The Ceramide-Dependent EV Secretome Differentially Affects Prostate Cancer Cell Migration
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Transfection
2.2. Real-Time qPCR
2.3. Extracellular Vesicle Purification
2.4. Immunohistochemistry
2.5. TMA Evaluation
2.6. Immunoblot
2.7. Nanoparticle Tracking Analysis
2.8. Viability Assay
2.9. Proliferation and Migration Assay
2.10. Mass Spectrometry
2.11. Bioinformatics Analysis
2.12. Statistics
3. Results
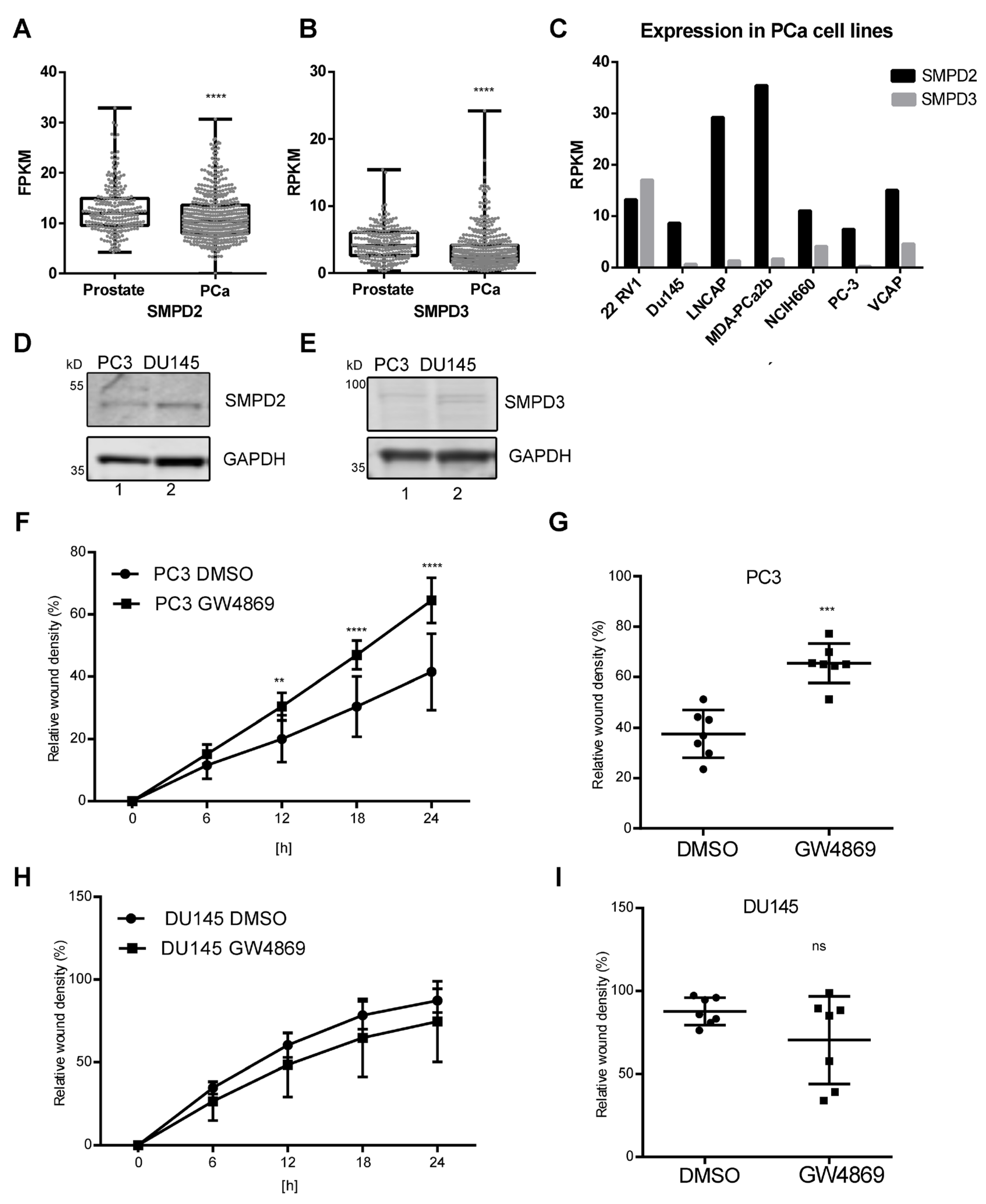
3.1. Reduced SMPD2 and SMPD3 Expression in Prostate Cancer
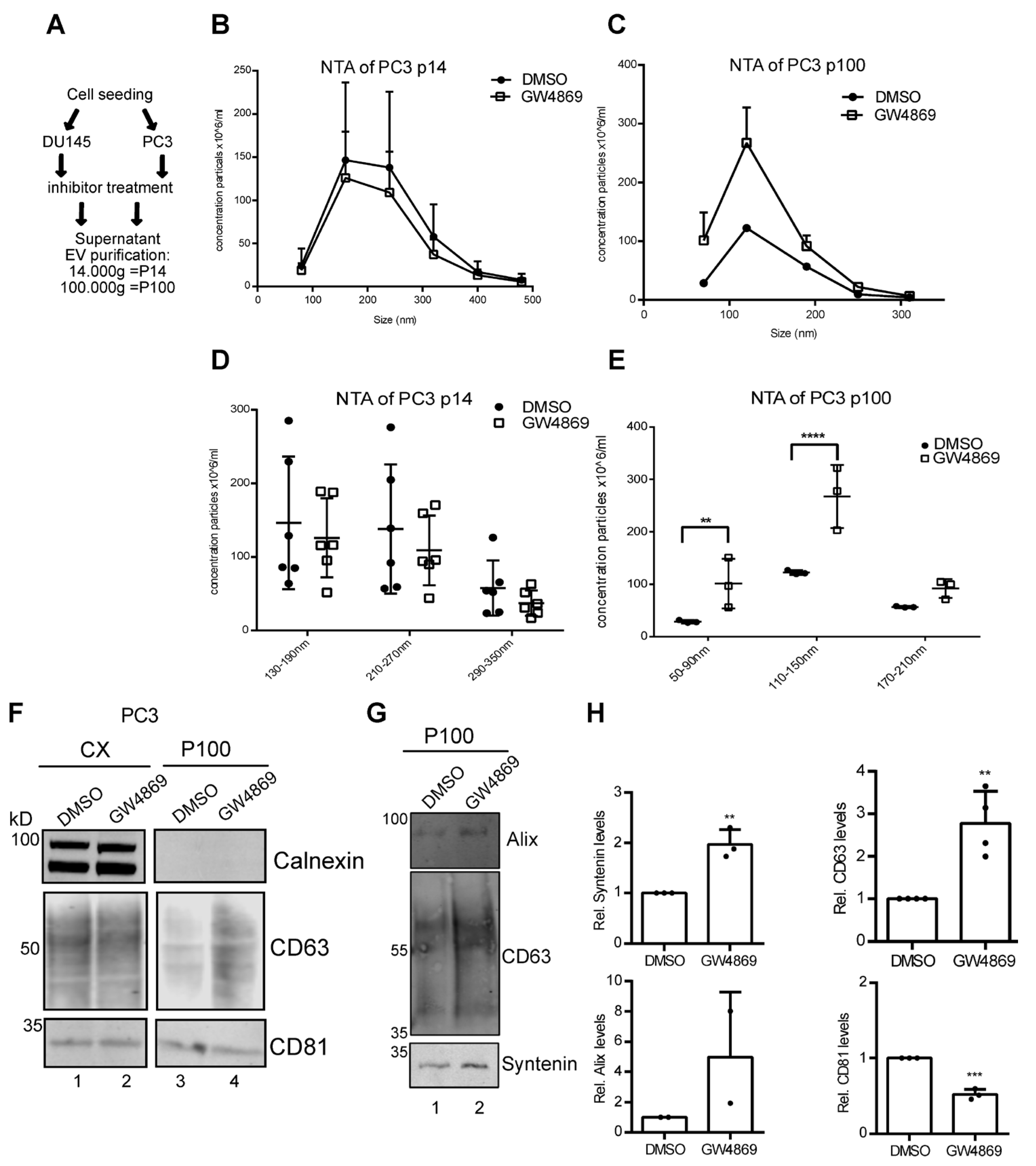
3.2. Ceramide-Dependent EV Secretion from PC3 Cells
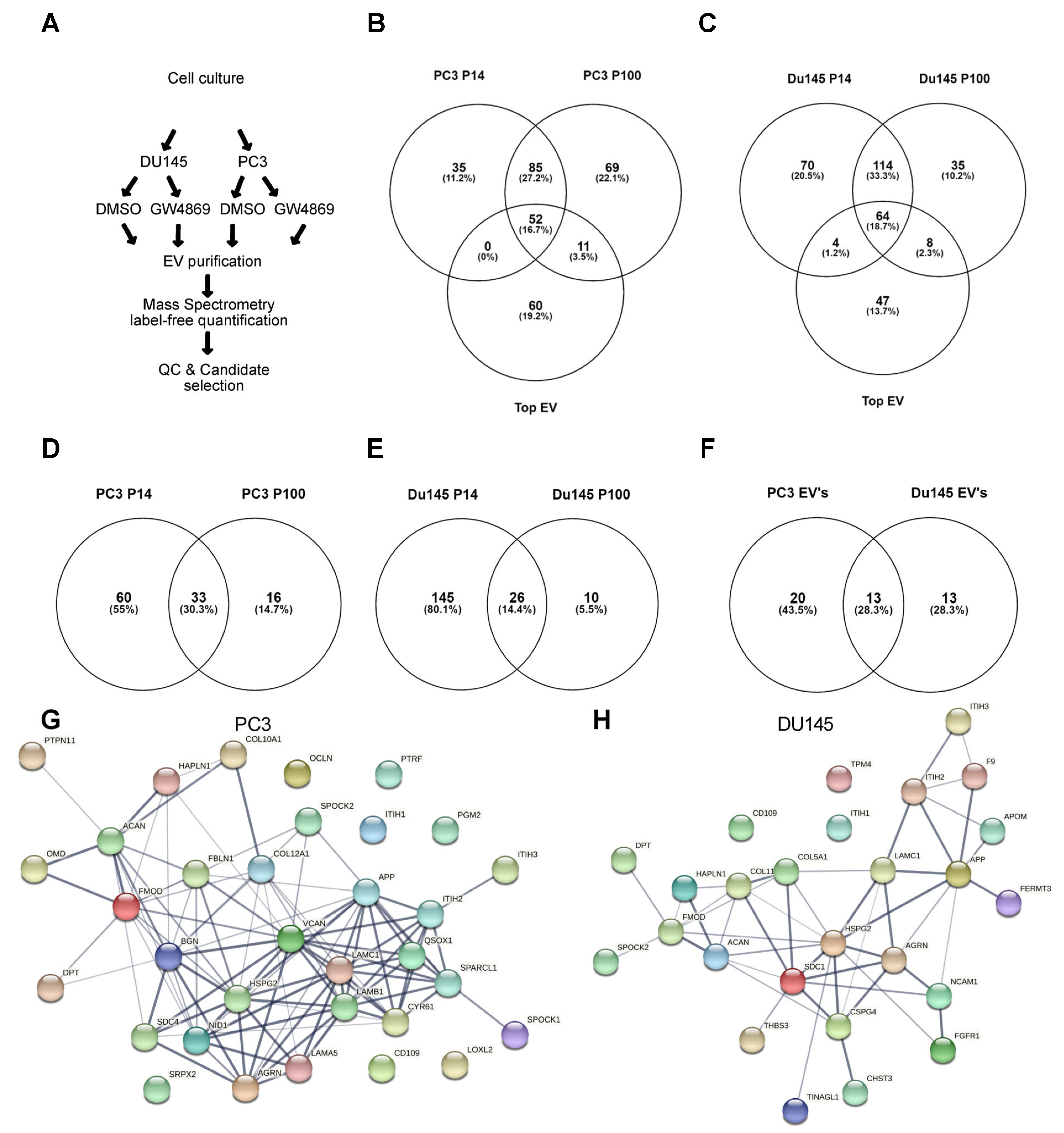
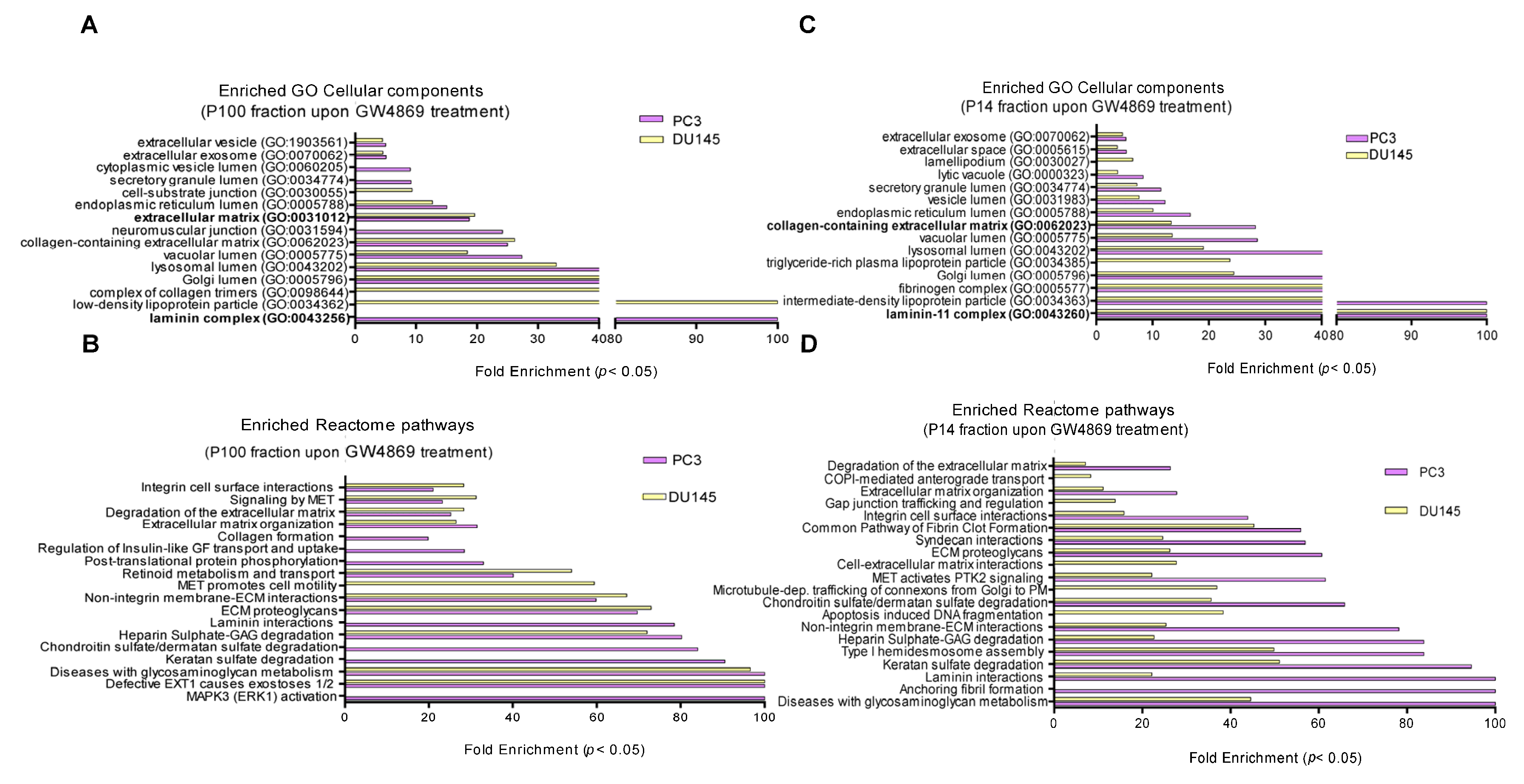
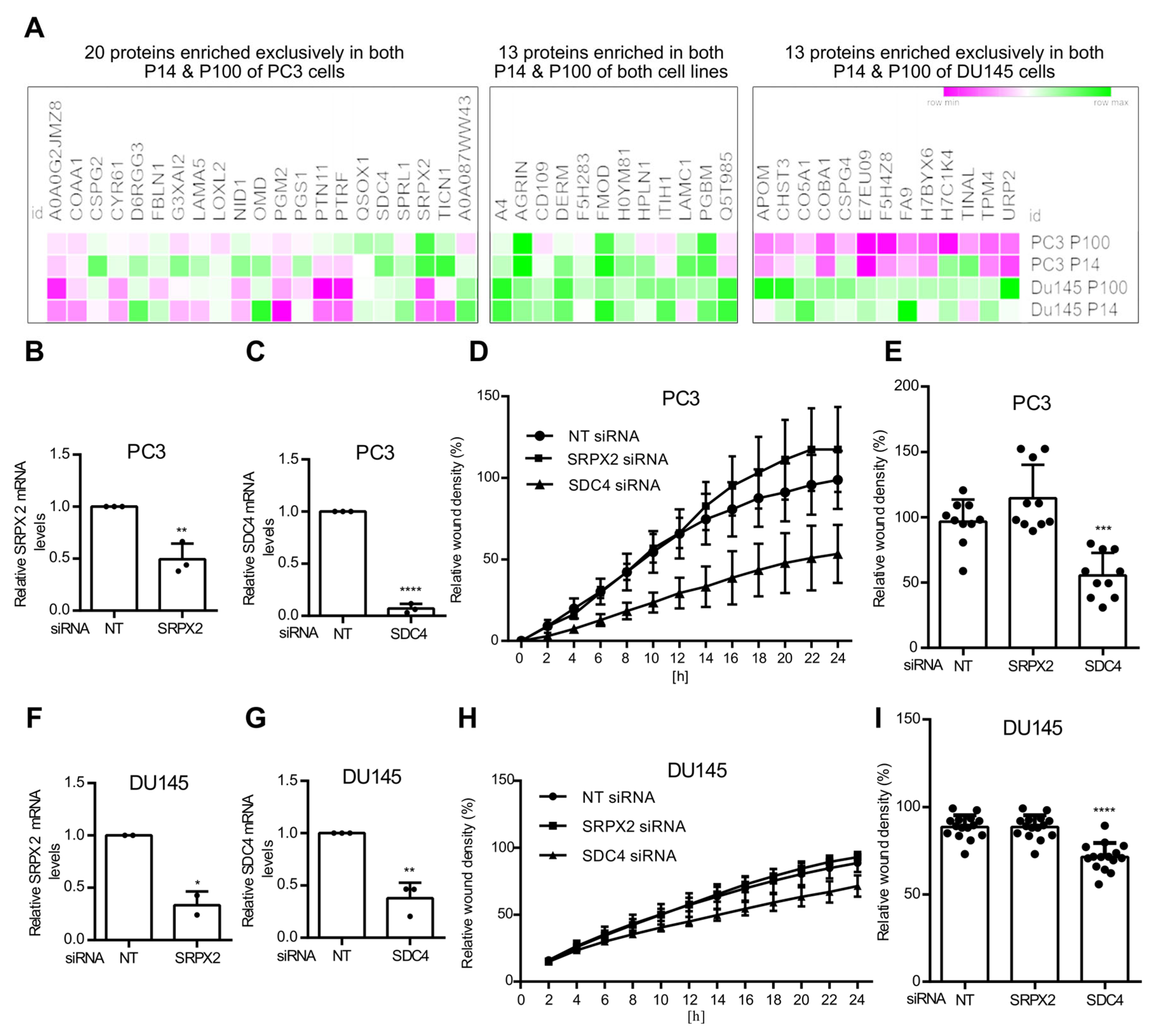
3.3. Ceramide-Dependent EV Proteome
3.4. Ceramide-Dependent Tumor Migration
4. Discussion
4.1. nSMase Suppression and Prostate Cancer Progression
4.2. EV Secretion and Cell Migration
4.3. NSMase-Dependent EV Proteome: Cell Type Specificity
4.4. Functional Roles of SDC4 and SRPX2
4.5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Yam, J.W.P.; Mao, X. The multifaceted roles of small extracellular vesicles in metabolic reprogramming in the tumor microenvironments. Proteomics 2024, 24, 2300021. [Google Scholar] [CrossRef]
- Conteduca, V.; Gurioli, G.; Brighi, N.; Lolli, C.; Schepisi, G.; Casadei, C.; Burgio, S.L.; Gargiulo, S.; Ravaglia, G.; Rossi, L.; et al. Plasma Androgen Receptor in Prostate Cancer. Cancers 2019, 11, 1719. [Google Scholar] [CrossRef]
- Casanova-Salas, I.; Aguilar, D.; Cordoba-Terreros, S.; Agundez, L.; Brandariz, J.; Herranz, N.; Mas, A.; Gonzalez, M.; Morales-Barrera, R.; Sierra, A.; et al. Circulating tumor extracellular vesicles to monitor metastatic prostate cancer genomics and transcriptomic evolution. Cancer Cell 2024, 42, 1301–1312.e7. [Google Scholar] [CrossRef]
- Li, Y.; Shi, X.; Jia, E.; Qin, S.; Yu, F. Extracellular vesicle biomarkers for prostate cancer diagnosis: A systematic review and meta-analysis. Urol. Oncol. Semin. Orig. Investig. 2023, 41, 440–453. [Google Scholar] [CrossRef]
- Vlaeminck-Guillem, V. Extracellular Vesicles in Prostate Cancer Carcinogenesis, Diagnosis, and Management. Front. Oncol. 2018, 8, 222. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Menck, K.; Sönmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J. Extracell. Vesicles 2017, 6, 1378056. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. CP Cell Biol. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef]
- Atanassov, I.; Urlaub, H. Increased proteome coverage by combining PAGE and peptide isoelectric focusing: Comparative study of gel-based separation approaches. Proteomics 2013, 13, 2947–2955. [Google Scholar] [CrossRef]
- Zhang, Y.; Bilbao, A.; Bruderer, T.; Luban, J.; Strambio-De-Castillia, C.; Lisacek, F.; Hopfgartner, G.; Varesio, E. The Use of Variable Q1 Isolation Windows Improves Selectivity in LC-SWATH-MS Acquisition. J. Proteome Res. 2015, 14, 4359–4371. [Google Scholar] [CrossRef]
- Lambert, J.-P.; Ivosev, G.; Couzens, A.L.; Larsen, B.; Taipale, M.; Lin, Z.-Y.; Zhong, Q.; Lindquist, S.; Vidal, M.; Aebersold, R.; et al. Mapping differential interactomes by affinity purification coupled with data-independent mass spectrometry acquisition. Nat. Methods 2013, 10, 1239–1245. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Worst, T.S.; von Hardenberg, J.; Gross, J.C.; Erben, P.; Schnolzer, M.; Hausser, I.; Bugert, P.; Michel, M.S.; Boutros, M. Database-augmented Mass Spectrometry Analysis of Exosomes Identifies Claudin 3 as a Putative Prostate Cancer Biomarker. Mol. Cell. Proteom. 2017, 16, 998–1008. [Google Scholar] [CrossRef]
- Ghosh, D.; Lim, T.K.; Basu, A.; Gross, J.C.; Lin, Q. Quantitative proteomics identifies possible flow of metastatic cues between progressive stages of colorectal cancer via transfer of ceramide-dependent exosomal cargoes. FEBS J. 2025. [Google Scholar] [CrossRef]
- Menck, K.; Klemm, F.; Gross, J.C.; Pukrop, T.; Wenzel, D.; Binder, C. Induction and transport of Wnt 5a during macrophage-induced malignant invasion is mediated by two types of extracellular vesicles. Oncotarget 2013, 4, 2057–2066. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.; O’Driscoll, L.; Thery, C.; Witwer, K.W. MISEV2023: An updated guide to EV research and applications. J. Extracell. Vesicles 2024, 13, e12416. [Google Scholar] [CrossRef]
- Lin, X.; Chang, W.; Wang, Y.; Tian, M.; Yu, Z. SRPX2, an independent prognostic marker, promotes cell migration and invasion in hepatocellular carcinoma. Biomed. Pharmacother. 2017, 93, 398–405. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Fan, Z.; Zhao, J.; Liu, S.; Zhang, M.; Li, H.; Goscinski, M.A.; Fan, H.; Suo, Z. High SRPX2 protein expression predicts unfavorable clinical outcome in patients with prostate cancer. OncoTargets Ther. 2018, 11, 3149–3157. [Google Scholar] [CrossRef]
- Luo, N.; Tan, Y.; Deng, H.; Wu, W.; Mei, L.; Huang, X.; Qin, Y.; Zhu, H.; Liu, C. SRPX2 Promotes Tumor Proliferation and Migration via the FAK Pathway in Papillary Thyroid Carcinoma. J. Oncol. 2022, 2022, 5821545. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, R.; Wu, J.; Li, S.; Yao, W.; Xu, J.; Zheng, C.; Lu, Y.; Zhang, H. SRPX2 promotes cancer cell proliferation and migration of papillary thyroid cancer. Clin. Exp. Med. 2023, 23, 4825–4834. [Google Scholar] [CrossRef] [PubMed]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Ghossoub, R.; Lembo, F.; Rubio, A.; Gaillard, C.B.; Bouchet, J.; Vitale, N.; Slavík, J.; Machala, M.; Zimmermann, P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat. Commun. 2014, 5, 3477. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Malagarie-Cazenave, S.; Olea, N.; Vara, D.; Chiloeches, A.; Díaz-Laviada, I. Apoptosis induced by capsaicin in prostate PC-3 cells involves ceramide accumulation, neutral sphingomyelinase, and JNK activation. Apoptosis 2007, 12, 2013–2024. [Google Scholar] [CrossRef]
- Yabu, T.; Shiba, H.; Shibasaki, Y.; Nakanishi, T.; Imamura, S.; Touhata, K.; Yamashita, M. Stress-induced ceramide generation and apoptosis via the phosphorylation and activation of nSMase1 by JNK signaling. Cell Death Differ. 2015, 22, 258–273. [Google Scholar] [CrossRef]
- Takanashi, Y.; Kahyo, T.; Sekihara, K.; Kawase, A.; Setou, M.; Funai, K. Prognostic potential of lipid profiling in cancer patients: A systematic review of mass spectrometry-based studies. Lipids Health Dis. 2024, 23, 154. [Google Scholar] [CrossRef]
- Lazniewska, J.; Li, K.L.; Johnson, I.R.D.; Sorvina, A.; Logan, J.M.; Martini, C.; Moore, C.; Ung, B.S.-Y.; Karageorgos, L.; Hickey, S.M.; et al. Dynamic interplay between sortilin and syndecan-1 contributes to prostate cancer progression. Sci. Rep. 2023, 13, 13489. [Google Scholar] [CrossRef]
- Sung, B.H.; Ketova, T.; Hoshino, D.; Zijlstra, A.; Weaver, A.M. Directional cell movement through tissues is controlled by exosome secretion. Nat. Commun. 2015, 6, 7164. [Google Scholar] [CrossRef]
- Sequeira, J.P.; Salta, S.; Freitas, R.; López-López, R.; Díaz-Lagares, Á.; Henrique, R.; Jerónimo, C. Biomarkers for Pre-Treatment Risk Stratification of Prostate Cancer Patients: A Systematic Review. Cancers 2024, 16, 1363. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yang, H.; Cho, S.; Jang, Y.; Han, I.-O.; Oh, E.-S. Correlation of syndecan gene amplification with metastatic potential and clinical outcomes in carcinomas. Am. J. Physiol. Cell Physiol. 2024, 327, C380–C386. [Google Scholar] [CrossRef] [PubMed]
- Poças, J.; Marques, C.; Gomes, C.; Otake, A.H.; Pinto, F.; Ferreira, M.; Silva, T.; Faria-Ramos, I.; Matos, R.; Ribeiro, A.R.; et al. Syndecan-4 is a maestro of gastric cancer cell invasion and communication that underscores poor survival. Proc. Natl. Acad. Sci. USA 2023, 120, e2214853120. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.J.; Barquilha, C.N.; Barbosa, I.C.; Macedo, R.T.; Lima, F.O.; Justulin, L.A.; Barbosa, G.O.; Carvalho, H.F.; Felisbino, S.L. Syndecan Family Gene and Protein Expression and Their Prognostic Values for Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 8669. [Google Scholar] [CrossRef]
- Urabe, F.; Kosaka, N.; Yamamoto, Y.; Ito, K.; Otsuka, K.; Soekmadji, C.; Egawa, S.; Kimura, T.; Ochiya, T. Metastatic prostate cancer-derived extracellular vesicles facilitate osteoclastogenesis by transferring the CDCP1 protein. J. Extracell. Vesicles 2023, 12, 12312. [Google Scholar] [CrossRef]

| Gene Symbol | Gene ID | Sequence |
|---|---|---|
| SDC4 | 6385 | UAGAGGAGAAUGAGGUUAU GAUCGGCCCUGAAGUUGUC CCAACAAGGUGUCAAUGUC GUGAGGAUGUGUCCAACAA |
| SRPX2 | 27286 | GUUGUGAGCUCUCCUGUGA UGAAAGCUACAAUGAAGUA CCUAUGAAGAUUAACGUCA GAUGAGAUGCCACGCACUA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choezom, D.; Plum, J.-M.; Karuna M., P.; Danieli-Mackay, A.; Lenz, C.; Brockmeyer, P.; Gross, J.C. The Ceramide-Dependent EV Secretome Differentially Affects Prostate Cancer Cell Migration. Cells 2025, 14, 547. https://doi.org/10.3390/cells14070547
Choezom D, Plum J-M, Karuna M. P, Danieli-Mackay A, Lenz C, Brockmeyer P, Gross JC. The Ceramide-Dependent EV Secretome Differentially Affects Prostate Cancer Cell Migration. Cells. 2025; 14(7):547. https://doi.org/10.3390/cells14070547
Chicago/Turabian StyleChoezom, Dolma, Jan-Moritz Plum, Pradhipa Karuna M., Adi Danieli-Mackay, Christof Lenz, Phillipp Brockmeyer, and Julia Christina Gross. 2025. "The Ceramide-Dependent EV Secretome Differentially Affects Prostate Cancer Cell Migration" Cells 14, no. 7: 547. https://doi.org/10.3390/cells14070547
APA StyleChoezom, D., Plum, J.-M., Karuna M., P., Danieli-Mackay, A., Lenz, C., Brockmeyer, P., & Gross, J. C. (2025). The Ceramide-Dependent EV Secretome Differentially Affects Prostate Cancer Cell Migration. Cells, 14(7), 547. https://doi.org/10.3390/cells14070547








