Abstract
Recent studies have revealed marked sex differences in pathophysiological roles of spinal microglia in neuropathic pain, with microglia contributing to pain exacerbation exclusively in males. However, the characteristics of pain-enhancing microglia, which are more prominent in males, remain poorly understood. Here, we reanalyzed a previously published single-cell RNA sequencing dataset and identified a microglial subpopulation that significantly increases in the spinal dorsal horn (SDH) of male mice following peripheral nerve injury. CC-chemokine ligand 4 (CCL4) was highly expressed in this subpopulation and its mRNA levels were increased in the SDH after partial sciatic nerve ligation (PSL) only in male mice. Notably, CCL4 expression was reduced in male mice following microglial depletion, indicating that microglia are the primary source of CCL4. Intrathecal administration of maraviroc, an inhibitor of the CCL4–CC-chemokine receptor 5 (CCR5) signaling pathway, after PSL, significantly suppressed mechanical allodynia only in male mice. Furthermore, intrathecal administration of CCL4 induced mechanical allodynia in both sexes, accompanied by increased expression of c-fos, a neuronal excitation marker, in the SDH. These findings highlight a sex-biased difference in the gene expression profile of spinal microglia following peripheral nerve injury, with elevated CCL4 expression in male mice potentially contributing to pain exacerbation.
1. Introduction
Pain serves as an essential manifestation of abnormalities in the body and helps in initiating protective actions [1,2]. However, pain exceeding the normal physiological range requires medical evaluation and intervention. Neuropathic pain, resulting from nervous system damage, often does not respond to conventional analgesics, and has prompted extensive research into the underlying molecular mechanisms [3,4]. Recent studies have highlighted activation of microglia in the spinal dorsal horn (SDH) and the role of pain-related inflammatory mediators, such as cytokines and chemokines, in exacerbating and prolonging the pain [5,6]. Emerging evidence is also revealing sex-based differences in the involvement of microglia in neuropathic pain [7,8]. We and other research groups have demonstrated that administering microglial inhibitors (e.g., minocycline and pexidartinib) to rodent models of neuropathic pain suppresses pain in males, but not in females [9,10,11]. These findings suggest that, in males, microglia play a direct role in pain exacerbation under pathological conditions and contribute to neuropathic pain. Conversely, this mechanism does not appear to operate in microglia of females, implying the involvement of alternative pathways in them.
Microglia express receptors that respond to various stimuli from their environment and neighboring cells, and their activation has been implicated in pain induction [12,13]. Several recent studies have revealed significant sex-based differences in this process. For example, intrathecal (i.t.) administration of lipopolysaccharide, a toll-like receptor 4 agonist, or of colony-stimulating factor 1 (CSF1), which is essential for the survival and proliferation of microglia, induces morphological activation of microglia and reduces the pain threshold in rodent models [14,15,16]. This effect is pronounced in male mice but absent in female mice. Additionally, we demonstrated that selective activation of spinal microglia using Gq-DREADD (designer receptors exclusively activated by designer drugs) induces pain in male mice, but not in female mice [17]. These findings underscore sex-dependent differences in spinal microglial responsiveness to specific stimuli and their involvement in pain under physiological conditions. Despite growing evidence gathered employing various tools used to manipulate microglial activity, the key molecules that define male-dominant microglial involvement in neuropathic pain have not been identified definitively. Identifying these molecules is essential for unraveling the sex-biased mechanisms of pain regulation.
Microglia typically produce inflammatory factors that act on surrounding cells [18,19]. Therefore, identifying the factors uniquely expressed in pain-enhancing microglia and characterizing these cells is critical. A previous study using single-cell RNA sequencing (scRNA-seq) identified unique subpopulations of spinal microglia predominantly arising in male mice following peripheral nerve injury [20]. Based on these findings, we reanalyzed spinal microglial transcriptomes at single-cell resolution to identify a key soluble factor, the chemokine CC-chemokine ligand 4 (CCL4), which is predominantly expressed in male microglia under pathological conditions. Furthermore, we validated the functional significance of CCL4 via conventional biochemical and behavioral assays and elucidated the mechanisms underlying sex differences in microglia-mediated neuropathic pain.
2. Materials and Methods
2.1. Mice
All animal experiments were approved by the Animal Research Committee of Wakayama Medical University and were conducted in accordance with the in-house guidelines for the care and use of laboratory animals at Wakayama Medical University, as well as the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Male and female C57BL/6 mice (6–8-weeks-old) were purchased from SLC (Hamamatsu, Japan) and used for the experiments at 8–12-weeks of age. All mice were housed in groups of 5–6 in plastic cages under controlled temperature (23–24 °C), relative humidity (60–70%), and a 12 h dark/light cycle, with free access to food and water.
2.2. Processing of scRNA-Seq Data
We analyzed scRNA-seq data from male and female mouse microglia using publicly available data from the gene expression omnibus data repository (accession number: GSE162807) [20]. This study included 142,905 cells collected before and after peripheral nerve injury (at 3 days, 14 days, and 5 months), which were aligned to the mouse mm10 reference transcriptome. To identify the cell types, we analyzed all microglia samples and integrated them using the Harmony method [21] in the Seurat single-cell analysis package (Version 5.1.0) [22]. Initially, cells expressing more than 4000 genes (potential doublets) or fewer than 500 genes, as well as those with more than 5% mitochondrial genes, were filtered out. After normalizing, selecting variable features, and scaling each dataset separately for the filtered 135,806 cells, we performed principal component analysis for dimensional reduction. We then used the IntegrateLayers function to combine all the sample datasets. The integrated dataset was processed using the FindNeighbors function (reduction = “harmony”, dims = 1:11) to obtain the nearest-neighbor graph, the FindClusters function (resolution = 0.3) to identify each cell population, and the RunUMAP function (reduction = “harmony”, dims = 1:11) for visualization of the integrated dataset using Uniform Manifold Approximation and Projection (UMAP) dimension reduction. This process resulted in the identification of 11 cell type clusters.
2.3. Gene Set Enrichment Analysis
To functionally annotate the marker genes in each cluster, we conducted gene set enrichment analysis (GSEA) [23] using the R package fgsea (version 1.32.0). First, we identified marker genes in each cell cluster in Seurat using the FindMarkers function with the parameters test.use = wilcox, min.pct = 0.01 and logfc.threshold = 0.1. For each cluster, we generated a gene list sorted in decreasing order of −log10 p-values and used this list for GSEA. After performing GSEA with the molecular signatures database (MSigDB) hallmark dataset [24], we considered gene sets enriched at an FDR < 0.05.
2.4. Partial Sciatic Nerve Ligation (PSL) Model
The mice were subjected to PSL, as previously described [25,26]. Briefly, under isoflurane anesthesia, the left common sciatic nerve of each mouse was exposed at the mid-thigh level by making a small skin incision on one side, hereafter referred to as the ipsilateral side. Approximately one-third of the sciatic nerve was tightly ligated with a silk suture (Natsume Seisakusho, Tokyo, Japan), followed by suturing of the muscle and skin layers and sterilization of the surgical area with povidone–iodine. The untreated right limb was considered the contralateral limb.
2.5. Administration of Pexidartinib (PLX3397)
To deplete macrophages and microglia in vivo, PLX3397 (MedChemExpress, Monmouth Junction, NJ, USA), a CSF1 receptor (CSF1R) inhibitor, was formulated into the AIN-76A rodent diet (Research Diets, New Brunswick, NJ, USA) at 290 mg/kg. The PLX3397 dose was established based on a previous report [27]. The mice had free access to the PLX3397-formulated diet for two weeks instead of normal food, as PLX3397 is orally active. The AIN-76A rodent diet was used as the control.
2.6. Immunohistochemistry
The lumbar (L4–5) spinal cord was harvested from euthanized mice following transcardial perfusion with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde/phosphate-buffer solution. The specimens were post-fixed in 4% paraformaldehyde and cryoprotected in 30% sucrose/PBS solution at 4 °C overnight. After embedding in a freezing compound (Sakura, Tokyo, Japan), frozen tissues were longitudinally sectioned at 30 µm thickness using a cryostat (Leica Microsystems, Wetzlar, Germany), and the sections were floated in PBS. The sections were treated with PBS containing 0.1% Triton X-100 (PBST) for 1 h and then blocked with 5% donkey serum at room temperature (15–25 °C) for 2 h. The sections were then incubated overnight at 4 °C with primary antibodies targeting IBA1 (rabbit polyclonal, 1:1000; Fujifilm Wako, Osaka, Japan), NeuN (mouse monoclonal, 1:500; Millipore, Billerica, MA, USA), and c-fos (rabbit polyclonal, 1:50; Santa Cruz Biotechnology, Dallas, TX, USA). The sections were rinsed in PBST and incubated with fluorescent dye-conjugated secondary antibodies (1:200; Thermo Fisher Scientific, Waltham, MA, USA) at room temperature for 2 h. Finally, the sections were washed with PBS, mounted on glass slides, and covered with coverslips using the DAPI-Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL, USA). Fluorescent images were acquired using a confocal laser-scanning microscope (Olympus, Tokyo, Japan). The number of IBA1+ cells within the lamina I-III of the SDH was measured in a square area (200 × 200 μm2) using the FLUOVIEW software (Version 2.5.1.228).
2.7. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
Mice were euthanized using isoflurane, and fresh dorsal horns of the lumbar (L4–5) SDH samples were collected in RNAlater solution (Thermo Fisher Scientific). Total RNA was isolated from tissues using the TRIzol Plus RNA Purification Kit (Thermo Fisher Scientific) following the manufacturer’s instructions. Briefly, tissues were placed in a 1.5 mL RNase-free tube and homogenized with TRIzol reagent. Chloroform was added to each sample, and the mixture was then centrifuged at 4 °C for 15 min. The aqueous phase containing RNA was transferred to a fresh tube, and RNA was isolated using a purification column. Total RNA extract (1 µg) was incubated with random primers (Promega, Madison, WI, USA) at 70 °C for 5 min and subsequently cooled on ice. Samples were converted into cDNA by incubation with M-MLV Reverse Transcriptase (Promega) and dNTP Mix (Promega). qPCR was performed using the AriaMx Real-Time PCR System (Agilent Technologies, Santa Clara, CA, USA) with template cDNA (10 ng), primers for each gene (Thermo Fisher Scientific), and SYBR Premix Ex Taq II (Takara Bio, Kusatsu, Japan). The reactions were performed under the following conditions: 3 min at 95 °C, followed by 45 cycles of step two comprising 10 s at 95 °C and 30 s at 60 °C. Fluorescence intensities were recorded and the data were normalized to β-actin expression (Actb). The primer sequences used were as follows: Actb, 5′-CAGCTGAGAGGGAAATCGTG-3′ and 5′-TCTCCAGGGAGGAAGAGGAT-3′; Cd11b, 5′-GTTTCTACTGTCCCCCAGCA-3′ and 5′-GTTGGAGCCGAACAAATAGC-3′; Ccl4, 5′-ATGAAGCTCTGCGTGTCTGC-3′ and 5′-GCCGGGAGGTGTAAGAGAAA-3′.
2.8. Drug Administration
A CC-chemokine receptor 5 (CCR5) antagonist (Maraviroc; Tocris Biosciences, Bristol, UK) was dissolved in dimethyl sulfoxide and diluted in sterile PBS for further use. Based on previous reports [9,28], 20 nmol maraviroc was administered intrathecally (i.t.) on day 7 after PSL. Recombinant CCL4 (BioLegend, San Diego, CA, USA) was dissolved in sterile PBS and i.t. administered at a dose of 1 or 10 pmol. Under isoflurane anesthesia, an i.t. injection was administered in the region between the spinal L5 and L6 vertebrae using a 30-gauge needle fitted with a Hamilton microsyringe [29].
2.9. Von Frey Test
The mechanical pain threshold was determined using the von Frey test, as previously described [17]. Briefly, mice were individually placed on a metal mesh grid floor (5 × 5 mm) and covered with an acrylic box. After a 2- to 3 h adaptation period, calibrated von Frey filaments (Neuroscience, Tokyo, Japan) were applied to the middle of the plantar surface of the hind paw through the mesh floor. The filament set used in this study consisted of nine calibrated von Frey filaments: 0.02, 0.04, 0.07, 0.16, 0.4, 0.6, 1.0, 1.4, and 2.0 g. Using the up–down method, testing began with the application of 0.4 g filament. Quick withdrawal, shaking, biting, or licking of the stimulated paw was considered a positive paw-withdrawal response. If no withdrawal response occurred, the next stronger stimulus was applied. Conversely, the next weaker stimulus was selected following paw withdrawal, in accordance with Chaplan’s procedure [30]. Once the response threshold was crossed (two responses were straddling the threshold), the 50% paw-withdrawal threshold was calculated based on these responses.
2.10. Gene Expression Analysis
Expression profiling of CCL4 (Ccl4) gene across a diverse range of normal tissues, organs, and cell lines in mice was visualized using BioGPS (http://biogps.org/).
2.11. Statistical Analysis
All data are presented as the mean ± standard error of the mean (SEM). To compare differences between two groups, a two-tailed Student’s t-test or Welch’s t-test was used. To compare the differences between the four groups with two factors, two-way ANOVA followed by Tukey’s multiple comparison test was used. Statistical analyses were performed using the GraphPad Prism software (GraphPad Software, Version 10.1.2, Boston, MA, USA), and statistical significance was set at p < 0.05.
3. Results
3.1. Expression of CCL4 by Male-Dominant Subpopulation of Spinal Microglia After Nerve Injury
To identify microglia-secreted pain-enhancing molecules in male-dominant subpopulations of activated microglia in the SDH after peripheral nerve injury (spared nerve injury: SNI), we reanalyzed a scRNA-seq dataset from a previously published study (Tansley et al.) [20]. Clustering analysis of microglia under the following conditions (day 3, day 14, 5 months after SNI and naïve controls for both sexes) revealed 11 distinct clusters (Figure 1A). Consistent with prior findings, canonical microglia genes, such as Tmem119, Fcrls, P2ry12, Cx3cr1, Trem2, and C1qa, were expressed in all cell type clusters, representing most of the analyzed microglia (Figure S1). In contrast, clusters 6 and 10, which accounted for a portion of the microglia, exhibited unique transcriptional profiles. The heatmap showed that the primary population in clusters 6 and 10 comprised microglia from male mice on day 3 after SNI, followed by female mice, whereas microglia from day 14 and 5 months after SNI, and naïve controls of both sexes were minimally represented (Figure 1B). Activated microglia play a crucial role in the development of neuropathic pain, highlighting the importance of early time points following nerve injury. Microglia categorized within clusters 6 and 10 showed an increase in both sexes on day 3 after SNI compared to naïve controls. However, the proportion of male microglia was greater than that of female microglia, suggesting that clusters 6 and 10 reflected sex-specific differences in activated microglia following nerve injury.
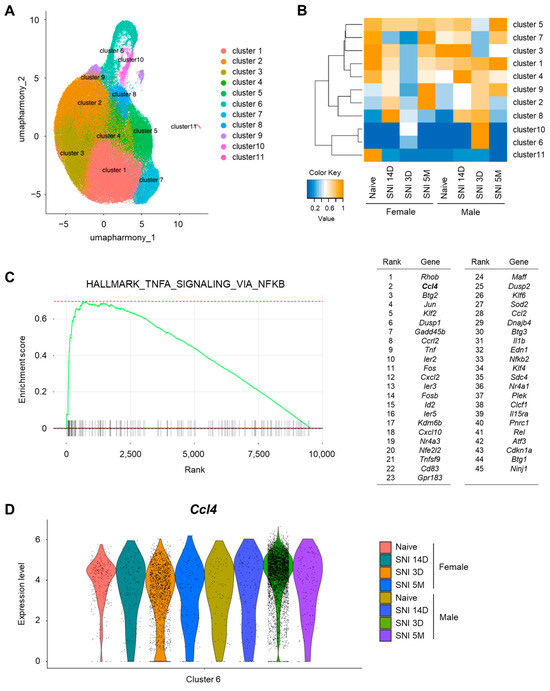
Figure 1.
Male-dominant subpopulation of spinal microglia after nerve injury express CCL4. (A) Uniform manifold approximation and projection (UMAP) visualization of mouse microglia (n = 2 control samples and n = 6 injury samples from male and female mice), colored by cell type cluster. The control naive sample included 33,330 cells, whereas the spared nerve injury (SNI) model samples included 102,476 cells collected on day 3, day 14, and 5 months after SNI. (B) Heatmap based on cell number ratio. This heatmap indicates the ratio of cells classified into each cluster relative to the total number of cells in each sample. The vertical axis represents the cell type cluster numbers, whereas the horizontal axis represents the sample types. (C) Result of gene set enrichment analysis (GSEA) of marker genes in males compared with those in females for the “HALLMARK_TNFA_SIGNALING_VIA_NFKB” gene set. List of 45 leading edge genes are shown. (D) Violin plot for Ccl4 genes, markers of cluster 6 cells.
Given the pivotal role of inflammation-related molecules in the pathophysiology of neuropathic pain, we analyzed the gene expression patterns in these clusters according to sex. GSEA revealed activation of the TNFA_SIGNALING_VIA_NFKB pathway in cluster 6 microglia. Furthermore, 45 hallmark genes were identified as characteristic of male-dominant microglia after nerve injury. Although Rhob exhibited the most pronounced difference between males and females among all genes in cluster 6, we prioritized the soluble inflammatory molecule CCL4, which exhibited the second greatest sex difference. (Figure 1C). A violin plot for Ccl4 expression levels across all microglia in cluster 6 confirmed that CCL4-expressing microglia were predominantly from male mice on day 3 after SNI (Figure 1D). Additionally, the number of CCL4-expressing microglia in males was greater than that in females in cluster 10 microglia (Figure S2), although the transcriptional profile of cluster 10 differed from that of cluster 6. These findings suggest that Ccl4 expression is a defining feature of male-dominant microglia involved in neuropathic pain.
3.2. Male Microglia-Dominant Upregulation of CCL4 in the Spinal Dorsal Horn
Using immunohistochemistry, we evaluated whether microglial activation differed in the SDH of male and female mice following PSL. On day 7 after PSL, the number of IBA1+ microglia was similarly increased on the ipsilateral side of the SDH in both male and female mice (Figure 2A,B). Next, we used RT-qPCR to assess the time course of Ccl4 expression in the SDH after PSL. Ccl4 was significantly upregulated on days 7 and 14 in male mice, but not in female mice, with expression levels being markedly higher in males at both time points (Figure 2C), whereas other pain-related genes, such as Ccl3, were similarly upregulated in both sexes [9]. As previously reported, treatment with PLX3397, an inhibitor of the CSF1 receptor, substantially reduced the expression of the microglial marker Cd11b in the SDH on day 7 after PSL in a sex-dependent manner. Consistently, Ccl4 expression was significantly decreased upon PLX3397 treatment in male mice but remained unchanged in female mice (Figure 2D). A database search using BioGPS confirmed that Ccl4 is expressed not only by peripheral macrophages but also by microglia (Figure S3). These findings indicate that Ccl4 is upregulated in SDH microglia after PSL in males but not in females.
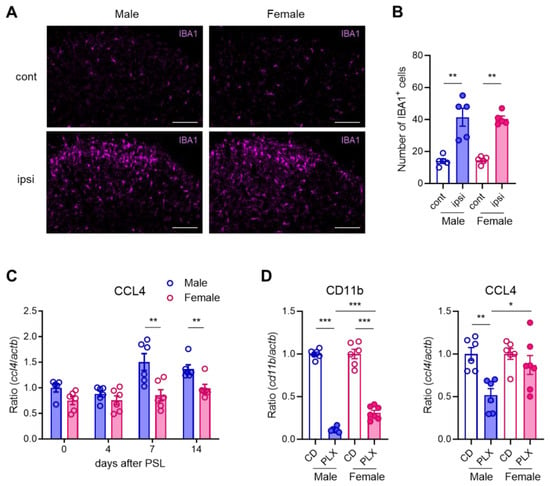
Figure 2.
Male microglia-dominant upregulation of CCL4 in the spinal dorsal horn. Male and female mice were subjected to partial sciatic nerve ligation (PSL), and the lumbar spinal dorsal horn (SDH) was collected. (A) IBA1+ microglia in the SDH on day 7 after PSL were visualized using immunohistochemistry. Scale bars = 100 μm. (B) Quantitative analysis of the number of IBA1+ cells in the lamina I-III in the SDH (n = 5, Welch’s t-test, ** p < 0.01). (C) mRNA levels of Ccl4 on days 0, 4, 7, and 14 after PSL were analyzed using RT-qPCR (n = 5–6, Student’s t-test, ** p < 0.01). (D) Mice were fed a control diet (CD) or PLX3397 diet for 7 days before PSL and subsequently subjected to PSL. The lumbar ipsilateral SDH was collected on day 7 after PSL. Fold changes in mRNA levels of Cd11b and Ccl4 in PLX3397-fed mice compared with those in control-fed mice. (n = 6–7, Student’s t-test, *** p < 0.001, ** p < 0.01, * p < 0.05).
3.3. Sexually Dimorphic Effect of CCR5 Antagonist in Relieving Neuropathic Pain
CCR5 is the principal receptor for CCL4, despite the complexity of the chemokine ligand–receptor system [31,32]. To assess whether the CCL4–CCR5 axis plays a sex-dependent pathophysiological role in neuropathic pain in the SDH, we investigated the effects of CCR5 blockade. Maraviroc, a CCR5 antagonist [33], was evaluated for its suppressive effects on PSL-induced neuropathic pain. Mechanical pain thresholds, assessed using the von Frey test, were significantly reduced on the ipsilateral side on day 7 after PSL in both male and female mice, confirming the development of mechanical allodynia. The i.t. administration of maraviroc (20 nmol) on day 7 transiently, but markedly, relieved PSL-induced mechanical allodynia 3 h after administration in male mice. In contrast, maraviroc did not suppress allodynia in female mice (Figure 3). These findings suggest that enhancement of the CCL4–CCR5 axis in the SDH plays a critical role in neuropathic pain in male mice.
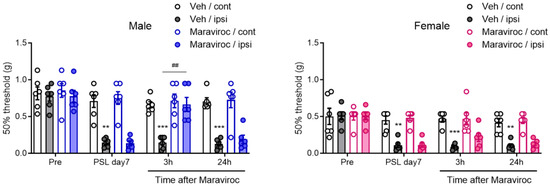
Figure 3.
Sexually dimorphic effect of CCR5 antagonist maraviroc in relieving neuropathic pain. Male and female mice were subjected to PSL, and maraviroc (20 nmol) was intrathecally (i.t.) administered once on day 7 after PSL. The 50% mechanical threshold on days 0 (pre) and 7 after PSL, and 3 and 24 h after i.t. administration of maraviroc in male and female mice were assessed employing the up–down method using the von Frey test (n = 6, two-way ANOVA followed by Tukey’s multiple comparison test, *** p < 0.001, ** p < 0.01 vs. Veh/cont, ## p < 0.01).
3.4. Sex-Independent Allodynic Effects of CCL4 in the Spinal Dorsal Horn
To determine whether an increase in CCL4 is sufficient to induce mechanical allodynia, we evaluated the effects of exogenous CCL4 on pain sensitivity in both male and female mice. A single i.t. administration of CCL4 (1 or 10 pmol) significantly reduced the mechanical pain threshold, indicating mechanical allodynia 6 h after administration in both sexes. The allodynic effects persisted for at least 72 h in a dose-dependent manner (Figure 4A). Further analysis showed that c-fos protein expression in the SDH was significantly increased in both male and female mice on day 1 after i.t. administration of CCL4 (10 pmol). These c-fos signals colocalized with NeuN, a marker of neuronal nuclei, indicating the activation of pain-processing neurons in the SDH (Figure 4B,C). These results indicate that CCL4 exerts a potent allodynic effect by reducing the mechanical pain thresholds in a sex-independent manner.
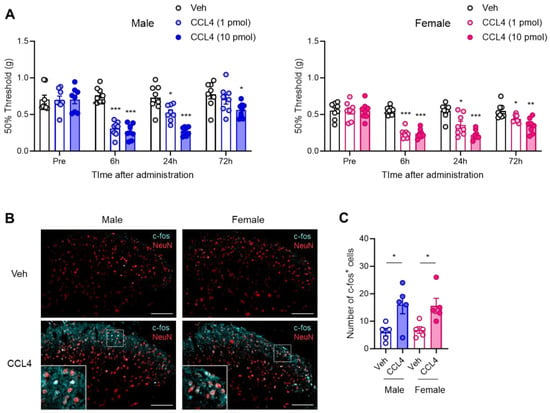
Figure 4.
Sex-independent allodynic effects of intrathecally administered CCL4. Recombinant CCL4 (1 or 10 pmol) was intrathecally administered to naïve male and female mice. (A) The 50% mechanical threshold before and 6, 24, and 72 h after administration in male and female mice were assessed employing the up–down method using the von Frey test (n = 8, two-way ANOVA followed by Tukey’s multiple comparison test, *** p < 0.001, ** p < 0.01, * p < 0.05 vs. Veh). (B) Expression of c-fos protein in the SDH on day 1 after administration of CCL4 (10 pmol) was visualized via immunohistochemistry. Scale bars = 100 μm. (C) Quantitative analysis of the number of c-fos+ cells in the lamina I–III of the SDH (n = 5, Student’s t-test, * p < 0.05).
4. Discussion
In this study, we identified sex-biased characteristics of spinal microglia that contribute to the etiology of neuropathic pain. Bioinformatic analysis of published scRNA-seq datasets for spinal microglia revealed male-dominant expression of the chemokine Ccl4 in specific microglial subpopulations following SNI. We further confirmed that Ccl4 mRNA was upregulated in the SDH of males, but not females, after PSL, despite both sexes exhibiting similar morphological activation of spinal microglia. The i.t. administration of maraviroc, an inhibitor of the CCL4-CCR5 signaling pathway, significantly suppressed PSL-induced mechanical allodynia in male mice. However, the anti-allodynic effects of maraviroc were not significant in female mice. Notably, the i.t. administration of recombinant CCL4 induced mechanical allodynia in both sexes, indicating that the sex-dependent pathophysiological roles of microglia-derived CCL4 in neuropathic pain are mediated via its expression following peripheral nerve injury.
Following the discovery of a causal link between microglial activation and neuropathic pain [34], numerous studies have highlighted the roles of soluble factors derived from activated microglia in pain hypersensitivity. For example, inflammatory cytokines (e.g., interleukin-1β and tumor necrosis factor-α), chemokines (e.g., CCL2 and CCL3), growth factors, and lipid mediators have been shown to play critical roles in neuropathic pain by enhancing neuronal excitability and/or amplifying neuroinflammation [5,13,35,36]. This process is accompanied by the activation of microglia and astrocytes, ultimately leading to hyperexcitation of pain-processing pathways [6,37]. Although accumulating evidence suggests pronounced sexually dimorphic characteristics of microglia in the etiology of neuropathic pain [7,8,38], the existence of sex differences in the pathophysiological roles of these molecules remains underexplored. Elucidating the mechanisms underlying sex differences in microglia-driven neuropathic pain requires identification of critical molecules that define male-dominant microglial contributions to neuropathic pain.
In a previous study, Tansley et al. classified spinal microglia from naïve, sham, and SNI mice into 11 distinct clusters [20]. Among these, unique subpopulations (original clusters 7–9) were predominantly composed of microglia from SNI day 3 in both sexes. On SNI day 3, morphological activation and proliferation of microglia in the SDH were significantly increased in both sexes, and these microglia exhibited distinct transcriptome profiles compared to other subpopulations. Notably, the number of proliferative microglia in male mice was greater than that in female mice, suggesting sex differences in microglial characteristics [20]. We hypothesized that further analysis of their transcriptome data would help identify novel soluble molecules that drive functional sex differences in microglia. Our findings revealed that the TNFα signaling via the NF-κB pathway was upregulated in cluster 6, aligning with the established role of neuroinflammatory process in the etiology of neuropathic pain. Importantly, the proportion of microglia in male SNI day 3 was greater than that in female SNI day 3 within cluster 6, and CCL4 expressing microglia were primarily observed in males. Given that cluster 6 in our analysis aligns with previous findings [20], CCL4 emerges as a key molecule associated with sex differences in activated microglia after nerve injury.
Several neuropathic pain models have been established to investigate the etiology of neuropathic pain and to evaluate the therapeutic potential of drugs [39,40]. Among the four main nerve injury models, spinal microglial activation is a common feature, and the inhibition of activated microglia has been shown to alleviate neuropathic pain across these models [11,25,41,42]. However, the temporal dynamics of microglial activation and expression profiles of microglia-derived molecules may vary between models. To elucidate the common pathophysiological mechanisms underlying neuropathic pain, it is essential to examine key phenomena across different neuropathic pain models. Several studies have demonstrated significant microglial activation and upregulation of inflammatory molecules, sustained for at least two weeks after nerve injury, in neuropathic pain models [9,36,43]. Therefore, although Tansley et al. employed the SNI model [20], their scRNA-seq transcriptome data can be used to investigate the mechanisms underlying PSL-induced neuropathic pain. Indeed, the male-dominant upregulation of CCL4 after PSL in our study indicates the existence of shared mechanisms between the SNI and PSL models.
CCL4, also known as macrophage inflammatory protein-1β, exerts diverse effects on various cell types via its interaction with CCR5. Several lines of evidence suggest that the CCL4–CCR5 axis facilitates inflammation that underlies intractable diseases, such as osteoarthritis and diabetes, and promotes tumor development and progression by modulating lymphocytes, macrophages, and tissue-resident cells [44,45,46,47]. Additionally, CCL4 is upregulated under pathological neurogenic conditions following nerve injury, and inhibition of macrophage- or Schwann cell-derived CCL4 has been shown to attenuate neuropathic pain [48]. CCL4 also plays a crucial role in several neuroinflammatory diseases of the central nervous system, including traumatic brain injury and Alzheimer’s disease [47,49,50]. Thus, CCL4 upregulation likely contributes to pain hypersensitivity at the spinal level. A previous study reported that CCL4 was upregulated in the SDH following chronic constriction injury and that i.t. administration of maraviroc attenuated neuropathic pain in male rats [28]. However, the study did not address sex-related differences. In the present study, we demonstrated that CCL4 was upregulated exclusively in male mice, and that the CCL4–CCR5 signaling pathway was enhanced in the SDH following peripheral nerve injury in a sex-dependent manner.
Given that perineurally administered maraviroc at the site surrounding the injured sciatic nerve exhibited anti-allodynic effects in both male and female PSL models, as previously reported [9], it is surprising that i.t. administered maraviroc showed anti-allodynic effects exclusively in male PSL models. Despite the sexually dimorphic anti-allodynic effects of i.t. administered maraviroc, the i.t. administration of CCL4 induced robust allodynia in both sexes, whereas a previous report presented an allodynic effect of i.t. administered CCL4 only in males [51]. These findings suggest that activation of CCR5 in the SDH leads to pain hypersensitivity in both males and females. CCR5 is highly expressed in immune cells, including spinal microglia, and is also known to be expressed in neurons [51,52,53,54]. Indeed, the induction of c-fos expression following CCL4 administration supports the excitation of pain-processing neurons. Interestingly, CCL3, another CCR5 ligand, was similarly upregulated in the SDH of both sexes after nerve injury, as previously reported [9]. However, CCL4 upregulation showed clear sex differences, being elevated only in males. This transcriptional disparity may underlie sex-biased characteristics of microglia. Understanding the mechanisms underlying these sex-related differences is crucial. We previously demonstrated that the sexually dimorphic characteristics of spinal microglia under neuropathic pain conditions are influenced by circulating androgens [9]. Further studies are needed to determine whether androgens modulate CCL4 expression and to elucidate the transcriptional mechanisms responsible for this regulation.
Overall, we demonstrated that the upregulation of CCL4 in the SDH following peripheral nerve injury occurs exclusively in males. Importantly, CCL4 may serve as a marker for male-dominant microglia involved in neuropathic pain. Although pharmacological inhibition of the CCL4–CCR5 pathway with i.t. maraviroc showed sexually dimorphic alleviation of neuropathic pain in the PSL model, activation of CCR5 via i.t. administration of exogenous CCL4 induced allodynia in both sexes. This suggests that the induction of CCL4 in activated microglia within the SDH may represent a crucial mechanism underlying sex differences in microglia-driven neuropathic pain. Considering that this sexual dimorphism in microglia is androgen-dependent, elucidating the regulatory mechanisms of spinal microglia mediated by androgen signaling can provide deeper insights into sex differences in pain processing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells14070484/s1, Figure S1: Expression of canonical microglia genes in all cell type clusters; Figure S2: Expression of Ccl4 in male-dominant subpopulation; Figure S3: Expression profiling of Ccl4 in mice.
Author Contributions
Conceptualization, T.S., S.H., K.S. and N.K.; methodology, T.S. and N.K.; software, T.S. and T.N.; validation, T.S., S.H., K.S. and N.K.; formal analysis, F.S., T.S., T.N. and N.K.; investigation, F.S., T.S., T.N. and N.K.; resources, T.S. and N.K.; data curation, F.S., T.S., T.N., Y.F., S.H., K.S. and N.K.; writing—original draft preparation, F.S., T.S. and N.K.; writing—review and editing, T.S., T.N., Y.F., S.H., K.S. and N.K.; visualization, T.S. and N.K.; supervision, N.K.; project administration, N.K.; funding acquisition, T.S., Y.F., K.S. and N.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI (Grant Number: 23K08371 to Y.F.), the Japan Agency for Medical Research and Development (Grant Number: JP21gk0210029 to N.K.), the Smoking Research Foundation (to N.K.), the Takeda Science Foundation (to N.K.), the Mochida Memorial Foundation (to N.K.), the Naito Foundation (to N.K.), the Daiichi Sankyo Foundation of Life Science (to N.K.), and the Program of the Joint Usage/Research Center for Developmental Medicine and High Depth Omics, IMEG, Kumamoto University (to T.S., K.S. and N.K.).
Institutional Review Board Statement
This study was approved by the Animal Research Committee of Wakayama Medical University (approval No. 781, 828, Tora 37, Tora90).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We are grateful to Mayumi Shibutani, Haruna Mano, Akari Minami, and Momo Kubota for their technical assistance. The authors thank the Medical Research Center organizers at Saitama Medical University for providing continuous support throughout this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CCL4 | CC-chemokine ligand 4 |
| CCR5 | CC-chemokine receptor 5 |
| CSF1 | colony-stimulating factor 1 |
| GSEA | gene set enrichment analysis |
| PSL | partial sciatic nerve ligation |
| RT-qPCR | reverse transcription-quantitative polymerase chain reaction |
| scRNA-seq | single-cell RNA sequencing |
| SDH | spinal dorsal horn |
| SNI | spared nerve injury |
| UMAP | uniform manifold approximation and projection |
References
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Chamessian, A.; Zhang, Y.Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef]
- Tsuda, M.; Masuda, T.; Kohno, K. Microglial diversity in neuropathic pain. Trends Neurosci. 2023, 46, 597–610. [Google Scholar] [CrossRef]
- Midavaine, E.; Cote, J.; Marchand, S.; Sarret, P. Glial and neuroimmune cell choreography in sexually dimorphic pain signaling. Neurosci. Biobehav. Rev. 2021, 125, 168–192. [Google Scholar] [CrossRef]
- Mapplebeck, J.C.S.; Beggs, S.; Salter, M.W. Sex differences in pain: A tale of two immune cells. Pain 2016, 157 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef]
- Saika, F.; Fukazawa, Y.; Hatano, Y.; Kishioka, S.; Hino, Y.; Hino, S.; Suzuki, K.; Kiguchi, N. Sexually dimorphic effects of pexidartinib on nerve injury-induced neuropathic pain in mice. Glia 2024, 72, 1402–1417. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]
- Taves, S.; Berta, T.; Liu, D.L.; Gan, S.; Chen, G.; Kim, Y.H.; Van de Ven, T.; Laufer, S.; Ji, R.R. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav. Immun. 2016, 55, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Weiner, H.L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 2018, 19, 622–635. [Google Scholar] [CrossRef]
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef]
- Kuhn, J.A.; Vainchtein, I.D.; Braz, J.; Hamel, K.; Bernstein, M.; Craik, V.; Dahlgren, M.W.; Ortiz-Carpena, J.; Molofsky, A.B.; Molofsky, A.V.; et al. Regulatory T-cells inhibit microglia-induced pain hypersensitivity in female mice. Elife 2021, 10, e69056. [Google Scholar] [CrossRef]
- Sorge, R.E.; LaCroix-Fralish, M.L.; Tuttle, A.H.; Sotocinal, S.G.; Austin, J.S.; Ritchie, J.; Chanda, M.L.; Graham, A.C.; Topham, L.; Beggs, S.; et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. 2011, 31, 15450–15454. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, H.; Lee, S.J. Distinct roles of GT1b and CSF-1 in microglia activation in nerve injury-induced neuropathic pain. Mol. Pain. 2021, 17, 17448069211020918. [Google Scholar] [CrossRef] [PubMed]
- Saika, F.; Matsuzaki, S.; Kishioka, S.; Kiguchi, N. Chemogenetic Activation of CX3CR1-Expressing Spinal Microglia Using Gq-DREADD Elicits Mechanical Allodynia in Male Mice. Cells 2021, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Distefano-Gagne, F.; Bitarafan, S.; Lacroix, S.; Gosselin, D. Roles and regulation of microglia activity in multiple sclerosis: Insights from animal models. Nat. Rev. Neurosci. 2023, 24, 397–415. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef]
- Tansley, S.; Uttam, S.; Urena Guzman, A.; Yaqubi, M.; Pacis, A.; Parisien, M.; Deamond, H.; Wong, C.; Rabau, O.; Brown, N.; et al. Single-cell RNA sequencing reveals time- and sex-specific responses of mouse spinal cord microglia to peripheral nerve injury and links ApoE to chronic pain. Nat. Commun. 2022, 13, 843. [Google Scholar] [CrossRef]
- Korsunsky, I.; Millard, N.; Fan, J.; Slowikowski, K.; Zhang, F.; Wei, K.; Baglaenko, Y.; Brenner, M.; Loh, P.R.; Raychaudhuri, S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 2019, 16, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C.; et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 2024, 42, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Birger, C.; Thorvaldsdottir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, N.; Kobayashi, D.; Saika, F.; Matsuzaki, S.; Kishioka, S. Inhibition of peripheral macrophages by nicotinic acetylcholine receptor agonists suppresses spinal microglial activation and neuropathic pain in mice with peripheral nerve injury. J. Neuroinflamm. 2018, 15, 96. [Google Scholar] [CrossRef]
- Seltzer, Z.; Dubner, R.; Shir, Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 1990, 43, 205–218. [Google Scholar] [CrossRef]
- Elmore, M.R.; Najafi, A.R.; Koike, M.A.; Dagher, N.N.; Spangenberg, E.E.; Rice, R.A.; Kitazawa, M.; Matusow, B.; Nguyen, H.; West, B.L.; et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014, 82, 380–397. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Jurga, A.; Slusarczyk, J.; Trojan, E.; Basta-Kaim, A.; Mika, J. Beneficial properties of maraviroc on neuropathic pain development and opioid effectiveness in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 68–78. [Google Scholar] [CrossRef]
- Kiguchi, N.; Uta, D.; Ding, H.; Uchida, H.; Saika, F.; Matsuzaki, S.; Fukazawa, Y.; Abe, M.; Sakimura, K.; Ko, M.C.; et al. GRP receptor and AMPA receptor cooperatively regulate itch-responsive neurons in the spinal dorsal horn. Neuropharmacology 2020, 170, 108025. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Comerford, I.; McColl, S.R. Atypical chemokine receptors in the immune system. Nat. Rev. Immunol. 2024, 24, 753–769. [Google Scholar] [CrossRef]
- Proudfoot, A.E. Chemokine receptors: Multifaceted therapeutic targets. Nat. Rev. Immunol. 2002, 2, 106–115. [Google Scholar] [CrossRef]
- Dorr, P.; Westby, M.; Dobbs, S.; Griffin, P.; Irvine, B.; Macartney, M.; Mori, J.; Rickett, G.; Smith-Burchnell, C.; Napier, C.; et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 2005, 49, 4721–4732. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Shigemoto-Mogami, Y.; Koizumi, S.; Mizokoshi, A.; Kohsaka, S.; Salter, M.W.; Inoue, K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003, 424, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Milligan, E.D.; Watkins, L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009, 10, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Tozaki-Saitoh, H.; Kojima, C.; Masuda, T.; Tsuda, M.; Inoue, K.; Hoka, S. Chemokine (C-C motif) receptor 5 is an important pathological regulator in the development and maintenance of neuropathic pain. Anesthesiology 2014, 120, 1491–1503. [Google Scholar] [CrossRef]
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef]
- Mogil, J.S. Qualitative sex differences in pain processing: Emerging evidence of a biased literature. Nat. Rev. Neurosci. 2020, 21, 353–365. [Google Scholar] [CrossRef]
- Mogil, J.S. Animal models of pain: Progress and challenges. Nat. Rev. Neurosci. 2009, 10, 283–294. [Google Scholar] [CrossRef]
- Calvo, M.; Dawes, J.M.; Bennett, D.L. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012, 11, 629–642. [Google Scholar] [CrossRef]
- Masuda, T.; Tsuda, M.; Yoshinaga, R.; Tozaki-Saitoh, H.; Ozato, K.; Tamura, T.; Inoue, K. IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep. 2012, 1, 334–340. [Google Scholar] [CrossRef]
- Guan, Z.; Kuhn, J.A.; Wang, X.; Colquitt, B.; Solorzano, C.; Vaman, S.; Guan, A.K.; Evans-Reinsch, Z.; Braz, J.; Devor, M.; et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat. Neurosci. 2016, 19, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, L.; Deng, H.; Chen, Y.; Zhou, H.; Liu, M.; Wang, S.; Zheng, L.; Zhu, L.; Lv, X. Anti-inflammatory protein TSG-6 secreted by bone marrow mesenchymal stem cells attenuates neuropathic pain by inhibiting the TLR2/MyD88/NF-kappaB signaling pathway in spinal microglia. J. Neuroinflamm. 2020, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.E.; Kunkel, S.L.; Shah, M.R.; Fu, R.; Mazarakis, D.D.; Haines, G.K.; Burdick, M.D.; Pope, R.M.; Strieter, R.M. Macrophage inflammatory protein-1 beta: A C-C chemokine in osteoarthritis. Clin. Immunol. Immunopathol. 1995, 77, 307–314. [Google Scholar] [CrossRef]
- Chang, T.T.; Chen, J.W. Emerging role of chemokine CC motif ligand 4 related mechanisms in diabetes mellitus and cardiovascular disease: Friends or foes? Cardiovasc. Diabetol. 2016, 15, 117. [Google Scholar] [CrossRef] [PubMed]
- Mukaida, N.; Sasaki, S.I.; Baba, T. CCL4 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1231, 23–32. [Google Scholar]
- Ciechanowska, A.; Mika, J. CC Chemokine Family Members’ Modulation as a Novel Approach for Treating Central Nervous System and Peripheral Nervous System Injury-A Review of Clinical and Experimental Findings. Int. J. Mol. Sci. 2024, 25, 3788. [Google Scholar] [CrossRef]
- Saika, F.; Kiguchi, N.; Kobayashi, Y.; Fukazawa, Y.; Kishioka, S. CC-chemokine ligand 4/macrophage inflammatory protein-1beta participates in the induction of neuropathic pain after peripheral nerve injury. Eur. J. Pain. 2012, 16, 1271–1280. [Google Scholar] [CrossRef]
- Xia, M.Q.; Qin, S.X.; Wu, L.J.; Mackay, C.R.; Hyman, B.T. Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer’s disease brains. Am. J. Pathol. 1998, 153, 31–37. [Google Scholar] [CrossRef]
- Ciechanowska, A.; Popiolek-Barczyk, K.; Pawlik, K.; Ciapala, K.; Oggioni, M.; Mercurio, D.; De Simoni, M.G.; Mika, J. Changes in macrophage inflammatory protein-1 (MIP-1) family members expression induced by traumatic brain injury in mice. Immunobiology 2020, 225, 151911. [Google Scholar] [CrossRef]
- Rojewska, E.; Zychowska, M.; Piotrowska, A.; Kreiner, G.; Nalepa, I.; Mika, J. Involvement of Macrophage Inflammatory Protein-1 Family Members in the Development of Diabetic Neuropathy and Their Contribution to Effectiveness of Morphine. Front. Immunol. 2018, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Sorce, S.; Myburgh, R.; Krause, K.H. The chemokine receptor CCR5 in the central nervous system. Prog. Neurobiol. 2011, 93, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Festa, B.P.; Siddiqi, F.H.; Jimenez-Sanchez, M.; Won, H.; Rob, M.; Djajadikerta, A.; Stamatakou, E.; Rubinsztein, D.C. Microglial-to-neuronal CCR5 signaling regulates autophagy in neurodegeneration. Neuron 2023, 111, 2021–2037.e12. [Google Scholar] [CrossRef]
- Dong, F.L.; Yu, L.; Feng, P.D.; Ren, J.X.; Bai, X.H.; Lin, J.Q.; Cao, D.L.; Deng, Y.T.; Zhang, Y.; Shen, H.H.; et al. An atlas of neuropathic pain-associated molecular pathological characteristics in the mouse spinal cord. Commun. Biol. 2025, 8, 70. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).