during Pseudomonas aeruginosa infection requires the bZIP protein ZIP-3. Proc. Natl. Acad. Sci. USA 2019, 116, 6146–6151. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Ma, Y.-F.; Wang, S.-T.; Chen, C.-S.; Teng, C.-H. Iron acquisition of urinary tract infection Escherichia coli involves pathogenicity in Caenorhabditis elegans. Microorganisms 2021, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Ragno, M.; Blackburn, S.A.; Fasseas, M.; Maitra, S.; Liang, M.; Rai, S.; Mastroianni, G.; Tholozan, F.; Thompson, R.; et al. New tools to monitor Pseudomonas aeruginosa infection and biofilms in vivo in C. elegans. Front. Cell. Infect. Microbiol. 2024, 14, 1478881. [Google Scholar] [CrossRef]
- Arnal, M.E.; Lallès, J.P. Gut epithelial inducible heat-shock proteins and their modulation by diet and the microbiota. Nutr. Rev. 2016, 74, 181–197. [Google Scholar] [CrossRef]
- Tizard, M.L.; Moss, M.T.; Sanderson, J.D.; Austen, B.M.; Hermon-Taylor, J. p43, the protein product of the atypical insertion sequence IS900, is expressed in Mycobacterium paratuberculosis. J. Gen. Microbiol. 1992, 138 Pt 8, 1729–1736. [Google Scholar] [CrossRef]
- Brydon, W.G.; Ferguson, A. Haemoglobin in gut lavage fluid as a measure of gastrointestinal blood loss. Lancet 1992, 340, 1381–1382. [Google Scholar] [CrossRef]
- Ankri, S. Entamoeba histolytica-Gut Microbiota Interaction: More Than Meets the Eye. Microorganisms 2021, 9, 581. [Google Scholar] [CrossRef]
- Garcia-Sanchez, A.M.; Miller, A.Z.; Caldeira, A.T.; Cutillas, C. Bacterial communities from Trichuris spp. A contribution to deciphering the role of parasitic nematodes as vector of pathogens. Acta Trop. 2022, 226, 106277. [Google Scholar] [CrossRef]
- Schachter, J.; Oliveira, D.A.D.; Silva, C.M.D.; Alencar, A.C.M.D.B.; Duarte, M.; Silva, M.M.P.D.; Ignácio, A.C.D.P.R.; Lopes-Torres, E.J. Whipworm Infection Promotes Bacterial Invasion, Intestinal Microbiota Imbalance, and Cellular Immunomodulation. Infect. Immun. 2020, 88, iai.00642-19. [Google Scholar] [CrossRef]
- Bancroft, A.J.; Levy, C.W.; Jowitt, T.A.; Hayes, K.S.; Thompson, S.; McKenzie, E.A.; Ball, M.D.; Dubaissi, E.; France, A.P.; Bellina, B.; et al. The major secreted protein of the whipworm parasite tethers to matrix and inhibits interleukin-13 function. Nat. Commun. 2019, 10, 2344. [Google Scholar] [CrossRef]
- Rooney, J.; Cantacessi, C.; Sotillo, J.; Cortes, A. Gastrointestinal worms and bacteria: From association to intervention. Parasite Immunol. 2023, 45, e12955. [Google Scholar] [CrossRef] [PubMed]
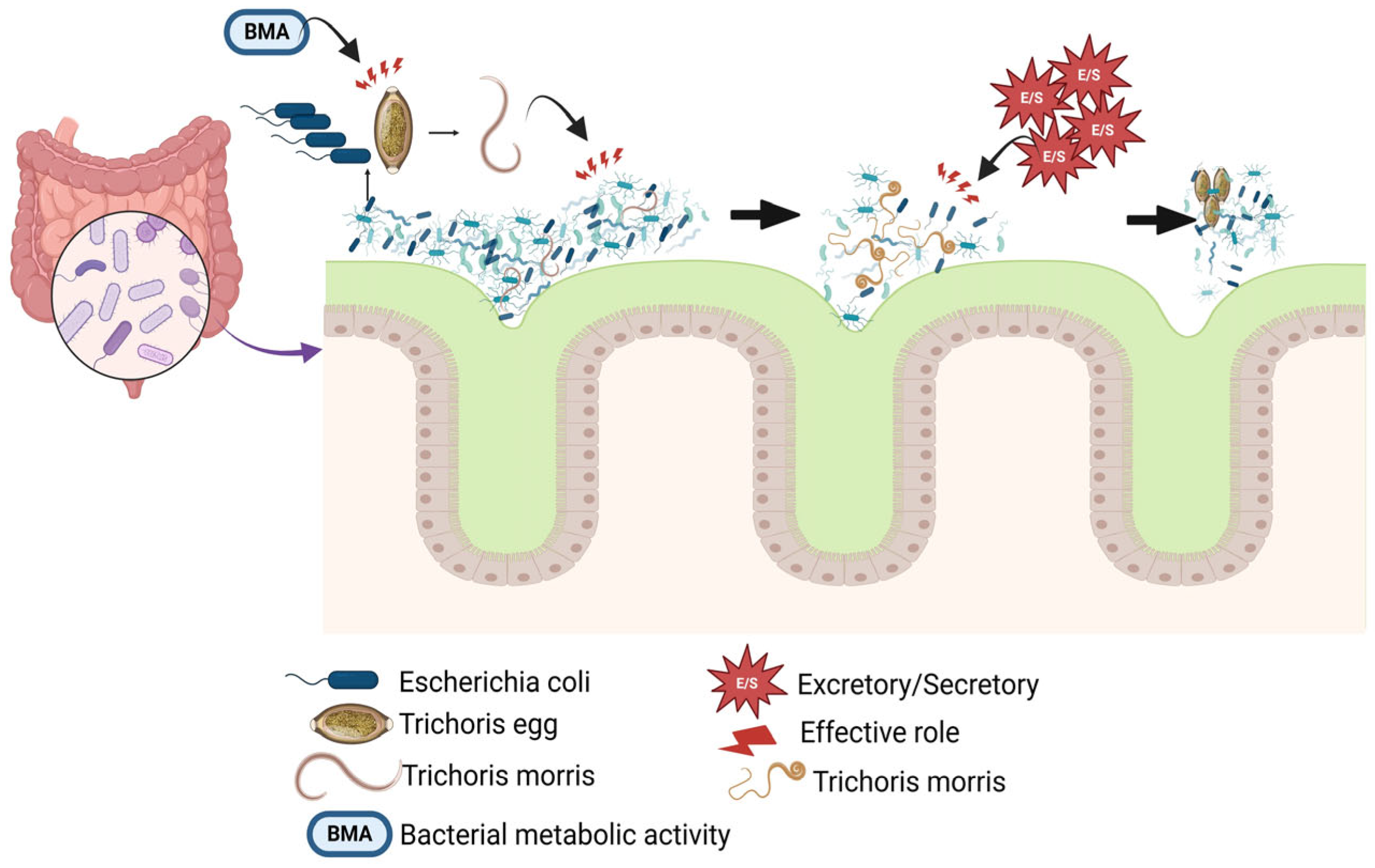
 Upregulation;
Upregulation;  , downregulation. This figure was generated using BioRender software version 04.
, downregulation. This figure was generated using BioRender software version 04.
 Upregulation;
Upregulation; 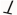 , downregulation. This figure was generated using BioRender software version 04.
, downregulation. This figure was generated using BioRender software version 04.


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khazaei, M.; Parsasefat, M.; Bahar, A.; Tahmasebi, H.; Oksenych, V. Behavioral Cooperation or Conflict of Human Intestinal Roundworms and Microbiomes: A Bio-Activity Perspective. Cells 2025, 14, 556. https://doi.org/10.3390/cells14070556
Khazaei M, Parsasefat M, Bahar A, Tahmasebi H, Oksenych V. Behavioral Cooperation or Conflict of Human Intestinal Roundworms and Microbiomes: A Bio-Activity Perspective. Cells. 2025; 14(7):556. https://doi.org/10.3390/cells14070556
Chicago/Turabian StyleKhazaei, Meisam, Malihe Parsasefat, Aisa Bahar, Hamed Tahmasebi, and Valentyn Oksenych. 2025. "Behavioral Cooperation or Conflict of Human Intestinal Roundworms and Microbiomes: A Bio-Activity Perspective" Cells 14, no. 7: 556. https://doi.org/10.3390/cells14070556
APA StyleKhazaei, M., Parsasefat, M., Bahar, A., Tahmasebi, H., & Oksenych, V. (2025). Behavioral Cooperation or Conflict of Human Intestinal Roundworms and Microbiomes: A Bio-Activity Perspective. Cells, 14(7), 556. https://doi.org/10.3390/cells14070556







