Diverse Roles of the Multiple Phosphodiesterases in the Regulation of Cyclic Nucleotide Signaling in Dictyostelium
Abstract
1. Introduction
2. Structural Properties of PDE Members
2.1. Class I PDEs
2.2. Class II PDEs
3. Enzymatic Properties of PDE Members
3.1. Extracellular cAMP PDEs
3.2. Intracellular cAMP PDEs
3.3. Intracellular cGMP PDEs
4. Structural Properties of Adenylyl and Guanylyl Cyclases
4.1. The Adenylyl Cyclases
4.2. The Guanylyl Cyclases
5. Cyclic Nucleotide Target Proteins
5.1. cAMP Targets
5.2. cGMP Targets

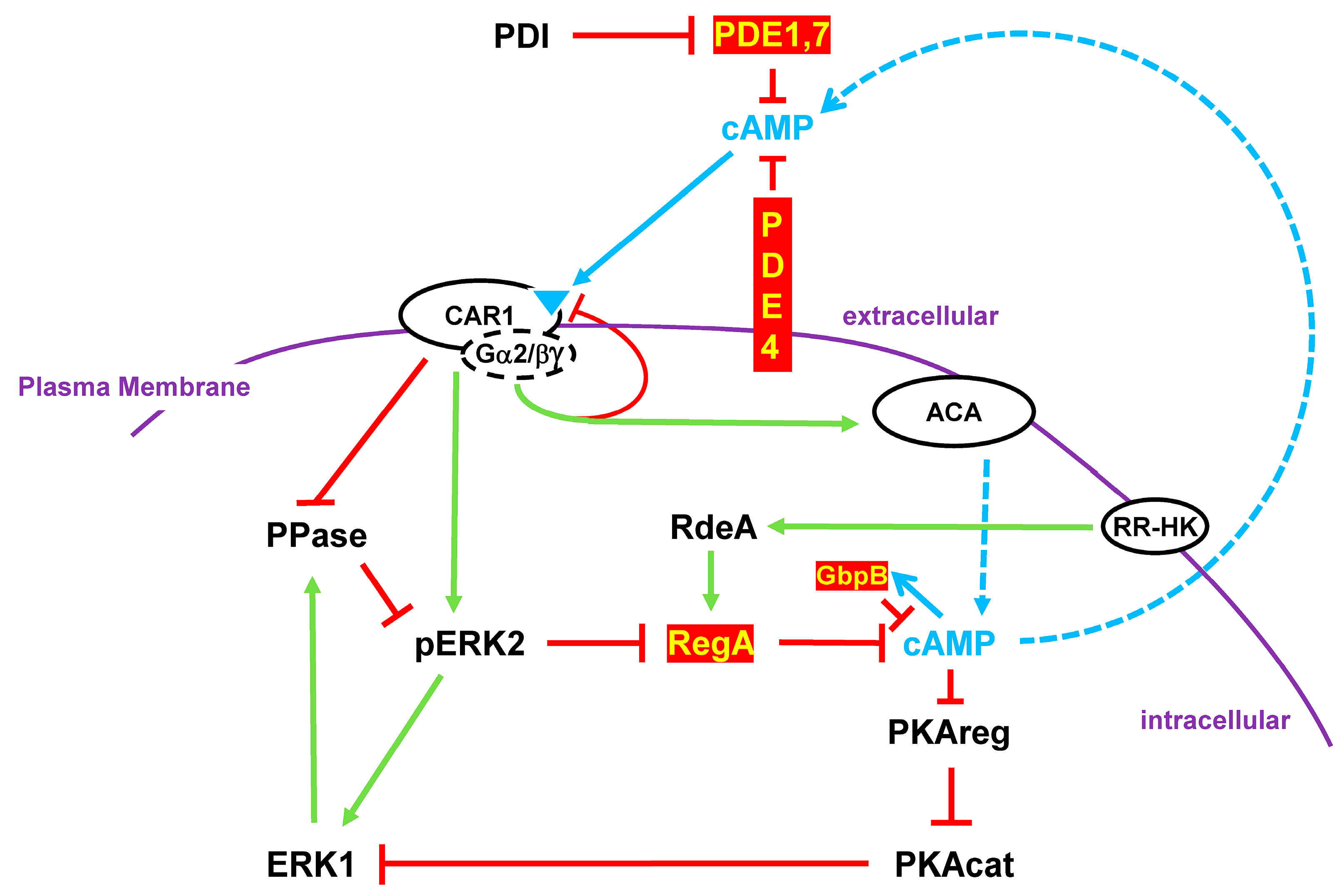
6. Good, Good, Good Oscillations
PDEs and cAMP Oscillations
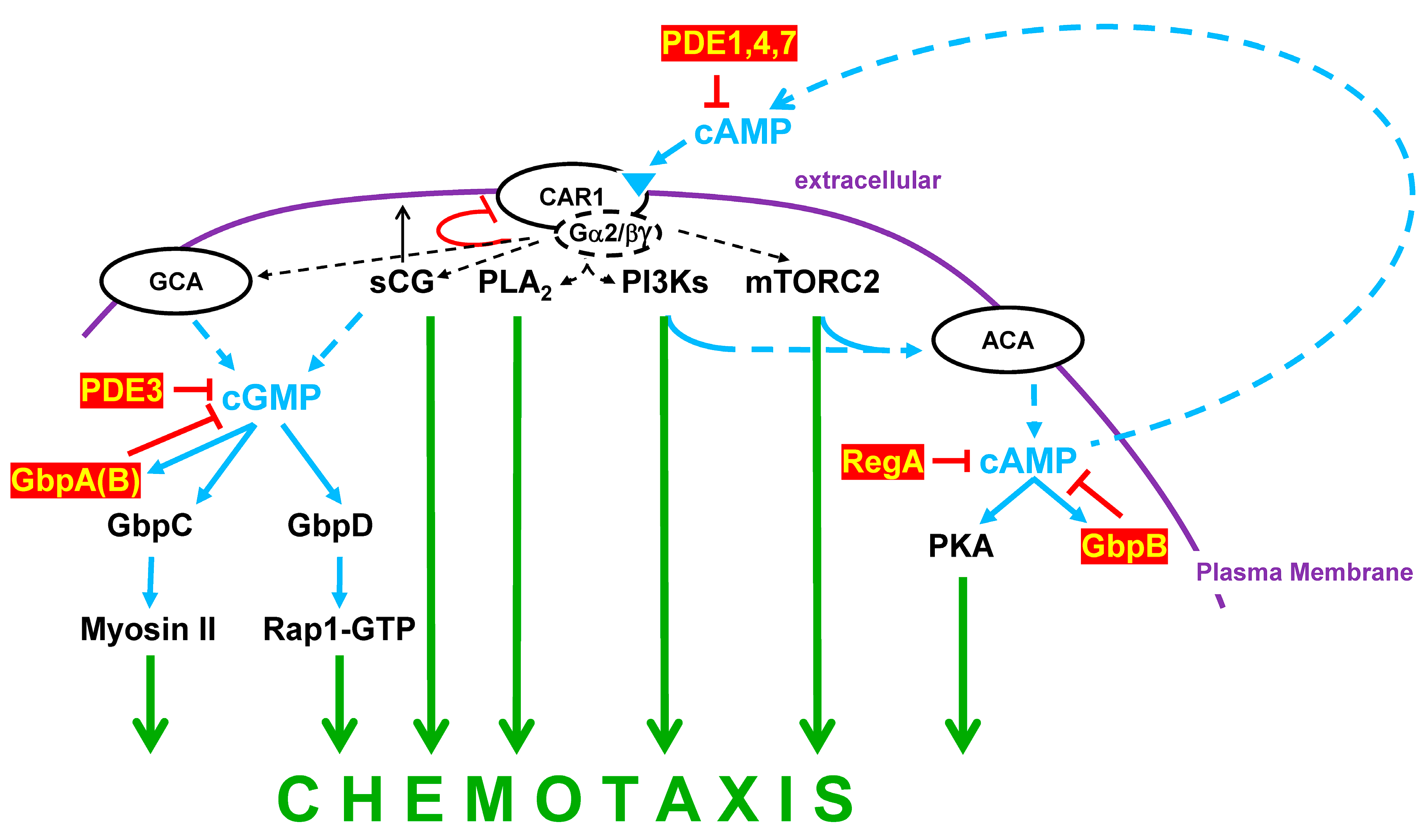
7. Turn, Turn, Turn
PDEs and Chemotaxis
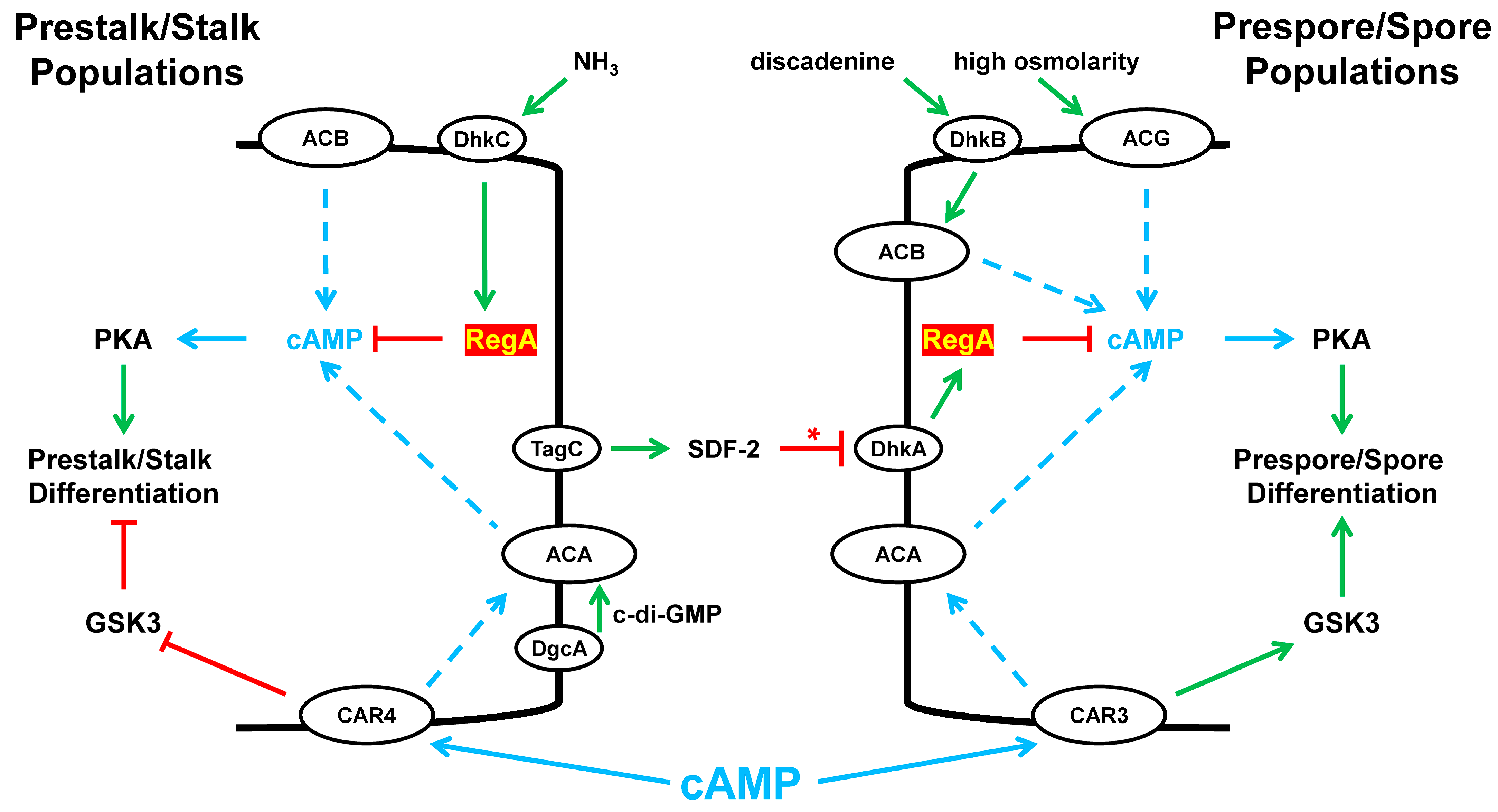
8. Instant Karma
RegA and Fate Specificity
9. This Is the End
PDEs, Disease, Therapeutics, and Drug Discovery
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaiswal, P.; Kimmel, A.R. mTORC1/AMPK responses define a core gene set for developmental cell fate switching. BMC Biol. 2019, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.; Majithia, A.R.; Rosel, D.; Liao, X.H.; Khurana, T.; Kimmel, A.R. Integrated actions of mTOR complexes 1 and 2 for growth and development of Dictyostelium. Int. J. Dev. Biol. 2019, 63, 521–527. [Google Scholar] [CrossRef] [PubMed]
- van Haastert, P.J.M.; Keizer-Gunnink, I.; Pots, H.; Ortiz-Mateos, C.; Veltman, D.; van Egmond, W.; Kortholt, A. Forty-five years of cGMP research in Dictyostelium: Understanding the regulation and function of the cGMP pathway for cell movement and chemotaxis. Mol. Biol. Cell 2021, 32, ar8. [Google Scholar] [CrossRef]
- Jaiswal, P.; Meena, N.P.; Chang, F.S.; Liao, X.H.; Kim, L.; Kimmel, A.R. An integrated, cross-regulation pathway model involving activating/adaptive and feed-forward/feed-back loops for directed oscillatory cAMP signal-relay/response during the development of Dictyostelium. Front. Cell Dev. Biol. 2023, 11, 1263316. [Google Scholar] [CrossRef]
- McMains, V.C.; Liao, X.H.; Kimmel, A.R. Oscillatory signaling and network responses during the development of Dictyostelium discoideum. Ageing Res. Rev. 2008, 7, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Saxe, C.L., 3rd; Johnson, R.L.; Devreotes, P.N.; Kimmel, A.R. Expression of a cAMP receptor gene of Dictyostelium and evidence for a multigene family. Genes. Dev. 1991, 5, 1–8. [Google Scholar] [CrossRef]
- Johnson, R.L.; Vaughan, R.A.; Caterina, M.J.; Van Haastert, P.J.; Devreotes, P.N. Overexpression of the cAMP receptor 1 in growing Dictyostelium cells. Biochemistry 1991, 30, 6982–6986. [Google Scholar] [CrossRef]
- Klein, P.S.; Sun, T.J.; Saxe, C.L.; Kimmel, A.R.; Johnson, R.L.; Devreotes, P.N. A Chemoattractant Receptor Controls Development in Dictyostelium discoideum. Science 1988, 241, 1467–1472. [Google Scholar] [CrossRef]
- Saxe, C.L., 3rd; Ginsburg, G.T.; Louis, J.M.; Johnson, R.; Devreotes, P.N.; Kimmel, A.R. CAR2, a prestalk cAMP receptor required for normal tip formation and late development of Dictyostelium discoideum. Genes. Dev. 1993, 7, 262–272. [Google Scholar] [CrossRef]
- Johnson, R.L.; Saxe, C.L., 3rd; Gollop, R.; Kimmel, A.R.; Devreotes, P.N. Identification and targeted gene disruption of cAR3, a cAMP receptor subtype expressed during multicellular stages of Dictyostelium development. Genes. Dev. 1993, 7, 273–282. [Google Scholar] [CrossRef]
- Kuhn, J.; Lin, Y.; Devreotes, P.N. Using Live-Cell Imaging and Synthetic Biology to Probe Directed Migration in Dictyostelium. Front. Cell Dev. Biol. 2021, 9, 740205. [Google Scholar] [CrossRef]
- Brzostowski, J.A.; Kimmel, A.R. Nonadaptive regulation of ERK2 in Dictyostelium: Implications for mechanisms of cAMP relay. Mol. Biol. Cell 2006, 17, 4220–4227. [Google Scholar] [CrossRef] [PubMed]
- Shaulsky, G.; Fuller, D.; Loomis, W.F. A cAMP-phosphodiesterase controls PKA-dependent differentiation. Development 1998, 125, 691–699. [Google Scholar] [CrossRef]
- Thomason, P.A.; Traynor, D.; Stock, J.B.; Kay, R.R. The RdeA-RegA system, a eukaryotic phospho-relay controlling cAMP breakdown. J. Biol. Chem. 1999, 274, 27379–27384. [Google Scholar] [CrossRef]
- Manahan, C.L.; Iglesias, P.A.; Long, Y.; Devreotes, P.N. Chemoattractant signaling in Dictyostelium discoideum. Annu. Rev. Cell Dev. Biol. 2004, 20, 223–253. [Google Scholar] [CrossRef]
- Bader, S.; Kortholt, A.; Van Haastert, P.J. Seven Dictyostelium discoideum phosphodiesterases degrade three pools of cAMP and cGMP. Biochem. J. 2007, 402, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Tulsian, N.K.; Chandramohan, A.; Anand, G.S. Parallel Allostery by cAMP and PDE Coordinates Activation and Termination Phases in cAMP Signaling. Biophys. J. 2015, 109, 1251–1263. [Google Scholar] [CrossRef]
- Kuburich, N.A.; Adhikari, N.; Hadwiger, J.A. Multiple phosphorylation sites on the RegA phosphodiesterase regulate Dictyostelium development. Cell. Signal. 2019, 57, 65–75. [Google Scholar] [CrossRef]
- Pitt, G.S.; Milona, N.; Borleis, J.; Lin, K.C.; Reed, R.R.; Devreotes, P.N. Structurally distinct and stage-specific adenylyl cyclase genes play different roles in Dictyostelium development. Cell 1992, 69, 305–315. [Google Scholar] [CrossRef]
- Martiel, J.-L.; Goldbeter, A. A Model Based on Receptor Desensitization for Cyclic AMP Signaling in Dictyostelium Cells. Biophys. J. 1987, 52, 807–828. [Google Scholar] [CrossRef]
- Maeda, M.; Lu, S.; Shaulsky, G.; Miyazaki, Y.; Kuwayama, H.; Tanaka, Y.; Kuspa, A.; Loomis, W.F. Periodic Signaling Controlled by an Oscillatory Circuit That Includes Protein Kinases ERK2 and PKA. Science 2004, 304, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, J.; Snippe, H.; Kleineidam, R.G.; Van Haastert, P.J. Guanylate cyclase in Dictyostelium discoideum with the topology of mammalian adenylate cyclase. Biochem. J. 2001, 354, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Gerisch, G.; Malchow, D.; Roos, W.; Wick, U. Oscillations of Cyclic Nucleotide Concentrations in Relation to the Excitability of Dictyostelium Cells. J. Exp. Biol. 1979, 81, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Wurster, B.; Schubiger, K.; Wick, U.; Gerisch, G. Cyclic GMP in Dictyostelium discoideum, Oscillations and pulses in response to folic acid and cyclic AMP signals. FEBS Lett. 1977, 76, 141–144. [Google Scholar] [CrossRef]
- Firtel, R.A.; Chapman, A.L. A role for cAMP-dependent protein kinase A in early Dictyostelium development. Genes. Dev. 1990, 4, 18–28. [Google Scholar] [CrossRef]
- Goldberg, J.M.; Bosgraaf, L.; Van Haastert, P.J.; Smith, J.L. Identification of four candidate cGMP targets in Dictyostelium. Proc. Natl. Acad. Sci. USA 2002, 99, 6749–6754. [Google Scholar] [CrossRef]
- Delhaye, S.; Bardoni, B. Role of phosphodiesterases in the pathophysiology of neurodevelopmental disorders. Mol. Psychiatry 2021, 26, 4570–4582. [Google Scholar] [CrossRef]
- Keravis, T.; Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br. J. Pharmacol. 2012, 165, 1288–1305. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, Y.; Yan, C.; Xiang, Y.K. Phosphodiesterase in heart and vessels: From physiology to diseases. Physiol. Rev. 2024, 104, 765–834. [Google Scholar] [CrossRef]
- Peng, T.; Gong, J.; Jin, Y.; Zhou, Y.; Tong, R.; Wei, X.; Bai, L.; Shi, J. Inhibitors of phosphodiesterase as cancer therapeutics. Eur. J. Med. Chem. 2018, 150, 742–756. [Google Scholar] [CrossRef]
- Shaulsky, G.; Escalante, R.; Loomis, W.F. Developmental signal transduction pathways uncovered by genetic suppressors. Proc. Natl. Acad. Sci. USA 1996, 93, 15260–15265. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Thomason, P.A.; Gross, J.D.; Newell, P.C. Evidence that the RdeA protein is a component of a multistep phosphorelay modulating rate of development in Dictyostelium. EMBO J. 1998, 17, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Thomason, P.A.; Traynor, D.; Cavet, G.; Chang, W.T.; Harwood, A.J.; Kay, R.R. An intersection of the cAMP/PKA and two-component signal transduction systems in Dictyostelium. EMBO J. 1998, 17, 2838–2845. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Söderbom, F.; Anjard, C.; Shaulsky, G.; Loomis, W.F. SDF-2 induction of terminal differentiation in Dictyostelium discoideum is mediated by the membrane-spanning sensor kinase DhkA. Mol. Cell Biol. 1999, 19, 4750–4756. [Google Scholar] [CrossRef]
- Anjard, C.; Loomis, W.F. Peptide signaling during terminal differentiation of Dictyostelium. Proc. Natl. Acad. Sci. USA 2005, 102, 7607–7611. [Google Scholar] [CrossRef]
- Wolanin, P.M.; Thomason, P.A.; Stock, J.B. Histidine protein kinases: Key signal transducers outside the animal kingdom. Genome Biol. 2002, 3, reviews3013.1. [Google Scholar] [CrossRef]
- Singleton, C.K.; Zinda, M.J.; Mykytka, B.; Yang, P. The histidine kinase dhkC regulates the choice between migrating slugs and terminal differentiation in Dictyostelium discoideum. Dev. Biol. 1998, 203, 345–357. [Google Scholar] [CrossRef]
- Kuwayama, H.; Snippe, H.; Derks, M.; Roelofs, J.; Van Haastert, P.J. Identification and characterization of DdPDE3, a cGMP-selective phosphodiesterase from Dictyostelium. Biochem. J. 2001, 353, 635–644. [Google Scholar] [CrossRef]
- Bader, S.; Kortholt, A.; Snippe, H.; Van Haastert, P.J.M. DdPDE4, a Novel cAMP-specific Phosphodiesterase at the Surface of Dictyostelium Cells. J. Biol. Chem. 2006, 281, 20018–20026. [Google Scholar] [CrossRef]
- Wu, L.; Franke, J. A developmentally regulated and cAMP-repressible gene of Dictyostelium discoideum: Cloning and expression of the gene encoding cyclic nucleotide phosphodiesterase inhibitor. Gene 1990, 91, 51–56. [Google Scholar] [CrossRef]
- Barra, J.; Barrand, P.; Blondelet, M.-H.; Brachet, P. pdsA, a gene involved in the production of active phosphodiesterase during starvation of Dictyostelium discoideum amoebae. Mol. General. Genet. MGG 1980, 177, 607–613. [Google Scholar] [CrossRef]
- Bosgraaf, L.; Russcher, H.; Snippe, H.; Bader, S.; Wind, J.; Van Haastert, P.J.M. Identification and Characterization of Two Unusual cGMP-stimulated Phoshodiesterases in Dictyostelium. Mol. Biol. Cell 2002, 13, 3878–3889. [Google Scholar] [CrossRef] [PubMed]
- Bosgraaf, L.; Russcher, H.; Smith, J.L.; Wessels, D.; Soll, D.R.; Van Haastert, P.J.M. A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium. EMBO J. 2002, 21, 4560–4570. [Google Scholar] [CrossRef]
- Meima, M.E.; Biondi, R.M.; Schaap, P. Identification of a novel type of cGMP phosphodiesterase that is defective in the chemotactic stmF mutants. Mol. Biol. Cell 2002, 13, 3870–3877. [Google Scholar] [CrossRef] [PubMed]
- Ott, A.; Oehme, F.; Keller, H.; Schuster, S.C. Osmotic stress response in Dictyostelium is mediated by cAMP. EMBO J. 2000, 19, 5782–5792. [Google Scholar] [CrossRef]
- Meima, M.E.; Weening, K.E.; Schaap, P. Characterization of a cAMP-stimulated cAMP Phosphodiesterase inDictyostelium discoideum. J. Biol. Chem. 2003, 278, 14356–14362. [Google Scholar] [CrossRef]
- Jaiswal, P.; Soldati, T.; Thewes, S.; Baskar, R. Regulation of aggregate size and pattern by adenosine and caffeine in cellular slime molds. BMC Dev. Biol. 2012, 12, 5. [Google Scholar] [CrossRef]
- Jaiswal, P.; Singh, S.P.; Aiyar, P.; Akkali, R.; Baskar, R. Regulation of multiple tip formation by caffeine in cellular slime molds. BMC Dev. Biol. 2012, 12, 26. [Google Scholar] [CrossRef]
- Alvarez-Curto, E.; Weening, K.E.; Schaap, P. Pharmacological profiling of the Dictyostelium adenylate cyclases ACA, ACB and ACG. Biochem. J. 2007, 401, 309–316. [Google Scholar] [CrossRef]
- Brenner, M.; Thoms, S.D. Caffeine blocks activation of cyclic AMP synthesis in Dictyostelium discoideum. Dev. Biol. 1984, 101, 136–146. [Google Scholar] [CrossRef]
- Pannbacker, R.G.; Bravard, L.J. Phosphodiesterase in Dictyostelium discoideum and the chemotactic response to cyclic adenosine monophosphate. Science 1972, 175, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Van Haastert, P.J.M.; Bijleveld, W.; Konijn, T.M. Phosphodiesterase induction in Dictyostelium discoideum by inhibition of extracellular phosphodiesterase activity. Dev. Biol. 1982, 94, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Robery, S.; Mukanowa, J.; Percie du Sert, N.; Andrews, P.L.R.; Williams, R.S.B. Investigating the Effect of Emetic Compounds on Chemotaxis in Dictyostelium Identifies a Non-Sentient Model for Bitter and Hot Tastant Research. PLoS ONE 2011, 6, e24439. [Google Scholar] [CrossRef]
- Garcia, G.L.; Rericha, E.C.; Heger, C.D.; Goldsmith, P.K.; Parent, C.A. The Group Migration of Dictyostelium Cells Is Regulated by Extracellular Chemoattractant Degradation. Mol. Biol. Cell 2009, 20, 3295–3304. [Google Scholar] [CrossRef]
- Alvarez-Curto, E.; Meima, M.E.; Schaap, P. Expression and role of adenylyl cyclases during late development in Dictyostelium discoideum. Int. J. Dev. Biol. 2001, 45, S147–S148. [Google Scholar]
- Söderbom, F.; Anjard, C.; Iranfar, N.; Fuller, D.; Loomis, W.F. An adenylyl cyclase that functions during late development of Dictyostelium. Development 1999, 126, 5463–5471. [Google Scholar] [CrossRef] [PubMed]
- Kriebel, P.W.; Parent, C.A. Adenylyl cyclase expression and regulation during the differentiation of Dictyostelium discoideum. IUBMB Life 2004, 56, 541–546. [Google Scholar] [CrossRef]
- Anjard, C.; Söderbom, F.; Loomis, W.F. Requirements for the adenylyl cyclases in the development of Dictyostelium. Development 2001, 128, 3649–3654. [Google Scholar] [CrossRef]
- Meima, M.E.; Schaap, P. Fingerprinting of Adenylyl Cyclase Activities during Dictyostelium Development Indicates a Dominant Role for Adenylyl Cyclase B in Terminal Differentiation. Dev. Biol. 1999, 212, 182–190. [Google Scholar] [CrossRef]
- van Es, S.; Virdy, K.J.; Pitt, G.S.; Meima, M.; Sands, T.W.; Devreotes, P.N.; Cotter, D.A.; Schaap, P. Adenylyl cyclase G, an osmosensor controlling germination of Dictyostelium spores. J. Biol. Chem. 1996, 271, 23623–23625. [Google Scholar] [CrossRef]
- Anjard, C.; Loomis, W.F. Cytokinins induce sporulation in Dictyostelium. Development 2008, 135, 819–827. [Google Scholar] [CrossRef]
- Basu, S.; Fey, P.; Jimenez-Morales, D.; Dodson, R.J.; Chisholm, R.L. dictyBase 2015: Expanding data and annotations in a new software environment. Genesis 2015, 53, 523–534. [Google Scholar] [CrossRef]
- Fey, P.; Dodson, R.J.; Basu, S.; Hartline, E.C.; Chisholm, R.L. dictyBase and the Dicty Stock Center (version 2.0)—A progress report. Int. J. Dev. Biol. 2019, 63, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.X.U.; Roberts, C.; Alexander, H.; Stewart, A.M.; Harwood, A.; Alexander, S.; Insall, R.H. Methanol and acriflavine resistance in Dictyostelium are caused by loss of catalase The GenBank accession number for the sequence reported in this paper is AF090443. Microbiology 2002, 148, 333–340. [Google Scholar] [CrossRef]
- Roelofs, J.; Van Haastert, P.J. Characterization of two unusual guanylyl cyclases from dictyostelium. J. Biol. Chem. 2002, 277, 9167–9174. [Google Scholar] [CrossRef]
- Veltman, D.M.; Roelofs, J.; Engel, R.; Visser, A.J.; Van Haastert, P.J. Activation of soluble guanylyl cyclase at the leading edge during Dictyostelium chemotaxis. Mol. Biol. Cell 2005, 16, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Veltman, D.M.; Van Haastert, P.J.M. Guanylyl Cyclase Protein and cGMP Product Independently Control Front and Back of Chemotaxing Dictyostelium Cells. Mol. Biol. Cell 2006, 17, 3921–3929. [Google Scholar] [CrossRef]
- Johnson, R.L.; Van Haastert, P.J.; Kimmel, A.R.; Saxe, C.L., 3rd; Jastorff, B.; Devreotes, P.N. The cyclic nucleotide specificity of three cAMP receptors in Dictyostelium. J. Biol. Chem. 1992, 267, 4600–4607. [Google Scholar] [PubMed]
- Devreotes, P.N. G protein-linked signaling pathways control the developmental program of Dictyostelium. Neuron 1994, 12, 235–241. [Google Scholar] [CrossRef]
- Sun, T.J.; Devreotes, P.N. Gene targeting of the aggregation stage cAMP receptor cAR1 in Dictyostelium. Genes. Dev. 1991, 5, 572–582. [Google Scholar] [CrossRef]
- Louis, J.M.; Ginsburg, G.T.; Kimmel, A.R. The cAMP receptor CAR4 regulates axial patterning and cellular differentiation during late development of Dictyostelium. Genes Dev. 1994, 8, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, G.T.; Kimmel, A.R. Autonomous and nonautonomous regulation of axis formation by antagonistic signaling via 7-span cAMP receptors and GSK3 in Dictyostelium. Genes Dev. 1997, 11, 2112–2123. [Google Scholar] [CrossRef]
- Kim, L.; Brzostowski, J.; Majithia, A.; Lee, N.S.; McMains, V.; Kimmel, A.R. Combinatorial cell-specific regulation of GSK3 directs cell differentiation and polarity in Dictyostelium. Development 2011, 138, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Harwood, A.; Kimmel, A.R. Receptor-Dependent and Tyrosine Phosphatase-Mediated Inhibition of GSK3 Regulates Cell Fate Choice. Dev. Cell 2002, 3, 523–532. [Google Scholar] [CrossRef]
- Kimmel, A.R.; Firtel, R.A. Breaking symmetries: Regulation of Dictyostelium development through chemoattractant and morphogen signal-response. Curr. Opin. Genet. Dev. 2004, 14, 540–549. [Google Scholar] [CrossRef]
- Kim, L.; Liu, J.; Kimmel, A.R. The Novel Tyrosine Kinase ZAK1 Activates GSK3 to Direct Cell Fate Specification. Cell 1999, 99, 399–408. [Google Scholar] [CrossRef]
- Turnham, R.E.; Scott, J.D. Protein kinase A catalytic subunit isoform PRKACA.; History, function and physiology. Gene 2016, 577, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Loomis William, F. Role of PKA in the Timing of Developmental Events in Dictyostelium Cells. Microbiol. Mol. Biol. Rev. 1998, 62, 684–694. [Google Scholar] [CrossRef]
- Mann, S.K.; Firtel, R.A. A developmentally regulated, putative serine/threonine protein kinase is essential for development in Dictyostelium. Mech. Dev. 1991, 35, 89–101. [Google Scholar] [CrossRef]
- Abe, K.; Saga, Y.; Okada, H.; Yanagisawa, K. Differentiation of Dictyostelium discoideum mutant cells in a shaken suspension culture and the effect of cyclic AMP. J. Cell Sci. 1981, 51, 131–142. [Google Scholar] [CrossRef]
- Taminato, A.; Bagattini, R.; Gorjão, R.; Chen, G.; Kuspa, A.; Souza, G.M. Role for YakA, cAMP, and protein kinase A in regulation of stress responses of Dictyostelium discoideum cells. Mol. Biol. Cell 2002, 13, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Kortholt, A.; Rehmann, H.; Kae, H.; Bosgraaf, L.; Keizer-Gunnink, I.; Weeks, G.; Wittinghofer, A.; Van Haastert, P.J.M. Characterization of the GbpD-activated Rap1 Pathway Regulating Adhesion and Cell Polarity in Dictyostelium discoideum. J. Biol. Chem. 2006, 281, 23367–23376. [Google Scholar] [CrossRef]
- Kortholt, A.; van Egmond, W.N.; Plak, K.; Bosgraaf, L.; Keizer-Gunnink, I.; van Haastert, P.J. Multiple regulatory mechanisms for the Dictyostelium Roco protein GbpC. J. Biol. Chem. 2012, 287, 2749–2758. [Google Scholar] [CrossRef]
- Marín, I.; van Egmond, W.N.; van Haastert, P.J.M. The Roco protein family: A functional perspective. FASEB J. 2008, 22, 3103–3110. [Google Scholar] [CrossRef]
- van Egmond, W.N.; Kortholt, A.; Plak, K.; Bosgraaf, L.; Bosgraaf, S.; Keizer-Gunnink, I.; van Haastert, P.J.M. Intramolecular Activation Mechanism of the Dictyostelium LRRK2 Homolog Roco Protein GbpC. J. Biol. Chem. 2008, 283, 30412–30420. [Google Scholar] [CrossRef]
- Bosgraaf, L.; Waijer, A.; Engel, R.; Visser, A.J.W.G.; Wessels, D.; Soll, D.; van Haastert, P.J.M. RasGEF-containing proteins GbpC and GbpD have differential effects on cell polarity and chemotaxis in Dictyostelium. J. Cell Sci. 2005, 118, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Zimprich, A.; Biskup, S.; Leitner, P.; Lichtner, P.; Farrer, M.; Lincoln, S.; Kachergus, J.; Hulihan, M.; Uitti, R.J.; Calne, D.B.; et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004, 44, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Gilsbach, B.K.; Ho, F.Y.; Vetter, I.R.; van Haastert, P.J.; Wittinghofer, A.; Kortholt, A. Roco kinase structures give insights into the mechanism of Parkinson disease-related leucine-rich-repeat kinase 2 mutations. Proc. Natl. Acad. Sci. USA 2012, 109, 10322–10327. [Google Scholar] [CrossRef]
- Wessels, D.J.; Zhang, H.; Reynolds, J.; Daniels, K.; Heid, P.; Lu, S.; Kuspa, A.; Shaulsky, G.; Loomis, W.F.; Soll, D.R. The internal phosphodiesterase RegA is essential for the suppression of lateral pseudopods during Dictyostelium chemotaxis. Mol. Biol. Cell 2000, 11, 2803–2820. [Google Scholar] [CrossRef]
- Faure, M.; Podgorski, G.J.; Franke, J.; Kessin, R.H. Disruption of Dictyostelium discoideum morphogenesis by overproduction of cAMP phosphodiesterase. Proc. Natl. Acad. Sci. USA 1988, 85, 8076–8080. [Google Scholar] [CrossRef]
- Weening, K.E.; Wijk, I.V.-V.; Thompson, C.R.; Kessin, R.H.; Podgorski, G.J.; Schaap, P. Contrasting activities of the aggregative and late PDSA promoters in Dictyostelium development. Dev. Biol. 2003, 255, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Kriebel, P.W.; Barr, V.A.; Rericha, E.C.; Zhang, G.; Parent, C.A. Collective cell migration requires vesicular trafficking for chemoattractant delivery at the trailing edge. J. Cell Biol. 2008, 183, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Veltman, D.M.; van Haastert, P.J. The role of cGMP and the rear of the cell in Dictyostelium chemotaxis and cell streaming. J. Cell Sci. 2008, 121, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Swaney, K.F.; Huang, C.H.; Devreotes, P.N. Eukaryotic chemotaxis: A network of signaling pathways controls motility, directional sensing, and polarity. Annu. Rev. Biophys. 2010, 39, 265–289. [Google Scholar] [CrossRef]
- Cai, H.; Devreotes, P.N. Moving in the right direction: How eukaryotic cells migrate along chemical gradients. Semin. Cell Dev. Biol. 2011, 22, 834–841. [Google Scholar] [CrossRef]
- Yu, Y.; Saxe, C.L., 3rd. Differential distribution of cAMP receptors cAR2 and cAR3 during Dictyostelium development. Dev. Biol. 1996, 173, 353–356. [Google Scholar] [CrossRef]
- Mohanty, S.; Lee, S.; Yadava, N.; Dealy, M.J.; Johnson, R.S.; Firtel, R.A. Regulated protein degradation controls PKA function and cell-type differentiation in Dictyostelium. Genes. Dev. 2001, 15, 1435–1448. [Google Scholar] [CrossRef]
- Chen, Z.H.; Schaap, P. The prokaryote messenger c-di-GMP triggers stalk cell differentiation in Dictyostelium. Nature 2012, 488, 680–683. [Google Scholar] [CrossRef]
- Richardson, D.L.; Loomis, W.F.; Kimmel, A.R. Progression of an inductive signal activates sporulation in Dictyostelium discoideum. Development 1994, 120, 2891–2900. [Google Scholar] [CrossRef]
- Balint-Kurti, P.; Ginsburg, G.T.; Liu, J.; Kimmel, A.R. Non-autonomous regulation of a graded, PKA-mediated transcriptional activation signal for cell patterning. Development 1998, 125, 3947–3954. [Google Scholar] [CrossRef]
- Anjard, C.; Loomis, W.F. GABA induces terminal differentiation of Dictyostelium through a GABAB receptor. Development 2006, 133, 2253–2261. [Google Scholar] [CrossRef]
- Schaap, P. Evolutionary crossroads in developmental biology: Dictyostelium discoideum. Development 2011, 138, 387–396. [Google Scholar] [CrossRef]
- Dunn, J.D.; Bosmani, C.; Barisch, C.; Raykov, L.; Lefrançois, L.H.; Cardenal-Muñoz, E.; López-Jiménez, A.T.; Soldati, T. Eat Prey, Live: Dictyostelium discoideum As a Model for Cell-Autonomous Defenses. Front. Immunol. 2018, 8, 1906. [Google Scholar] [CrossRef]
- Francione, L.M.; Annesley, S.J.; Carilla-Latorre, S.; Escalante, R.; Fisher, P.R. The Dictyostelium model for mitochondrial disease. Semin. Cell Dev. Biol. 2011, 22, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Haver, H.N.; Scaglione, K.M. Dictyostelium discoideum as a Model for Investigating Neurodegenerative Diseases. Front. Cell Neurosci. 2021, 15, 759532. [Google Scholar] [CrossRef]
- Huber, R.J.; Williams, R.S.; Müller-Taubenberger, A. Editorial: Dictyostelium: A Tractable Cell and Developmental Model in Biomedical Research. Front. Cell Dev. Biol. 2022, 10, 909619. [Google Scholar] [CrossRef]
- Ludtmann, M.H.; Otto, G.P.; Schilde, C.; Chen, Z.H.; Allan, C.Y.; Brace, S.; Beesley, P.W.; Kimmel, A.R.; Fisher, P.; Killick, R.; et al. An ancestral non-proteolytic role for presenilin proteins in multicellular development of the social amoeba Dictyostelium discoideum. J. Cell Sci. 2014, 127, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- McMains, V.C.; Myre, M.; Kreppel, L.; Kimmel, A.R. Dictyostelium possesses highly diverged presenilin/gamma-secretase that regulates growth and cell-fate specification and can accurately process human APP: A system for functional studies of the presenilin/gamma-secretase complex. Dis. Model. Mech. 2010, 3, 581–594. [Google Scholar] [CrossRef]
- Myre, M.A.; Lumsden, A.L.; Thompson, M.N.; Wasco, W.; MacDonald, M.E.; Gusella, J.F. Deficiency of huntingtin has pleiotropic effects in the social amoeba Dictyostelium discoideum. PLoS Genet. 2011, 7, e1002052. [Google Scholar] [CrossRef]
- Storey, C.L.; Williams, R.S.B.; Fisher, P.R.; Annesley, S.J. Dictyostelium discoideum: A Model System for Neurological Disorders. Cells 2022, 11, 463. [Google Scholar] [CrossRef]
- Bozzaro, S.; Bucci, C.; Steinert, M. Phagocytosis and Host–Pathogen Interactions in Dictyostelium with a Look at Macrophages. In International Review of Cell and Molecular Biology; Academic Press: San Diego, CA, USA, 2008; Chapter 6; Volume 271, pp. 253–300. [Google Scholar]
- Cosson, P.; Soldati, T. Eat, kill or die: When amoeba meets bacteria. Curr. Opin. Microbiol. 2008, 11, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.J.; Lin, T.L.; Hsu, C.R.; Wang, J.T. Use of a Dictyostelium model for isolation of genetic loci associated with phagocytosis and virulence in Klebsiella pneumoniae. Infect. Immun. 2011, 79, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Pukatzki, S.; Kessin, R.H.; Mekalanos, J.J. The human pathogen Pseudomonas aeruginosa utilizes conserved virulence pathways to infect the social amoeba Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 2002, 99, 3159–3164. [Google Scholar] [CrossRef]
- Solomon, J.M.; Rupper, A.; Cardelli, J.A.; Isberg, R.R. Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host-pathogen interactions. Infect. Immun. 2000, 68, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- Miyata Sarah, T.; Kitaoka, M.; Brooks Teresa, M.; McAuley Steven, B.; Pukatzki, S. Vibrio cholerae Requires the Type VI Secretion System Virulence Factor VasX To Kill Dictyostelium discoideum. Infect. Immun. 2011, 79, 2941–2949. [Google Scholar] [CrossRef]
- Hagedorn, M.; Rohde, K.H.; Russell, D.G.; Soldati, T. Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science 2009, 323, 1729–1733. [Google Scholar] [CrossRef]
- Henske, E.P.; Jóźwiak, S.; Kingswood, J.C.; Sampson, J.R.; Thiele, E.A. Tuberous sclerosis complex. Nat. Rev. Dis. Primers 2016, 2, 16035. [Google Scholar] [CrossRef]
- Salussolia, C.L.; Klonowska, K.; Kwiatkowski, D.J.; Sahin, M. Genetic Etiologies, Diagnosis, and Treatment of Tuberous Sclerosis Complex. Annu. Rev. Genomics Hum. Genet. 2019, 20, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.S. Presenilin, γ-Secretase, and the Search for Pathogenic Triggers of Alzheimer’s Disease. Biochemistry 2025. [Google Scholar] [CrossRef]
- Mätlik, K.; Pressl, C.; Heintz, N. Cell Type-Specific Studies of Human Tissue for Investigation of the Molecular Cell Biology of Late-Onset Neurodegenerative Disease. Annu. Rev. Neurosci. 2025, 48. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Flower, M.D.; Ross, C.A.; Wild, E.J. Huntington disease: New insights into molecular pathogenesis and therapeutic opportunities. Nat. Rev. Neurol. 2020, 16, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Steimle, P.A.; Ren, Y.; Ross, C.A.; Robinson, D.N.; Egelhoff, T.T.; Sesaki, H.; Iijima, M. Dictyostelium huntingtin controls chemotaxis and cytokinesis through the regulation of myosin II phosphorylation. Mol. Biol. Cell 2011, 22, 2270–2281. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Kotera, J. Overview of PDEs and Their Regulation. Circ. Res. 2007, 100, 309–327. [Google Scholar] [CrossRef]
- Page, C.P.; Spina, D. Selective PDE inhibitors as novel treatments for respiratory diseases. Curr. Opin. Pharmacol. 2012, 12, 275–286. [Google Scholar] [CrossRef]
- Azevedo, M.F.; Faucz, F.R.; Bimpaki, E.; Horvath, A.; Levy, I.; de Alexandre, R.B.; Ahmad, F.; Manganiello, V.; Stratakis, C.A. Clinical and molecular genetics of the phosphodiesterases (PDEs). Endocr. Rev. 2014, 35, 195–233. [Google Scholar] [CrossRef]
- Zheng, L.; Zhou, Z.-Z. An overview of phosphodiesterase 9 inhibitors: Insights from skeletal structure, pharmacophores, and therapeutic potential. Eur. J. Med. Chem. 2023, 259, 115682. [Google Scholar] [CrossRef]
- Johnstone, T.B.; Smith, K.H.; Koziol-White, C.J.; Li, F.; Kazarian, A.G.; Corpuz, M.L.; Shumyatcher, M.; Ehlert, F.J.; Himes, B.E.; Panettieri, R.A., Jr.; et al. PDE8 Is Expressed in Human Airway Smooth Muscle and Selectively Regulates cAMP Signaling by β(2)-Adrenergic Receptors and Adenylyl Cyclase 6. Am. J. Respir. Cell Mol. Biol. 2018, 58, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Epstein, P.M.; Basole, C.; Brocke, S. The Role of PDE8 in T Cell Recruitment and Function in Inflammation. Front. Cell Dev. Biol. 2021, 9, 636778. [Google Scholar] [CrossRef]
- Dong, H.; Claffey, K.P.; Brocke, S.; Epstein, P.M. Inhibition of breast cancer cell migration by activation of cAMP signaling. Breast Cancer Res. Treat. 2015, 152, 17–28. [Google Scholar] [CrossRef]

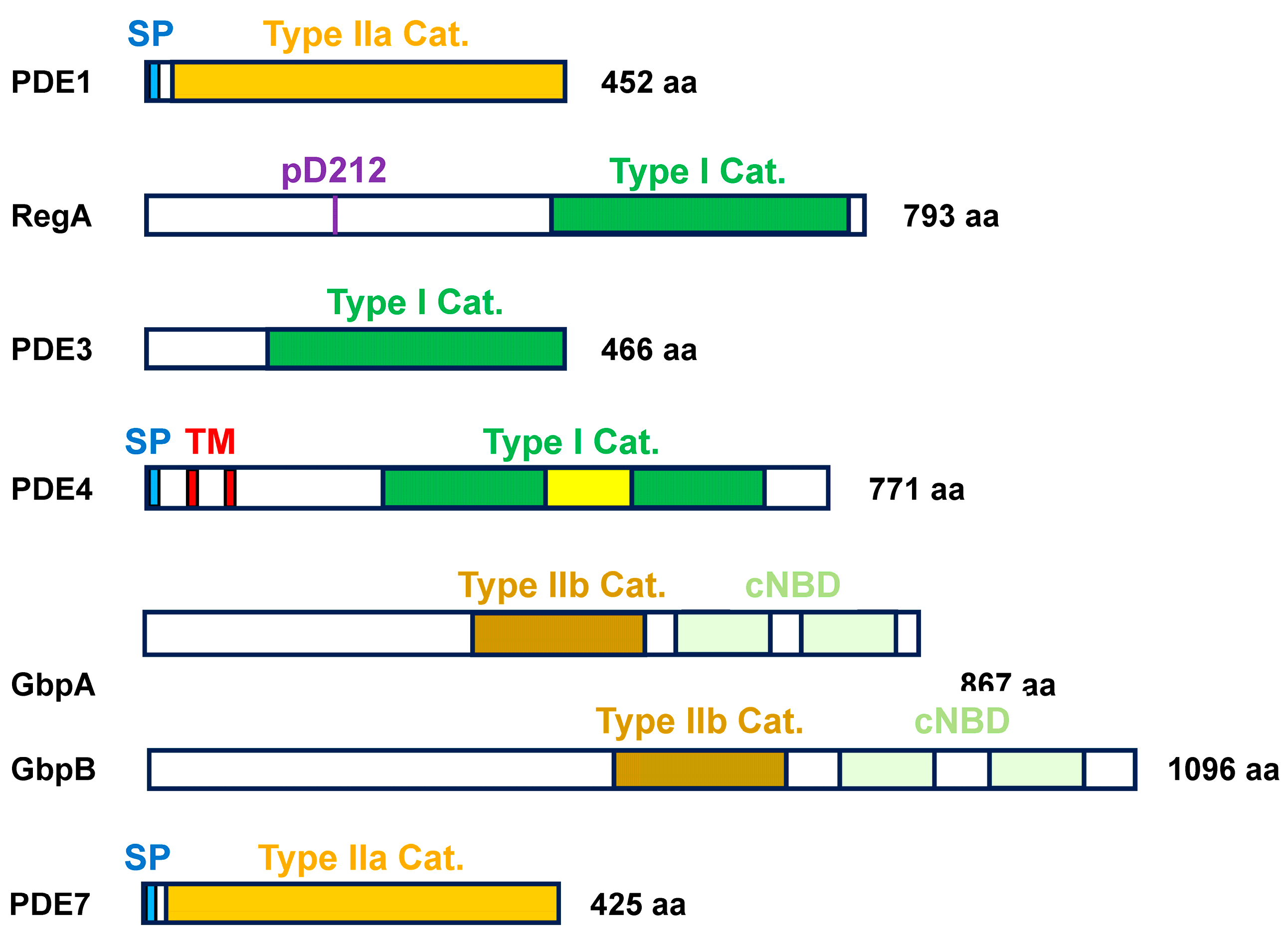
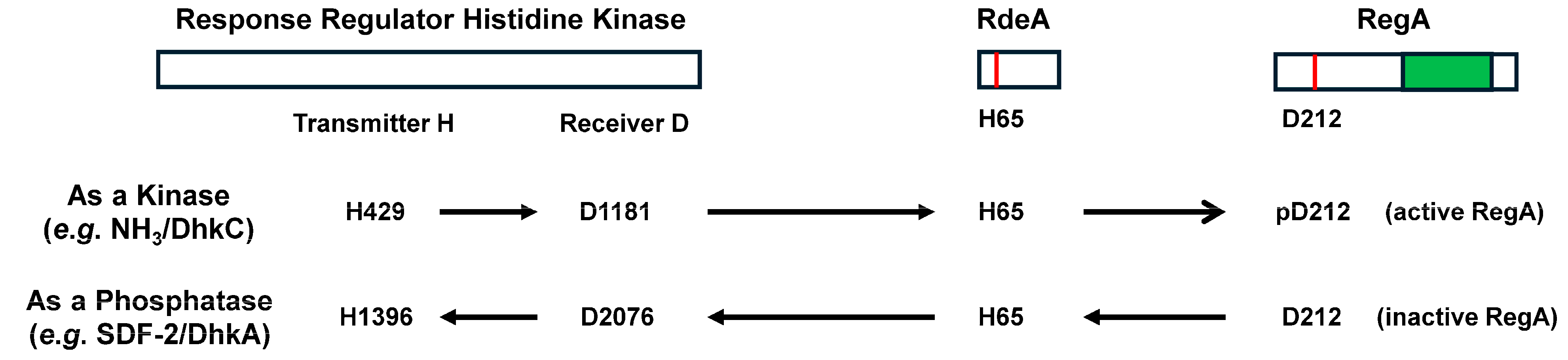

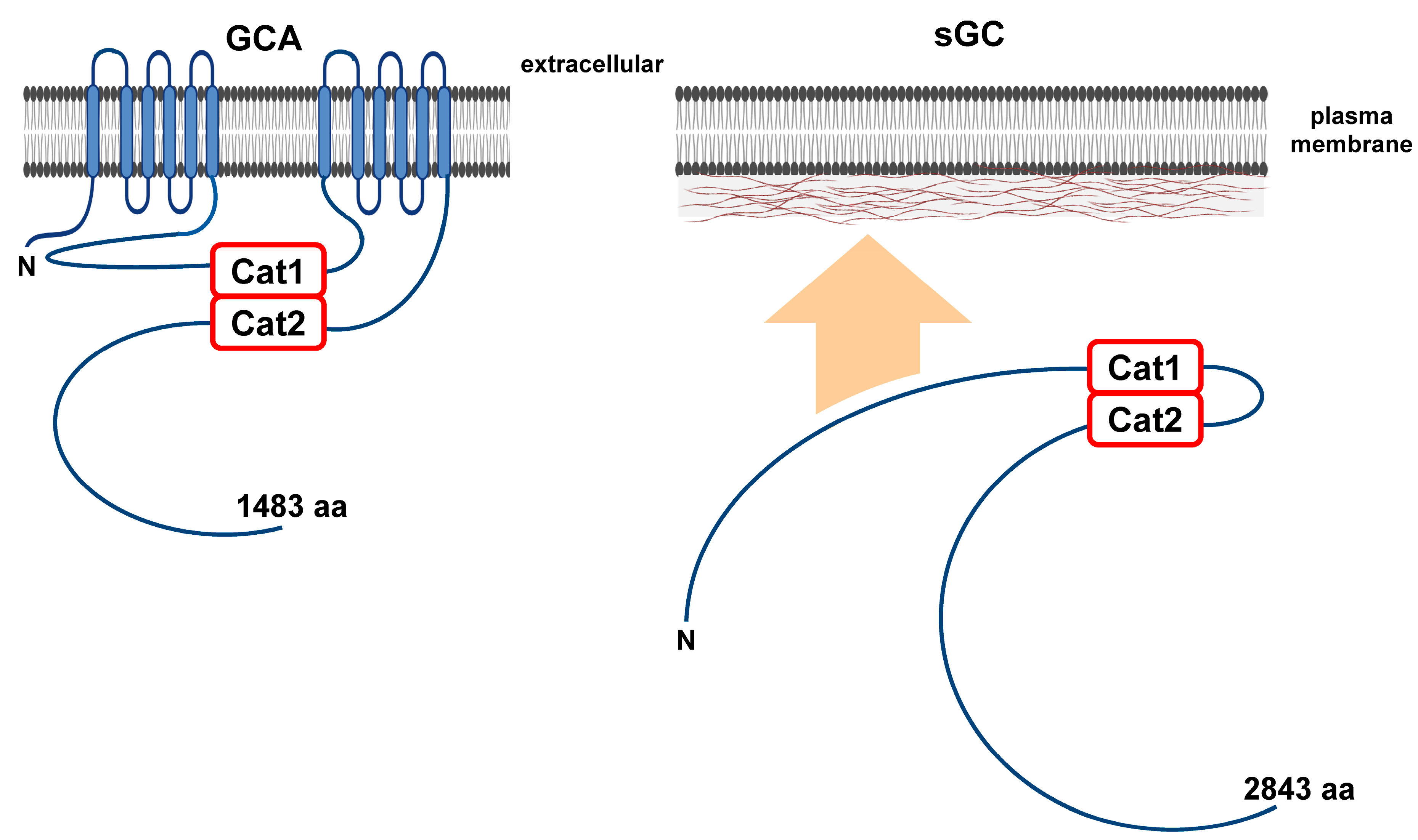
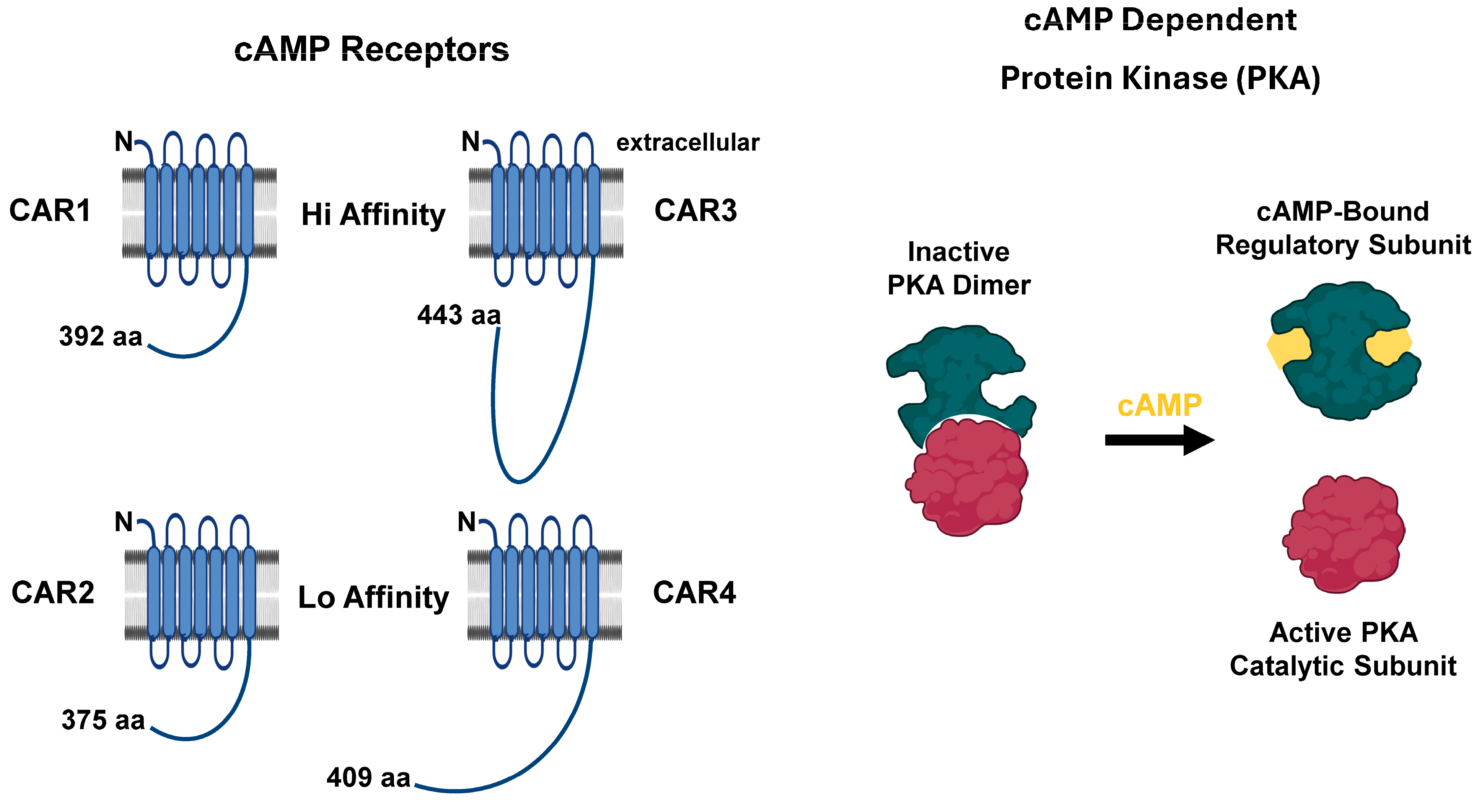
| PDE | Class Type | Localization | Substrate | Pharmacological Inhibition | Cellular Activation | Cellular Inhibition |
|---|---|---|---|---|---|---|
| PDE1 (PsdA) | IIa ^ γ-proteobacteria | Extracellular | cAMP (cGMP *) | DTT | - | PDI |
| RegA | I | Cytosol | cAMP | IBMX | Response Regulator | Activated ERK2 |
| PDE3 | I | Cytosol | cGMP | IBMX | - | - |
| PDE4 | I | Extracellular Facing Membrane-bound | cAMP | IBMX | - | - |
| GbpA | IIb ^ metallo-β-lactamase | Cytosol | cGMP | - | cGMP | - |
| GbpB | IIb ^ metallo-β-lactamase | Cytosol | cAMP cGMP | - | cAMP cGMP | - |
| PDE7 | IIa ^ γ-proteobacteria | Extracellular | cAMP (cGMP *) | DTT | - | (PDI) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaiswal, P.; Kimmel, A.R. Diverse Roles of the Multiple Phosphodiesterases in the Regulation of Cyclic Nucleotide Signaling in Dictyostelium. Cells 2025, 14, 522. https://doi.org/10.3390/cells14070522
Jaiswal P, Kimmel AR. Diverse Roles of the Multiple Phosphodiesterases in the Regulation of Cyclic Nucleotide Signaling in Dictyostelium. Cells. 2025; 14(7):522. https://doi.org/10.3390/cells14070522
Chicago/Turabian StyleJaiswal, Pundrik, and Alan R. Kimmel. 2025. "Diverse Roles of the Multiple Phosphodiesterases in the Regulation of Cyclic Nucleotide Signaling in Dictyostelium" Cells 14, no. 7: 522. https://doi.org/10.3390/cells14070522
APA StyleJaiswal, P., & Kimmel, A. R. (2025). Diverse Roles of the Multiple Phosphodiesterases in the Regulation of Cyclic Nucleotide Signaling in Dictyostelium. Cells, 14(7), 522. https://doi.org/10.3390/cells14070522






