Induced Pluripotent Stem Cell-Derived Exosomes Promote Peripheral Nerve Regeneration in a Rat Sciatic Nerve Crush Injury Model: A Safety and Efficacy Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Sample Collection
2.2. Peripheral Blood Mononuclear Cells (PBMCs)
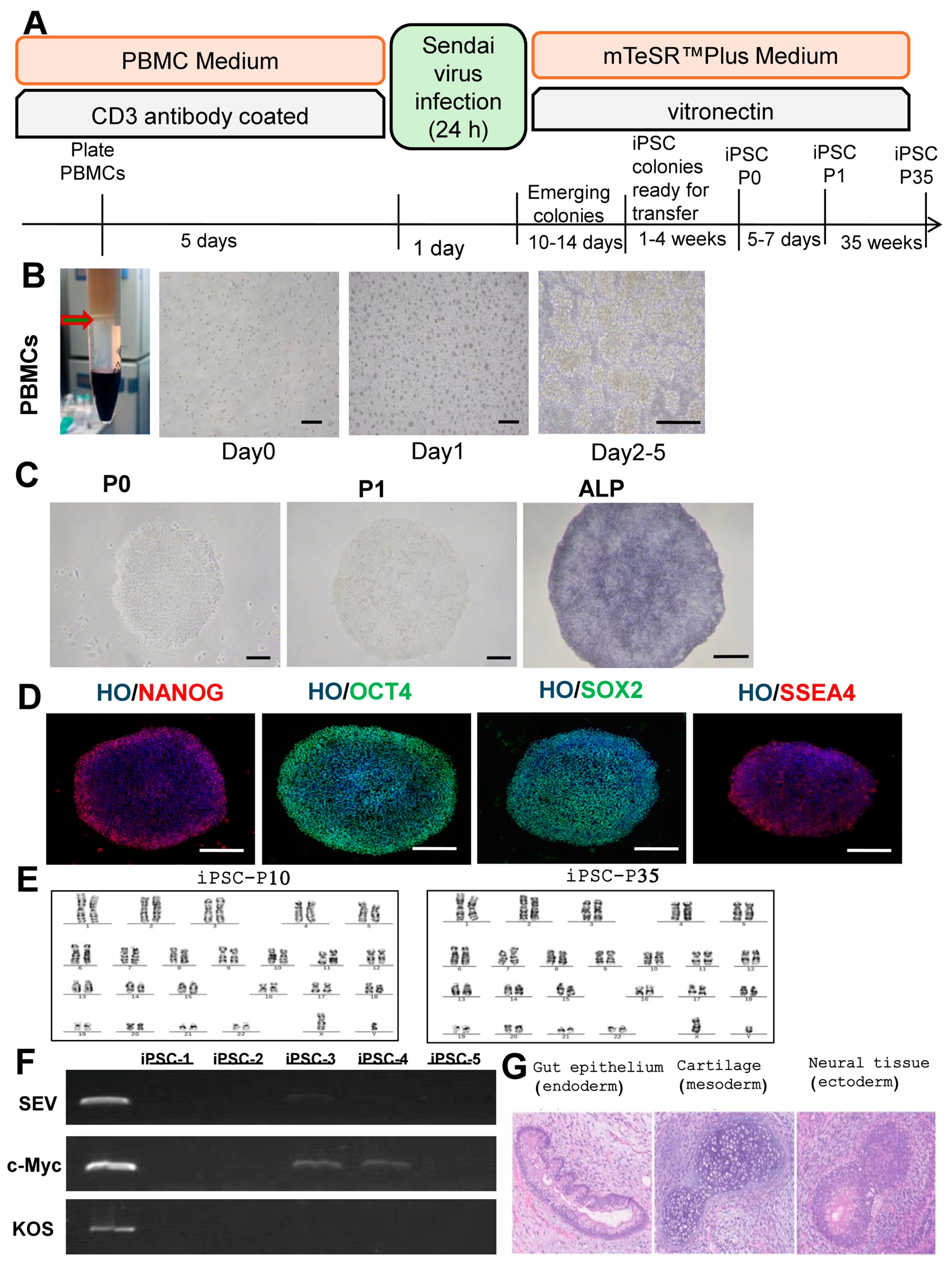
2.3. Reprogramming PBMCs to iPSC (Feeder-Free)
2.4. iPSC Characterisation
2.5. Extraction and Identification of iPSC-Exos
2.6. Transmission Electron Microscopy (TEM)
2.7. Nanoparticle Tracking Analysis (NTA)
2.8. Western Blot Analysis
2.9. Exosome Labeling and Uptake Assay
2.10. Cell Viability Assay
2.11. Animals
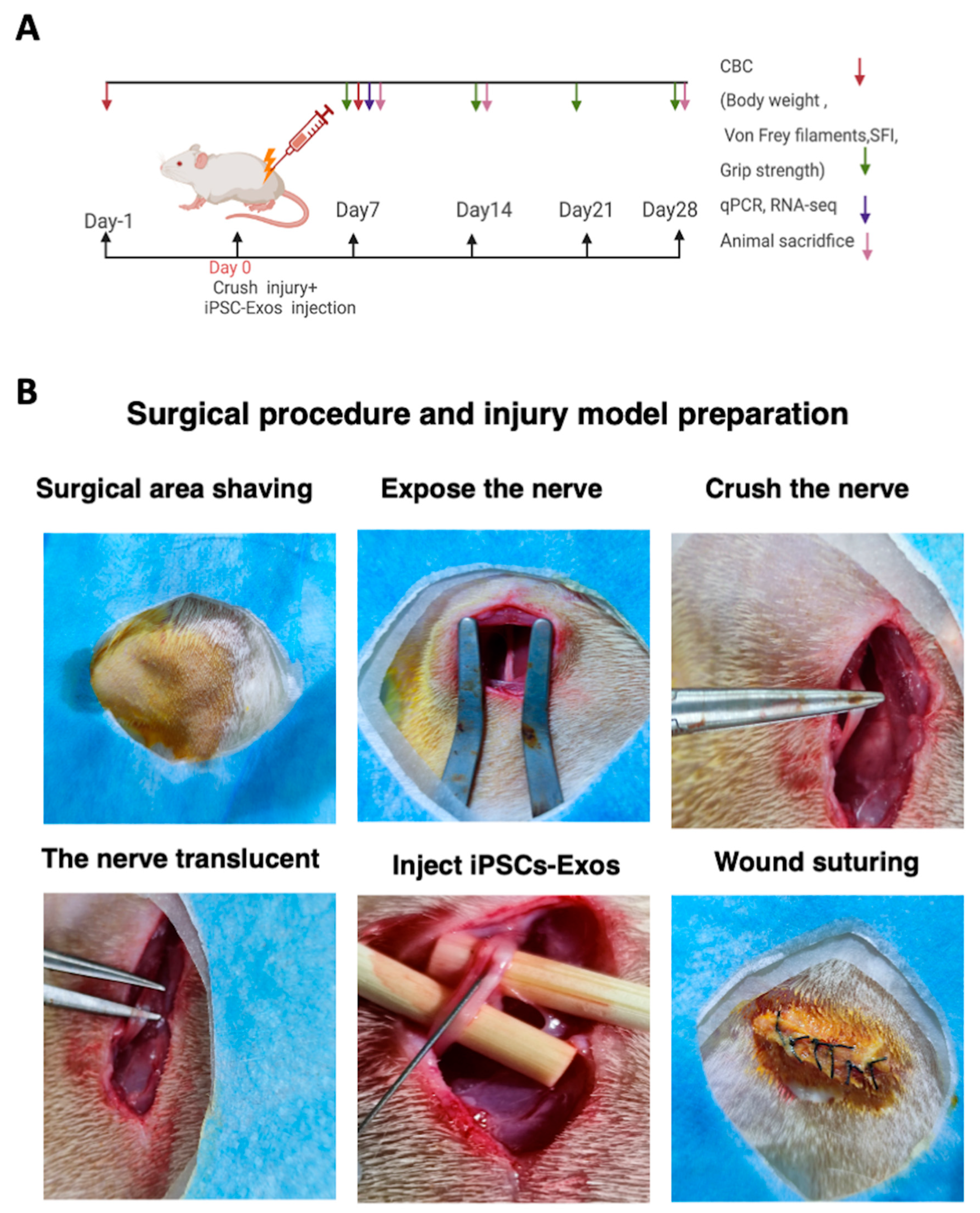
2.12. Nerve Crush Injury and Exosomes Implantation
2.13. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.14. Rat Sciatic Functional Index (SFI) Analysis
2.15. Grip Strength Measurement
2.16. Assessment of Nociceptive Responses
2.17. Systemic and Local Biocompatibility of iPSC-Exos Following Nerve Injury
2.18. Muscle Wet Weight, and Histological Examination of the Gastrocnemius Muscle
2.19. TEM, H&E Staining
2.20. Immunofluorescence Staining
2.21. RNA-Seq Library Construction
2.22. RNA-Seq Data Processing
2.23. Image Analysis
2.24. Statistical Analysis
3. Results
3.1. Reprogramming and Characterization of iPSCs
3.2. Characterization of iPSC-Exos

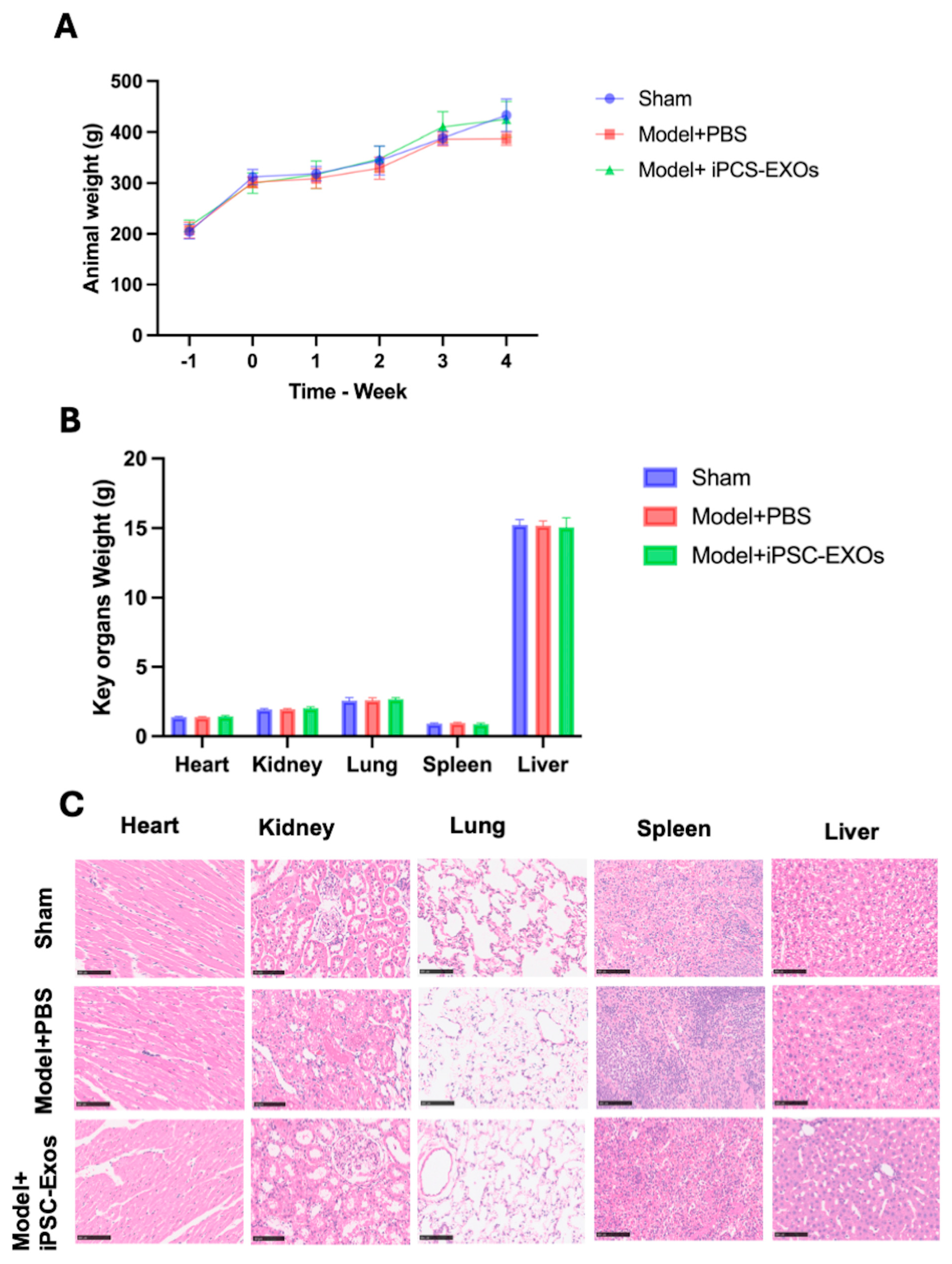
3.3. iPSC-Exosome Injection Is Safe, with No Evidence of Toxicity Observed
3.4. iPSC-Exos Upregulates the Expression of Genes Related to Nerve Regeneration
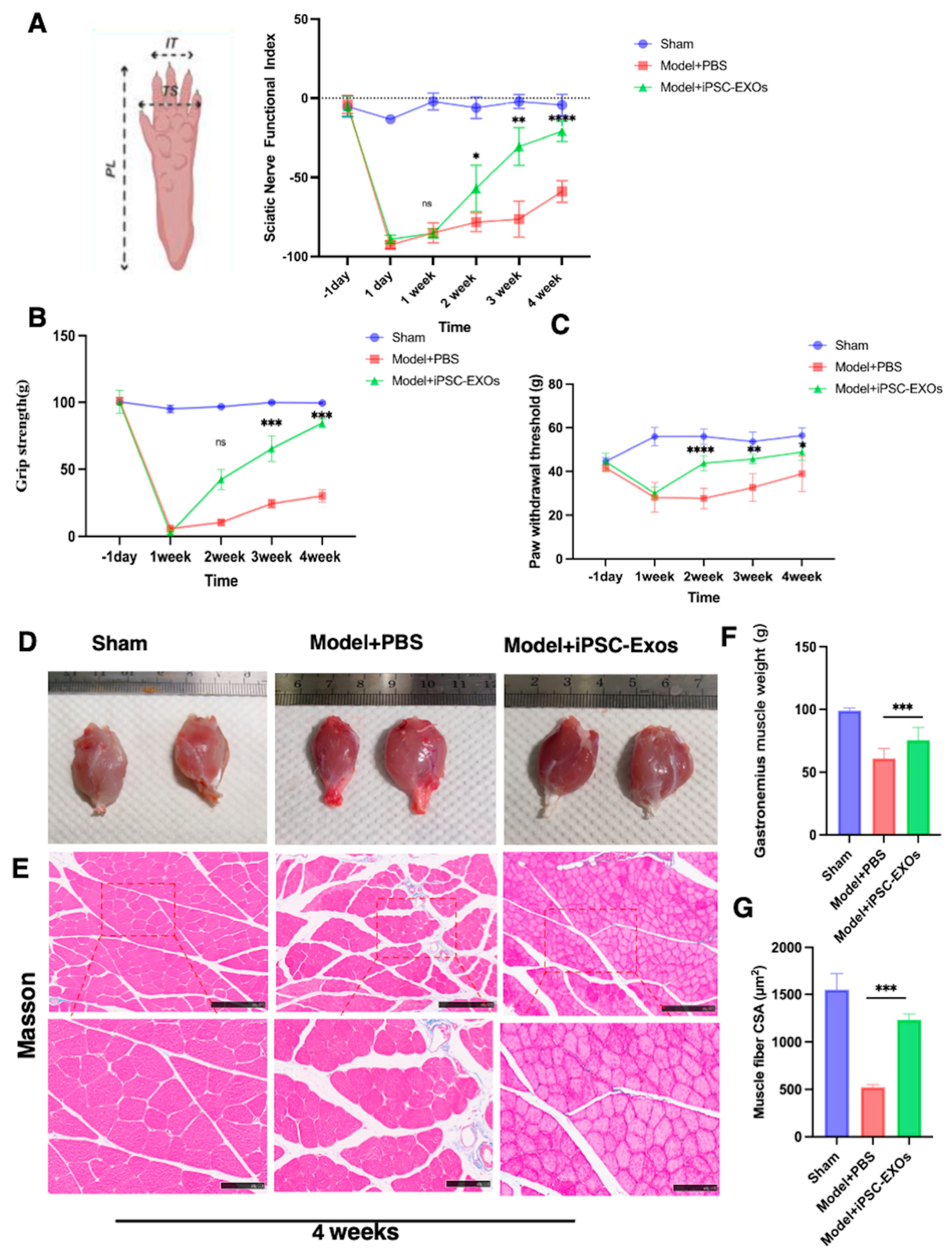
3.5. iPSC-Exos Promotes Functional Recovery and Attenuates Mechanical Pain Following
3.6. iPSC-Exos Prevented Gastrocnemius Muscle Atrophy Following Sciatic Nerve Injury
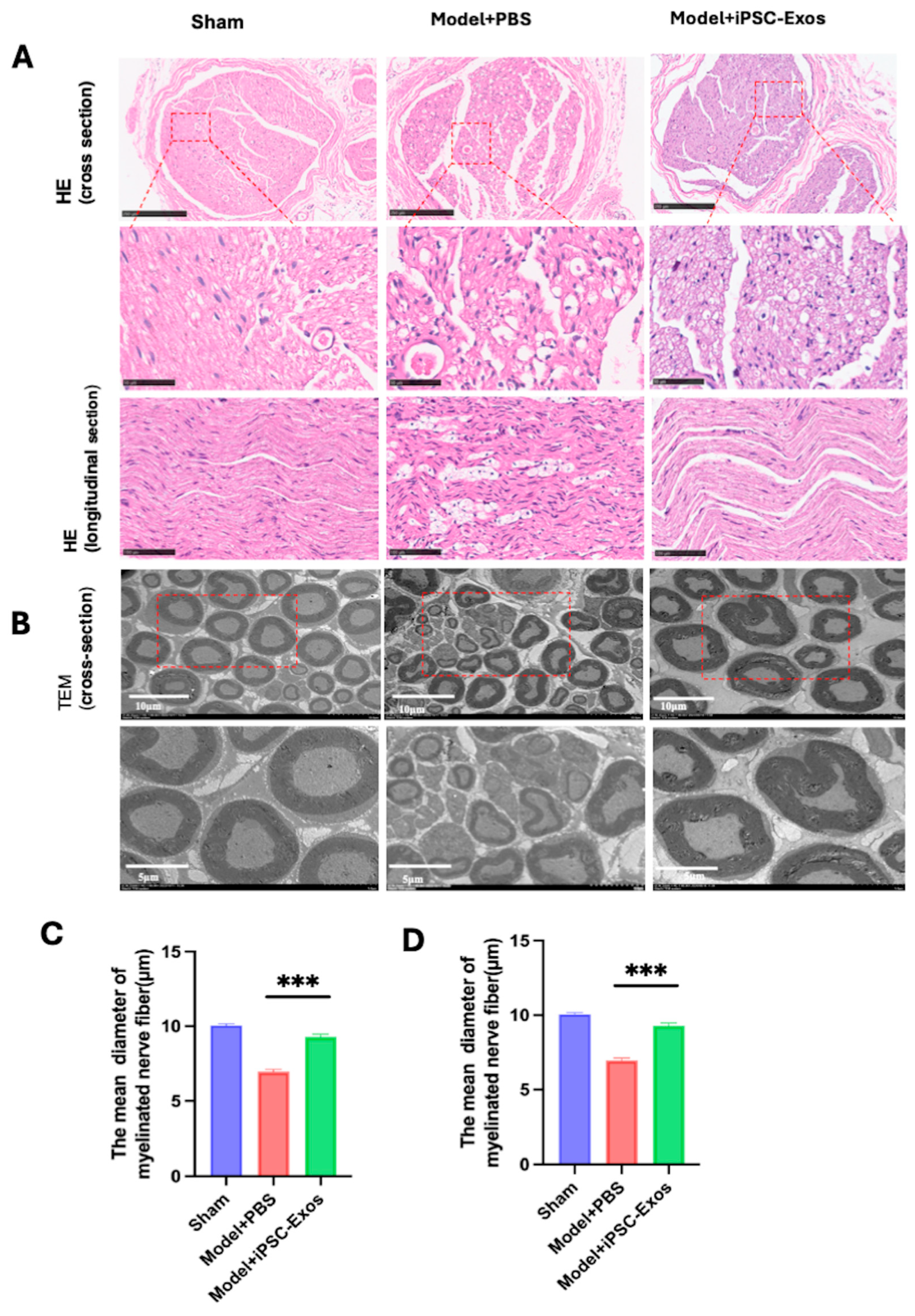
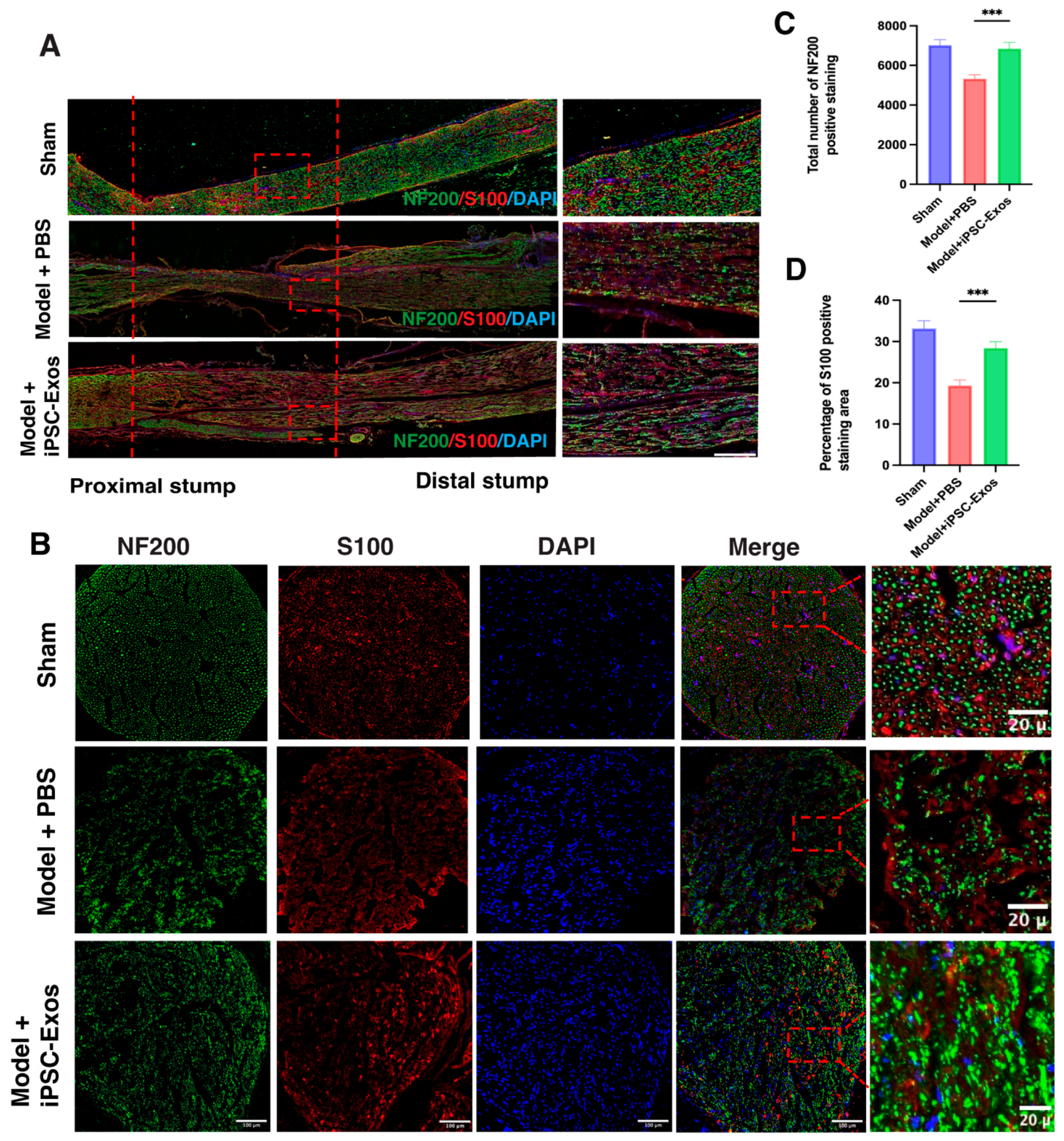
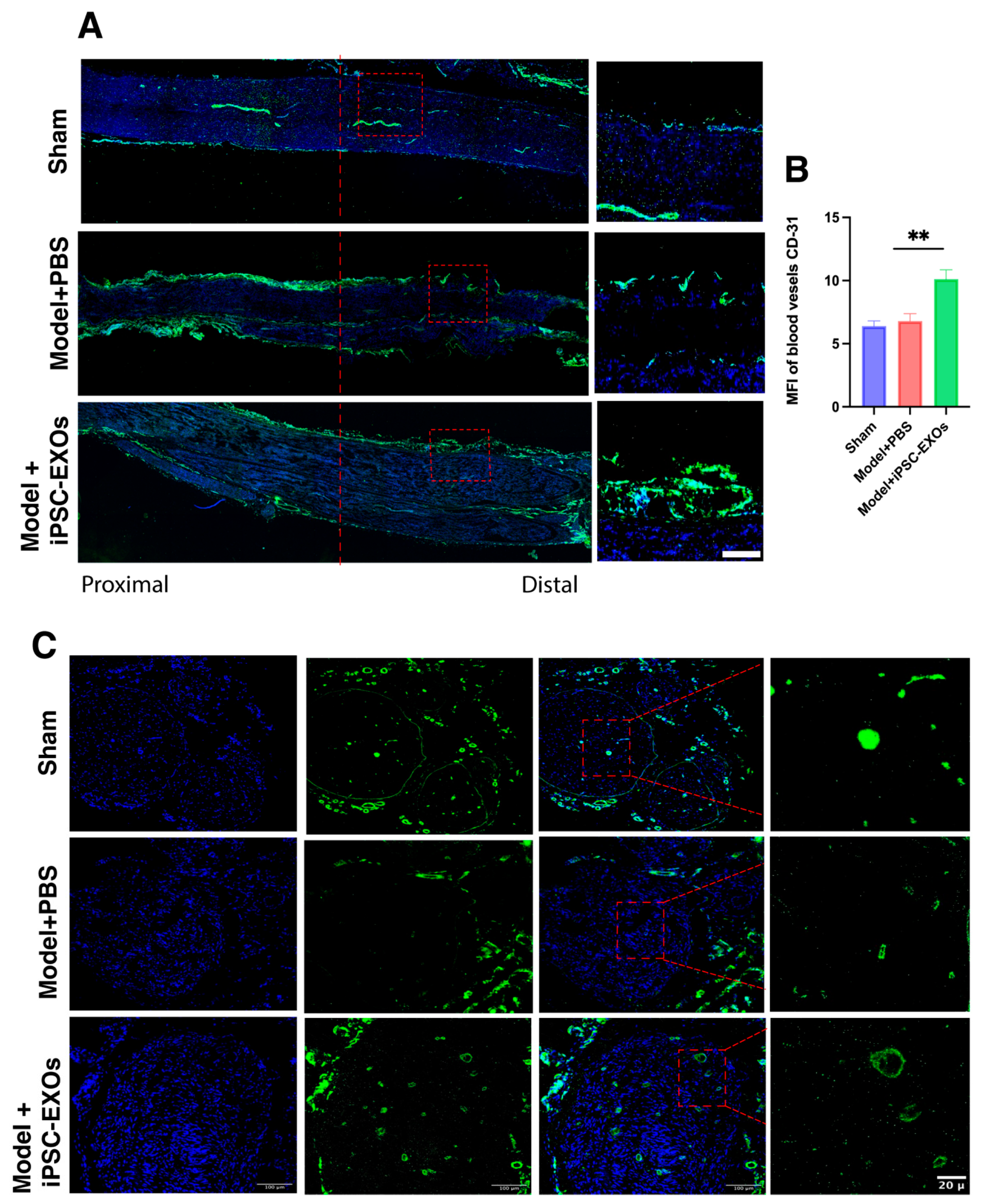
3.7. iPSC-Exos Promotes Axon Regeneration, Myelination, and Angiogenesis Following Sciatic Nerve Injury
3.8. iPSC-Exos Boost Nerve Repair by Modulating Key Molecular Pathways: A Transcriptomic Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT1 | AKT Serine/Threonine Kinase 1 |
| ANOVA | Analysis of Variance |
| BDNF | Brain-Derived Neurotrophic Factor |
| CD31 | Cluster of Differentiation 31 (Platelet Endothelial Cell Adhesion Molecule) |
| IF | Immunofluorescence |
| ECM | Extracellular matrix |
| IL-10 | Interleukin-10 |
| iPSCs | Induced Pluripotent Stem Cells |
| iPSCs-Exos | Induced Pluripotent Stem Cells-Derived Exosomes |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MBP | Myelin Basic Protein |
| MPZ | Myelin Protein Zero |
| MSCs | Mesenchymal Stem Cells |
| NF200 | Neurofilament 200 |
| NTA | Nanoparticle Tracking Analysis |
| PMP22 | Peripheral Myelin Protein 22 |
| PNI | Peripheral Nerve Injury |
| SCs | Schwann Cells |
| SD | Standard Deviation |
| SEM | Mean ± Standard Error of the Mean |
| S100β | S100 Calcium-Binding Protein Beta |
| SFI | Sciatic Nerve Functional Index |
| SNI | Sciatic Nerve Injury |
| TEM | Transmission Electron Microscopy |
| VEGFA | Vascular Endothelial Growth Factor A |
| WT | Withdrawal Threshold |
References
- Modrak, M.; Talukder, M.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2020, 98, 780–795. [Google Scholar] [PubMed]
- Novak, C.B.; Anastakis, D.J.; Beaton, D.E.; Mackinnon, S.E.; Katz, J. Biomedical and psychosocial factors associated with disability after peripheral nerve injury. JBJS 2011, 93, 929–936. [Google Scholar]
- Wellington, B. Quality of life issues for patients following traumatic brachial plexus injury—Part 2 research project. Int. J. Orthop. Trauma Nurs. 2010, 14, 5–11. [Google Scholar]
- Li, R.; Liu, Z.; Pan, Y.; Chen, L.; Zhang, Z.; Lu, L. Peripheral nerve injuries treatment: A systematic review. Cell Biochem. Biophys. 2014, 68, 449–454. [Google Scholar]
- Jiang, L.; Jones, S.; Jia, X. Stem cell transplantation for peripheral nerve regeneration: Current options and opportunities. Int. J. Mol. Sci. 2017, 18, 94. [Google Scholar] [CrossRef]
- Case, L.C.; Tessier-Lavigne, M. Regeneration of the adult central nervous system. Curr. Biol. 2005, 15, R749–R753. [Google Scholar]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.; Maurício, A.C. Peripheral nerve injury treatments and advances: One health perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien. Med. Wochenschr. 2019, 169, 240. [Google Scholar]
- Gordon, T.; Tyreman, N.; Raji, M.A. The basis for diminished functional recovery after delayed peripheral nerve repair. J. Neurosci. 2011, 31, 5325–5334. [Google Scholar]
- Zhang, P.-X.; Han, N.; Kou, Y.-H.; Zhu, Q.-T.; Liu, X.-L.; Quan, D.-P.; Chen, J.-G.; Jiang, B.-G. Tissue engineering for the repair of peripheral nerve injury. Neural Regen. Res. 2019, 14, 51–58. [Google Scholar]
- Scheib, J.; Höke, A. Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 2013, 9, 668–676. [Google Scholar] [CrossRef]
- Moskow, J.; Ferrigno, B.; Mistry, N.; Jaiswal, D.; Bulsara, K.; Rudraiah, S.; Kumbar, S.G. Bioengineering approach for the repair and regeneration of peripheral nerve. Bioact. Mater. 2019, 4, 107–113. [Google Scholar] [PubMed]
- Kariminekoo, S.; Movassaghpour, A.; Rahimzadeh, A.; Talebi, M.; Shamsasenjan, K.; Akbarzadeh, A. Implications of mesenchymal stem cells in regenerative medicine. Artif. Cells Nanomed. Biotechnol. 2016, 44, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar]
- Tanna, T.; Sachan, V. Mesenchymal stem cells: Potential in treatment of neurodegenerative diseases. Curr. Stem Cell Res. Ther. 2014, 9, 513–521. [Google Scholar]
- Matsuse, D.; Kitada, M.; Kohama, M.; Nishikawa, K.; Makinoshima, H.; Wakao, S.; Fujiyoshi, Y.; Heike, T.; Nakahata, T.; Akutsu, H. Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration. J. Neuropathol. Exp. Neurol. 2010, 69, 973–985. [Google Scholar]
- Zhang, Q.; Nguyen, P.D.; Shi, S.; Burrell, J.C.; Cullen, D.K.; Le, A.D. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci. Rep. 2018, 8, 6634. [Google Scholar]
- Takeya, H.; Itai, S.; Kimura, H.; Kurashina, Y.; Amemiya, T.; Nagoshi, N.; Iwamoto, T.; Sato, K.; Shibata, S.; Matsumoto, M. Schwann cell-encapsulated chitosan-collagen hydrogel nerve conduit promotes peripheral nerve regeneration in rodent sciatic nerve defect models. Sci. Rep. 2023, 13, 11932. [Google Scholar] [CrossRef]
- Mathot, F.; Rbia, N.; Bishop, A.T.; Hovius, S.E.; Shin, A.Y. Adipose derived mesenchymal stem cells seeded onto a decellularized nerve allograft enhances angiogenesis in a rat sciatic nerve defect model. Microsurgery 2020, 40, 585–592. [Google Scholar]
- Hopf, A.; Schaefer, D.J.; Kalbermatten, D.F.; Guzman, R.; Madduri, S. Schwann cell-like cells: Origin and usability for repair and regeneration of the peripheral and central nervous system. Cells 2020, 9, 1990. [Google Scholar] [CrossRef]
- Kaminska, A.; Radoszkiewicz, K.; Rybkowska, P.; Wedzinska, A.; Sarnowska, A. Interaction of neural stem cells (NSCs) and mesenchymal stem cells (MSCs) as a promising approach in brain study and nerve regeneration. Cells 2022, 11, 1464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chi, Y.; Wei, Y.; Zhang, W.; Wang, F.; Zhang, L.; Zou, L.; Song, B.; Zhao, X.; Han, Z. Bone marrow-derived mesenchymal stem/stromal cells in patients with acute myeloid leukemia reveal transcriptome alterations and deficiency in cellular vitality. Stem Cell Res. Ther. 2021, 12, 365. [Google Scholar] [CrossRef] [PubMed]
- Thirabanjasak, D.; Tantiwongse, K.; Thorner, P.S. Angiomyeloproliferative lesions following autologous stem cell therapy. J. Am. Soc. Nephrol. 2010, 21, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Duan, Y.; Nie, H.; Cui, X.; Du, J.; Yao, Y. Mesenchymal stem cells: Biological characteristics and application in disease therapy. Biochimie 2021, 185, 9–21. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 2016, 17, 183–193. [Google Scholar]
- Tonge, P.D.; Corso, A.J.; Monetti, C.; Hussein, S.M.; Puri, M.C.; Michael, I.P.; Li, M.; Lee, D.-S.; Mar, J.C.; Cloonan, N. Divergent reprogramming routes lead to alternative stem-cell states. Nature 2014, 516, 192–197. [Google Scholar] [CrossRef]
- Omole, A.E.; Fakoya, A.O.J.; Nnawuba, K.C.; Haider, K.H. Common ethical considerations of human-induced pluripotent stem cell research. In Handbook of Stem Cell Therapy; Springer: Singapore, 2022; pp. 1–17. [Google Scholar]
- Madrid, M.; Sumen, C.; Aivio, S.; Saklayen, N. Autologous induced pluripotent stem cell–based cell therapies: Promise, progress, and challenges. Curr. Protoc. 2021, 1, e88. [Google Scholar] [CrossRef]
- Gomes, K.M.S.; Costa, I.C.; Santos, J.F.d.; Dourado, P.M.M.; Forni, M.F.; Ferreira, J.C.B. Induced pluripotent stem cells reprogramming: Epigenetics and applications in the regenerative medicine. Rev. Assoc. Médica Bras. 2017, 63, 180–189. [Google Scholar] [CrossRef]
- Doss, M.X.; Sachinidis, A. Current challenges of iPSC-based disease modeling and therapeutic implications. Cells 2019, 8, 403. [Google Scholar] [CrossRef]
- Okano, H.; Morimoto, S. iPSC-based disease modeling and drug discovery in cardinal neurodegenerative disorders. Cell Stem Cell 2022, 29, 189–208. [Google Scholar]
- Koniusz, S.; Andrzejewska, A.; Muraca, M.; Srivastava, A.K.; Janowski, M.; Lukomska, B. Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front. Cell. Neurosci. 2016, 10, 109. [Google Scholar]
- Namini, M.S.; Daneshimehr, F.; Beheshtizadeh, N.; Mansouri, V.; Ai, J.; Jahromi, H.K.; Ebrahimi-Barough, S. Cell-free therapy based on extracellular vesicles: A promising therapeutic strategy for peripheral nerve injury. Stem Cell Res. Ther. 2023, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [PubMed]
- Li, J.; Zhang, Y.; Dong, P.-Y.; Yang, G.-M.; Gurunathan, S. A comprehensive review on the composition, biogenesis, purification, and multifunctional role of exosome as delivery vehicles for cancer therapy. Biomed. Pharmacother. 2023, 165, 115087. [Google Scholar]
- Di Bella, M.A. Overview and update on extracellular vesicles: Considerations on exosomes and their application in modern medicine. Biology 2022, 11, 804. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Z.; He, L.; Liu, C.; Wang, N.; Rong, L.; Liu, B. Exosomes derived from miR-26a-modified MSCs promote axonal regeneration via the PTEN/AKT/mTOR pathway following spinal cord injury. Stem Cell Res. Ther. 2021, 12, 1–15. [Google Scholar]
- Bucan, V.; Vaslaitis, D.; Peck, C.-T.; Strauß, S.; Vogt, P.M.; Radtke, C. Effect of exosomes from rat adipose-derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Mol. Neurobiol. 2019, 56, 1812–1824. [Google Scholar]
- Hu, T.; Chang, S.; Qi, F.; Zhang, Z.; Chen, J.; Jiang, L.; Wang, D.; Deng, C.; Nie, K.; Xu, G. Neural grafts containing exosomes derived from Schwann cell-like cells promote peripheral nerve regeneration in rats. Burn. Trauma 2023, 11, tkad013. [Google Scholar]
- Ge, Y.; Wu, J.; Zhang, L.; Huang, N.; Luo, Y. A new strategy for the regulation of neuroinflammation: Exosomes derived from mesenchymal stem cells. Cell. Mol. Neurobiol. 2024, 44, 24. [Google Scholar]
- Cong, M.; Hu, J.-J.; Yu, Y.; Li, X.-L.; Sun, X.-T.; Wang, L.-T.; Wu, X.; Zhu, L.-J.; Yang, X.-J.; He, Q.-R. miRNA-21-5p is an important contributor to the promotion of injured peripheral nerve regeneration using hypoxia-pretreated bone marrow–derived neural crest cells. Neural Regen. Res. 2025, 20, 277–290. [Google Scholar]
- Salehpour, A.; Karimi, Z.; Ghasemi Zadeh, M.; Afshar, M.; Kameli, A.; Mooseli, F.; Zare, M.; Afshar, A. Therapeutic potential of mesenchymal stem cell-derived exosomes and miRNAs in neuronal regeneration and rejuvenation in neurological disorders: A mini review. Front. Cell. Neurosci. 2024, 18, 1427525. [Google Scholar]
- Shi, L.; Zhou, Y.; Yin, Y.; Zhang, J.; Chen, K.; Liu, S.; Chen, P.; Jiang, H.; Liu, J.; Wu, Y. Advancing Tissue Damage Repair in Geriatric Diseases: Prospects of Combining Stem Cell-Derived Exosomes with Hydrogels. Int. J. Nanomed. 2024, 19, 3773–3804. [Google Scholar]
- Ahn, S.-H.; Ryu, S.-W.; Choi, H.; You, S.; Park, J.; Choi, C. Manufacturing therapeutic exosomes: From bench to industry. Mol. Cells 2022, 45, 284–290. [Google Scholar] [PubMed]
- Li, W.-Y.; Zhu, Q.-B.; Jin, L.-Y.; Yang, Y.; Xu, X.-Y.; Hu, X.-Y. Exosomes derived from human induced pluripotent stem cell-derived neural progenitor cells protect neuronal function under ischemic conditions. Neural Regen. Res. 2021, 16, 2064–2070. [Google Scholar]
- Li, J.; Jing, Y.; Bai, F.; Wu, Y.; Wang, L.; Yan, Y.; Jia, Y.; Yu, Y.; Jia, B.; Ali, F. Induced pluripotent stem cells as natural biofactories for exosomes carrying miR-199b-5p in the treatment of spinal cord injury. Front. Pharmacol. 2023, 13, 1078761. [Google Scholar]
- Garcia, G.; Pinto, S.; Ferreira, S.; Lopes, D.; Serrador, M.J.; Fernandes, A.; Vaz, A.R.; Mendonça, A.d.; Edenhofer, F.; Malm, T. Emerging role of miR-21-5p in neuron–glia dysregulation and exosome transfer using multiple models of Alzheimer’s disease. Cells 2022, 11, 3377. [Google Scholar] [CrossRef]
- Seki, T.; Yuasa, S.; Fukuda, K. Generation of induced pluripotent stem cells from a small amount of human peripheral blood using a combination of activated T cells and Sendai virus. Nat. Protoc. 2012, 7, 718–728. [Google Scholar]
- Xu, J.; Huang, L.-J.; Fang, Z.; Luo, H.-M.; Chen, Y.-Q.; Li, Y.-J.; Gong, C.-Z.; Chen, H. Spinal dI4 interneuron differentiation from human pluripotent stem cells. Front. Mol. Neurosci. 2022, 15, 845875. [Google Scholar]
- Xian, P.; Hei, Y.; Wang, R.; Wang, T.; Yang, J.; Li, J.; Di, Z.; Liu, Z.; Baskys, A.; Liu, W. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics 2019, 9, 5956. [Google Scholar]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Gardiner, C.; Ferreira, Y.J.; Dragovic, R.A.; Redman, C.W.; Sargent, I.L. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J. Extracell. Vesicles 2013, 2, 19671. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Fu, X.; Li, X.; Li, J.; Han, W.; Wang, Y. Modification of adipose mesenchymal stem cells-derived small extracellular vesicles with fibrin-targeting peptide CREKA for enhanced bone repair. Bioact. Mater. 2023, 20, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Bauder, A.R.; Ferguson, T.A. Reproducible mouse sciatic nerve crush and subsequent assessment of regeneration by whole mount muscle analysis. J. Vis. Exp. JoVE 2012, 3606. [Google Scholar] [CrossRef]
- Varejão, A.S.; Melo-Pinto, P.; Meek, M.F.; Filipe, V.M.; Bulas-Cruz, J. Methods for the experimental functional assessment of rat sciatic nerve regeneration. Neurol. Res. 2004, 26, 186–194. [Google Scholar]
- Brown, C.; Mackinnon, S.; Evans, P.; Bain, J.; Makino, A.; Hunter, O.; Hare, G. Self-evaluation of walking-track measurement using a sciatic function index. Microsurgery 1989, 10, 226–235. [Google Scholar]
- Bain, J.; Mackinnon, S.; Hunter, D. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast. Reconstr. Surg. 1989, 83, 129–136. [Google Scholar]
- Ruhl, T.; Christer, T.; Rhode, S.C.; Beier, J.P. Time course of functional recovery after 1 cm sciatic nerve resection in rats with or without surgical intervention-measured by grip strength and locomotor activity. Neurosci. Res. 2023, 190, 78–84. [Google Scholar]
- Phan, T.T.; Jayathilake, N.J.; Lee, K.P.; Park, J.M. BDNF/TrkB Signaling Inhibition Suppresses Astrogliosis and Alleviates Mechanical Allodynia in a Partial Crush Injury Model. Exp. Neurobiol. 2023, 32, 343. [Google Scholar] [CrossRef]
- Zu, R.; Wu, L.; Zhou, R.; Cao, B.; Liu, S.; Yang, G.; Leng, P.; Li, Y.; Zhang, L.; Song, X. A new classifier constructed with platelet features for malignant and benign pulmonary nodules based on prospective real-world data. J. Cancer 2022, 13, 2515. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.; Han, Y.; He, Q. ClusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Soekmadji, C.; Hill, A.F.; Wauben, M.H.; Buzás, E.I.; Di Vizio, D.; Falcon-Perez, J.M.; Gardiner, C.; Hochberg, F.; Kurochkin, I.V. Updating the MISEV minimal requirements for extracellular vesicle studies: Building bridges to reproducibility. J. Extracell. Vesicles 2017, 6, 1396823. [Google Scholar]
- Jimenez-Andrade, J.M.; Herrera, M.B.; Ghilardi, J.R.; Vardanyan, M.; Melemedjian, O.K.; Mantyh, P.W. Vascularization of the dorsal root ganglia and peripheral nerve of the mouse: Implications for chemical-induced peripheral sensory neuropathies. Mol. Pain 2008, 4, 10. [Google Scholar] [CrossRef]
- Sharma, K.; Zhang, Y.; Paudel, K.R.; Kachelmeier, A.; Hansbro, P.M.; Shi, X. The emerging role of pericyte-derived extracellular vesicles in vascular and neurological health. Cells 2022, 11, 3108. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.-L.G. Current status of therapeutic approaches against peripheral nerve injuries: A detailed story from injury to recovery. Int. J. Biol. Sci. 2020, 16, 116. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef]
- Saffari, S.; Saffari, T.M.; Ulrich, D.J.; Hovius, S.E.; Shin, A.Y. The interaction of stem cells and vascularity in peripheral nerve regeneration. Neural Regen. Res. 2021, 16, 1510–1517. [Google Scholar]
- Teli, P.; Kale, V.; Vaidya, A. Extracellular vesicles isolated from mesenchymal stromal cells primed with neurotrophic factors and signaling modifiers as potential therapeutics for neurodegenerative diseases. Curr. Res. Transl. Med. 2021, 69, 103286. [Google Scholar] [CrossRef]
- Flamant, S.; Loinard, C.; Tamarat, R. MSC beneficial effects and limitations, and MSC-derived extracellular vesicles as a new cell-free therapy for tissue regeneration in irradiated condition. Environ. Adv. 2023, 13, 100408. [Google Scholar]
- Fox, I.J.; Daley, G.Q.; Goldman, S.A.; Huard, J.; Kamp, T.J.; Trucco, M. Use of differentiated pluripotent stem cells in replacement therapy for treating disease. Science 2014, 345, 1247391. [Google Scholar] [PubMed]
- Aldoghachi, A.F.; Loh, J.-K.; Wang, M.-L.; Yang, Y.-P.; Chien, C.-S.; Teh, H.X.; Omar, A.H.; Cheong, S.-K.; Yeap, S.K.; Ho, W.Y. Current developments and therapeutic potentials of exosomes from induced pluripotent stem cells-derived mesenchymal stem cells. J. Chin. Med. Assoc. 2023, 86, 356–365. [Google Scholar] [PubMed]
- Zhang, Q.; Liu, J.; Wang, W.; Lin, W.; Ahmed, W.; Duan, W.; Huang, S.; Zhu, Z.; Chen, L. The role of exosomes derived from stem cells in nerve regeneration: A contribution to neurological repair. Exp. Neurol. 2024, 380, 114882. [Google Scholar]
- Zhong, L.; Wang, J.; Wang, P.; Liu, X.; Liu, P.; Cheng, X.; Cao, L.; Wu, H.; Chen, J.; Zhou, L. Neural stem cell-derived exosomes and regeneration: Cell-free therapeutic strategies for traumatic brain injury. Stem Cell Res. Ther. 2023, 14, 198. [Google Scholar]
- Tsukamoto, M.; Kimura, K.; Tanaka, M.; Kuwamura, M.; Ohtaka, M.; Nakanishi, M.; Sugiura, K.; Hatoya, S. Generation of footprint-free canine induced pluripotent stem cells from peripheral blood mononuclear cells using Sendai virus vector. Mol. Reprod. Dev. 2020, 87, 663–665. [Google Scholar]
- Andrews, P.W.; Gokhale, P.J. A short history of pluripotent stem cells markers. Stem Cell Rep. 2023, 19, 1–10. [Google Scholar]
- Fusaki, N.; Ban, H.; Nishiyama, A.; Saeki, K.; Hasegawa, M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn. Acad. Ser. B 2009, 85, 348–362. [Google Scholar]
- Potapova, T.A.; Zhu, J.; Li, R. Aneuploidy and chromosomal instability: A vicious cycle driving cellular evolution and cancer genome chaos. Cancer Metastasis Rev. 2013, 32, 377–389. [Google Scholar]
- Hu, X.-M.; Wang, C.-C.; Xiao, Y.; Liu, Y.; Huang, H.-R.; Jiang, P.; Wang, Y.-K.; Lin, Y.-J.; Li, L.-C.; Qi, Z.-Q. Non-Clinical Safety Evaluation of Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells in Cynomolgus Monkeys. Int. J. Nanomed. 2024, 19, 4923–4939. [Google Scholar]
- Lee, H.; Blaufox, M. Blood volume in the rat. J. Nucl. Med. 1985, 26, 72–76. [Google Scholar] [PubMed]
- Jiang, W.; Tan, Y.; Cai, M.; Zhao, T.; Mao, F.; Zhang, X.; Xu, W.; Yan, Z.; Qian, H.; Yan, Y. Human umbilical cord MSC-derived exosomes suppress the development of CCl4-induced liver injury through antioxidant effect. Stem Cells Int. 2018, 2018, 6079642. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhou, Y.; Jiao, Z.; Wang, X.; Zhao, Y.; Li, Y.; Chen, H.; Yang, L.; Zhu, H.; Li, Y. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth through hedgehog signaling pathway. Cell. Physiol. Biochem. 2017, 42, 2242–2254. [Google Scholar] [CrossRef] [PubMed]
- Rahman, E.; Webb, W.R.; Rao, P.; Abu-Farsakh, H.N.; Upton, A.E.; Yu, N.; Garcia, P.E.; Ioannidis, S.; Sayed, K.; Philipp-Dormston, W.G. Exosomes Exposed: Overview Systematic Review on Evidence Versus Expectation in Aesthetic and Regenerative Medicine. Aesthetic Plast. Surg. 2025, 49, 557–568. [Google Scholar] [CrossRef]
- Aldali, F.; Deng, C.; Nie, M.; Chen, H. Advances in therapies using mesenchymal stem cells and their exosomes for treatment of peripheral nerve injury: State of the art and future perspectives. Neural Regen. Res. 2025, 20, 3151–3171. [Google Scholar] [CrossRef]
- Ioannides, P.; Giedzinski, E.; Limoli, C.L. Evaluating different routes of extracellular vesicle administration for cranial therapies. J. Cancer Metastasis Treat. 2020, 6, 10-20517. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Fu, X.; Han, N. Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes as Nanomedicine for Peripheral Nerve Injury. Int. J. Mol. Sci. 2024, 25, 7882. [Google Scholar] [CrossRef]
- Zuniga, J.R.; Radwan, A.M. Classification of nerve injuries. Trigeminal Nerve Inj. 2013, 17–25. [Google Scholar]
- Zhao, J.; Ding, Y.; He, R.; Huang, K.; Liu, L.; Jiang, C.; Liu, Z.; Wang, Y.; Yan, X.; Cao, F. Dose-effect relationship and molecular mechanism by which BMSC-derived exosomes promote peripheral nerve regeneration after crush injury. Stem Cell Res. Ther. 2020, 11, 360. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, M.; Yi, X.; Tao, J.; Li, S.; Jiang, Z.; Cheng, B.; Yuan, H.; Zhang, F. Acellular nerve grafts supplemented with induced pluripotent stem cell-derived exosomes promote peripheral nerve reconstruction and motor function recovery. Bioact. Mater. 2022, 15, 272–287. [Google Scholar] [CrossRef]
- Rau, C.-S.; Kuo, P.-J.; Wu, S.-C.; Huang, L.-H.; Lu, T.-H.; Wu, Y.-C.; Wu, C.-J.; Lin, C.-W.; Tsai, C.-W.; Hsieh, C.-H. Enhanced nerve regeneration by exosomes secreted by adipose-derived stem cells with or without FK506 stimulation. Int. J. Mol. Sci. 2021, 22, 8545. [Google Scholar] [CrossRef] [PubMed]
- Kmiotek-Wasylewska, K.; Łabędź-Masłowska, A.; Bobis-Wozowicz, S.; Karnas, E.; Noga, S.; Sekuła-Stryjewska, M.; Woźnicka, O.; Madeja, Z.; Dawn, B.; Zuba-Surma, E.K. Induced pluripotent stem cell-derived extracellular vesicles enriched with miR-126 induce proangiogenic properties and promote repair of ischemic tissue. FASEB J. 2024, 38, e23415. [Google Scholar] [PubMed]
- Poongodi, R.; Yang, T.-H.; Huang, Y.-H.; Yang, K.D.; Chen, H.-Z.; Chu, T.-Y.; Wang, T.-Y.; Lin, H.-C.; Cheng, J.-K. Stem cell exosome-loaded Gelfoam improves locomotor dysfunction and neuropathic pain in a rat model of spinal cord injury. Stem Cell Res. Ther. 2024, 15, 143. [Google Scholar] [CrossRef]
- Hsu, J.-M.; Shiue, S.-J.; Yang, K.D.; Shiue, H.-S.; Hung, Y.-W.; Pannuru, P.; Poongodi, R.; Lin, H.-Y.; Cheng, J.-K. Locally applied stem cell exosome-scaffold attenuates nerve injury-induced pain in rats. J. Pain Res. 2020, 13, 3257–3268. [Google Scholar]
- Yavuz, B.; Mutlu, E.C.; Ahmed, Z.; Ben-Nissan, B.; Stamboulis, A. Applications of stem cell-derived extracellular vesicles in nerve regeneration. Int. J. Mol. Sci. 2024, 25, 5863. [Google Scholar] [CrossRef]
- Isaacman-Beck, J. Molecular Analysis of Target Specific Peripheral Nerve Regeneration; University of Pennsylvania: Philadelphia, PA, USA, 2015. [Google Scholar]
- Gonzalez-Perez, F.; Udina, E.; Navarro, X. Extracellular matrix components in peripheral nerve regeneration. Int. Rev. Neurobiol. 2013, 108, 257–275. [Google Scholar]
- Huang, J.; Zhang, G.; Li, S.; Li, J.; Wang, W.; Xue, J.; Wang, Y.; Fang, M.; Zhou, N. Endothelial cell-derived exosomes boost and maintain repair-related phenotypes of Schwann cells via miR199-5p to promote nerve regeneration. J. Nanobiotechnol. 2023, 21, 10. [Google Scholar]
- Chen, J.; Ren, S.; Duscher, D.; Kang, Y.; Liu, Y.; Wang, C.; Yuan, M.; Guo, G.; Xiong, H.; Zhan, P. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. J. Cell. Physiol. 2019, 234, 23097–23110. [Google Scholar]
- Cai, W.; Liu, Y.; Zhang, T.; Ji, P.; Tian, C.; Liu, J.; Zheng, Z. GDNF facilitates the differentiation of ADSCs to Schwann cells and enhances nerve regeneration through GDNF/MTA1/Hes1 axis. Arch. Biochem. Biophys. 2024, 753, 109893. [Google Scholar]


| Gene | Direction | Primer Sequence |
|---|---|---|
| Akt1 | Forward | GTGGCAAGATGTGTATGAG |
| Reverse | CTGGCTGAGTAGGAGAAC | |
| PMP22 | Forward | TCGCGGTGCTAGTGTTGC |
| Reverse | GACAGGACGCTGAAGATGACA | |
| IL-10 | Forward | CAGTCAGCCAGACCCACAT |
| Reverse | GCTCCACTGCCTTGCTTT | |
| S100b | Forward | TTGCCCTCATTGATGTCTTCCA |
| Reverse | TCTGCCTTGATTCTTACAGGTGAC | |
| MBP | Forward | CCC CAG CTA AAT CTG CTG AG |
| Reverse | CCC CAG CTA AAT CTG CTG AG | |
| MPZ | Forward | CCT TCA AAT ATG CCT GGG T |
| Reverse | CAG CAC AGT CAG CTT GAG AG | |
| BDNF | Forward | CGTGGGGAGCTGAGCGTGTG |
| Reverse | GCCCCTGCAGCCTTCCTTC | |
| Col4a5 | Forward | GATCTCCAGGTGACCAAGGA |
| Reverse | CCTGAAATGCCAGTTCCAA | |
| FGF2 | Forward | ACCCGGCCACTTCAAGG |
| Reverse | GATGCGCAGGAAGAAGCC | |
| ANGPT4 | Forward | GCAAGGCACCACCTAACAGA |
| Reverse | GATGGACTGCTCCAGCTTCA | |
| CSF3R | Forward | GTTCTGCTGCAAGCAAAGCA |
| Reverse | GCAGCTGGAAGGTTTCCTCT | |
| GAPDH | Forward | TGC TGA GTA TGT CGT GGA G |
| Reverse | GTC TTC TGA GTG GCA GTG AT |
| Variables | Sham | Model + PBS | Model + iPCS-Exos | Reference Range | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| WBC | 11.47 | 3.43 | 11.72 | 3.31 | 9.18 | 1.37 | 8.9–13.9 |
| RBC | 7.29 | 13.88 | 7.02 | 1.65 | 8.11 | 0.27 | 9.6–11.3 |
| HGB | 143.75 | 0.67 | 135.83 | 37.05 | 159 | 6.68 | 137–160 |
| HCT | 38.9 | 4.60 | 38.36 | 8.74 | 42.55 | 1.72 | 42.2–49.5 |
| MCV | 53.25 | 0.23 | 54.88 | 2.09 | 52.45 | 0.75 | 43.1–44.1 |
| MCH | 19.72 | 1.188 | 19.15 | 1.13 | 19.57 | 0.23 | 13.6–15.3 |
| MCHC | 370.7 | 11.87 | 350 | 31.4 | 373 | 3.74 | 317–330.4 |
| PLT | 372.5 | 182 | 360.1 | 281.4 | 422.25 | 195.93 | 929–1089.5 |
| Lym% | 61.27 | 8.2 | 67.4 | 7.54 | 68.97 | 10.40 | 51.5–66.5 |
| Neu% | 30.5 | 7.86 | 23.6 | 7.55 | 19.87 | 6.89 | 10–30 |
| Mon% | 3.5 | 1.56 | 4.72 | 2.163 | 7.55 | 2.63 | 2–6 |
| Eos% | 4.5 | 1.47 | 3.85 | 1.33 | 3.42 | 1.03 | 0.5–4.5 |
| Bas% | 0.675 | 0.63 | 1.05 | 0.59 | 0.17 | 0.09 | 0.00–0.089 |
| Neu# | 3.41 | 1.18 | 2.8 | 1.29 | 1.88 | 0.94 | 1.83–3.6 |
| Lym# | 7.1 | 2.53 | 7.85 | 2.16 | 6.25 | 0.66 | 6.3–10.6 |
| Mon# | 0.36 | 0.24 | 0.49 | 0.30 | 0.71 | 0.36 | 0.1–0.6 |
| Eos# | 0.51 | 0.05 | 0.44 | 0.16 | 0.31 | 0.12 | 0.158–2.01 |
| Bas# | 0.07 | 0.05 | 0.125 | 0.08 | 0.017 | 0.009 | 0.0–0.67 |
| RDW-SD | 28.5 | 1.42 | 29.5 | 1.336 | 27.27 | 0.65 | 38–50 |
| RDW-CV | 14.4 | 0.46 | 14.45 | 0.45 | 14.1 | 0.31 | 13.0–18.5 |
| MPV | 8.3 | 0.52 | 7.43 | 0.37 | 7.27 | 0.20 | 13.8–15.4 |
| PLCR | 18.82 | 4.5 | 12.36 | 2.72 | 11.6 | 1.49 | 20–35 |
| PDW | 15.3 | 0.09 | 15.15 | 0.23 | 15 | 0.081 | - |
| PCT | 0.31 | 0.14 | 0.26 | 0.201 | 0.3075 | 0.138 | - |
| PCT | 0.31 | 0.14 | 0.26 | 0.201 | 0.3075 | 0.138 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldali, F.; Yang, Y.; Deng, C.; Li, X.; Cao, X.; Xu, J.; Li, Y.; Ding, J.; Chen, H. Induced Pluripotent Stem Cell-Derived Exosomes Promote Peripheral Nerve Regeneration in a Rat Sciatic Nerve Crush Injury Model: A Safety and Efficacy Study. Cells 2025, 14, 529. https://doi.org/10.3390/cells14070529
Aldali F, Yang Y, Deng C, Li X, Cao X, Xu J, Li Y, Ding J, Chen H. Induced Pluripotent Stem Cell-Derived Exosomes Promote Peripheral Nerve Regeneration in a Rat Sciatic Nerve Crush Injury Model: A Safety and Efficacy Study. Cells. 2025; 14(7):529. https://doi.org/10.3390/cells14070529
Chicago/Turabian StyleAldali, Fatima, Yujie Yang, Chunchu Deng, Xiangling Li, Xiaojian Cao, Jia Xu, Yajie Li, Jianlin Ding, and Hong Chen. 2025. "Induced Pluripotent Stem Cell-Derived Exosomes Promote Peripheral Nerve Regeneration in a Rat Sciatic Nerve Crush Injury Model: A Safety and Efficacy Study" Cells 14, no. 7: 529. https://doi.org/10.3390/cells14070529
APA StyleAldali, F., Yang, Y., Deng, C., Li, X., Cao, X., Xu, J., Li, Y., Ding, J., & Chen, H. (2025). Induced Pluripotent Stem Cell-Derived Exosomes Promote Peripheral Nerve Regeneration in a Rat Sciatic Nerve Crush Injury Model: A Safety and Efficacy Study. Cells, 14(7), 529. https://doi.org/10.3390/cells14070529







