BDNF Signaling and Pain Modulation
Abstract
1. Introduction
2. BDNF Signaling
2.1. BDNF Synthesis, Source, and Release
2.2. Targets and Downstream Signaling
2.3. Synaptic Plasticity
2.4. Neuroimmune Signaling
2.4.1. Expression of BDNF on Neuroimmune Cell Types
2.4.2. BDNF and Neuroimmune Signaling
3. Peripheral Nociception
3.1. Expression and Localization
3.2. Cellular Functions
3.3. Behavioral Studies
4. Spinal Nociception
4.1. Expression and Localization
4.2. Cellular Functions
4.3. Behavioral Studies
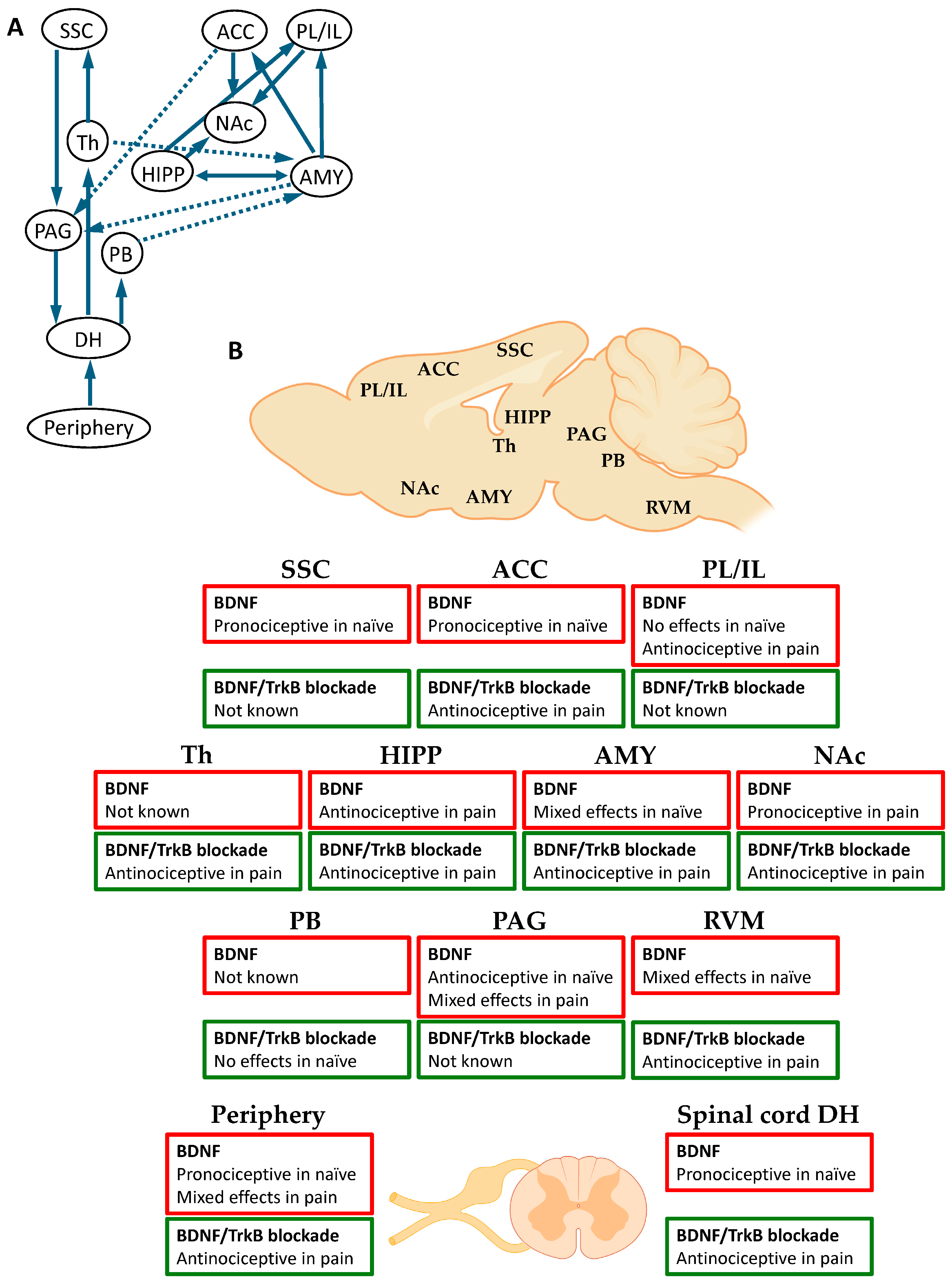
5. Pain Processing and Modulation in the Brain
5.1. Expression and Localization
5.2. Cellular Functions
5.3. Behavioral Studies
6. Other Brain Disorders
6.1. Depression
6.2. Schizophrenia
6.3. Neurodegeneration
7. Conclusions
| Intervention | Region and Assay | Species | Pain Model | Effect | Reference |
|---|---|---|---|---|---|
| Periphery | |||||
| BDNF | DRG culture (patch-clamp) | Rat | STZ-induced neuropathy | ↓neuronal properties | [158] |
| DRG culture | Rat | TNF-α treatment | ↑substance P and CGRP release | [159] | |
| Avil-CreERT2 (condition BDNF knockout from primary sensory neurons) | Spinal WDR neurons (in vivo electrophysiology) | Mouse | Naïve | No effects | [165] |
| Spinal cord | |||||
| BDNF | Isolated hemisected spinal cord | Rat | Naïve | ↑NMDA-induced, C- and A-fiber evoked responses | [154] |
| BDNF | Lamina II neurons in slice (patch-clamp) | Rat | Naïve | ↑C-fiber evoked responses (EPSCs) | [179] |
| BDNF | Lamina II neurons in slice (Ca2+ imaging) | Rat | Naïve | ↑Ca2+ oscillations | [144] |
| Capsaicin challenge | ↓Ca2+ oscillations | ||||
| Lamina II neurons in slice (patch-clamp) | Naïve | ↑EPSC frequency; No effects on EPSC decay or amplitude | |||
| TrkB-IgG | Lamina II neurons in slice (patch-clamp) | Rat | NGF-induced inflammation | ↓C-fiber evoked responses | [154] |
| TrkB-Fc chimera | Lamina II neurons in slice (patch-clamp) | Rat | Bone cancer-induced pain | ↓DRG evoked EPSCs | [176] |
| BDNF | Isolated dorsal horn with dorsal root attached | Rat | Naïve | ↓electrical- or capsaicin-induced substance P release | [180] |
| ↑K+-mediated GABA release | |||||
| SNL | ↑GABA release | [181] | |||
| BDNF | Lamina I neurons in slice (patch-clamp) | Rat | Naïve | ↑GABA-mediated Ca2+ responses; depolarized Eanion | [30] |
| anti-TrkB | PNI | hyperpolarized Eanion | |||
| Brain and brainstem | |||||
| TrkB-Fc | MT (in vivo electrophysiology) | Rat | CPSP | ↓SNS-electrically evoked neuronal response | [192] |
| BDNF | NRM (patch-clamp) | Rat | Naïve | ↑frequency and amplitude of AMPA mEPSCs | [203] |
| TrkB-IgG | CFA | ↓AMPA EPSCs | |||
| BDNF | NRM (patch-clamp) | Rat | Naïve | ↓mIPSC frequency | [208] |
| Depolarizing shift in EPSC and ↑excitability in MOR-expressing neurons | [204] | ||||
| TrkB-IgG | CFA | Hyperpolarizing shift in EPSC and ↓excitability in MOR-expressing neurons | |||
| BDNF | No effect on mIPSC frequency | [208] | |||
| pAAV2-hSyn-Cre-GFP, (AAV2-Retro) + pAAV2-CAG-DIO-BDNF-mCherry-3∗flag(vCA1-IL pathway-specific overexpression of BDN) | IL (in vivo electrophysiology) | Rat | CFA | ↑spontaneous neuronal firing, power spectral density in low gamma band, gPDC | [201] |
| p156sinRRLpptCAG-BDNF (BDNF lentiviral vector) | S1 (hindlimb part) | Rat | CFA | ↑LTP | [109] |
| Cx3cr1CreER/+;Bdnffl/fl (systemic depletion of microglial BDNF) | Layer 5 S1 (in vivo two-photon imaging) | Mouse | SNI | ↓spontaneous and mechanically induced Ca2+ activity | [108] |
| EE-induced BDNF increase | Hippocampus | Mouse | CCI | ↑LTP maintenance (fEPSP) | [207] |
| Intervention | Region/Assay | Species | Pain Model | Effect | Reference | |
|---|---|---|---|---|---|---|
| Periphery | ||||||
| Avil-CreERT2 (condition BDNF knockout from primary sensory neurons) | Nocifensive behaviors | Mouse | Formalin test | ↓second phase | [37,165] | |
| Mechanical allodynia | SNI- or paclitaxel-induced neuropathy | No effects | [37] | |||
| Mechanical and thermal hypersensitivity | CFA inflammatory pain | |||||
| Mechanical allodynia | pSNL | [165] | ||||
| SNL | ↓ | |||||
| Mechanical hypersensitivity | Hyperalgesic priming | |||||
| BDNF | Mechanical allodynia | Mouse | Normal | ↑ | [97] | |
| SNC | No effects | |||||
| Thermal hypersensitivity | Rat | Normal | ↑ | [164] | ||
| Weight-bearing deficits and mechanical allodynia | Rat | Normal | No effects | [168] | ||
| MIA MNX | ↓ | |||||
| Mechanical allodynia | Rat | Normal | ↑ | [160] | ||
| Anti-BDNF antibody | L5 spinal nerve lesion model | ↓ | ||||
| Ad-proBDNF (adenovirus vector-encoding proBDNF gene) | Mechanical allodynia | Mouse | Normal | ↑ | [167] | |
| Flitching and licking (second phase) | Formalin (0.5%) | |||||
| Spinal cord | ||||||
| Anti- BDNF antibody | Thermal hyperalgesia | Rat | SNL | ↓ | [153] | |
| BDNF | [181] | |||||
| Normal | [180] | |||||
| Mechanical allodynia and thermal hyperalgesia | Mouse | ↑ | [175] [182] | |||
| Mechanical allodynia | Rat | [30] | ||||
| adBDNF | ||||||
| neuroptrophin-4/5 | Thermal hyperalgesia | Mouse | [182] | |||
| Antisense oligonucleotide against BDNF mRNA | Carrageenan inflammatory pain | ↓ | ||||
| Mechanical allodynia | pSNL | [183] [175] | ||||
| TrK-Fc | Mechanical allodynia and thermal hyperalgesia | |||||
| Rat | Bone cancer pain | [176] | ||||
| Bilateral cervical facet joint distraction | [156] | |||||
| Mechanical allodynia | Rat | PNI | [30] | |||
| ATP-challenged microglia with anti-TrkB, TrkB-Fc BDNF siRNA | Normal | No effect | ||||
| TrKB-IgG | Nocifensive behaviors | Rat | NGF primed in formalin test | ↓ | [154] | |
| Y1036, TrkB-Fc, Cx3cr1CreER × loxP-Bdnf (tamoxifen-induced Cre-loxP–mediated deletion of the Bdnf gene in CX3CR1-positive cells) | Mechanical allodynia | Mouse | SNL | ↓ | [186] | |
| Brain and brainstem | ||||||
| BDNF, p156sinRRLpptCAG-BDNF (BDNF lentiviral vector) | ACC | Cold hypersensitivity, CPA, clonidine-induced CPP | Rat | Naïve | ↑ | [109,196,197,198] |
| Tat-CTX-B | CFA, SNI, bone cancer pain | ↓ | ||||
| BDNF | IL | Thermal hyperalgesia and mechanical allodynia | Rat | Naïve | No effects | [199] |
| CFA | ↓ | |||||
| pAAV2-hSyn-Cre-GFP, (AAV2-Retro) + pAAV2-CAG-DIO-BDNF-mCherry-3∗flag (vCA1-IL pathway-specific overexpression of BDNF) | vCA1-IL | Spontaneous nociceptive behaviors, thermal hyperalgesia, mechanical allodynia, anxiety-like behaviors | Rat | ↓ | [201] | |
| pAAV-CMV-MCS-EGFP-3Flag (BDNF-specific overexpression) | ventral DG | Thermal hyperalgesia, mechanical allodynia, anxiety-like behaviors | Mouse | [200] | ||
| BDNF | NAc | Thermal hyperalgesia | Mouse | CUMS | ↑ | [215] |
| TrkB-Fc | Morphine-induced | ↓ | ||||
| CCI | [195] | |||||
| ANA-12 | Optogenetically induced hypersensitivity | [216] | ||||
| BDNF | CeA | Thermal hyperalgesia | Mouse | Naïve | ↑ | [191] |
| TrkB-IgG | CFA | ↓ | ||||
| TrkB-Fc | CeA | Morphine-induced analgesia | Mouse | Naïve | ↓ | [205] |
| oe-BDNF lentivirus (BDNF overexpression) | Parafascicular nucleus of thalamus | Anxiety-like behaviors and mechanical allodynia | Mouse | CRS | ↓ | [194] |
| sh-BDNF lentivirus (BDNF knockdown) | ↑ | |||||
| TrkB-Fc, CTX-B | MT | Mechanical allodynia | Rat | CPSP | ↓ | [192] |
| Thermal hyperalgesia | No effects | |||||
| K252a | NRM | Mechanical allodynia | Rat | CFA | ↓ | [208] |
| BDNF | Midbrain (PAG-DRN) | Thermal hyperalgesia | Rat | Normal | ↓ | [211,212] |
| Nociceptive responses | Formalin | [213] | ||||
| BDNF | PAG | tDCS-induced analgesic effects | Rat | MIA | ↓ | [214] |
| AAV-eGFP-Cre virus | PB | Thermal hyperalgesia and mechanical allodynia | Floxed-BDNF mouse | Normal | No effects | [205] |
| BDNF | RVM | Thermal hyper-algesia and mechanical allodynia | Rat | Naïve | ↑ | [187,189] |
| Thermal hyperalgesia | CFA | ↓ | [187] | |||
| RVM 5-HT-depleted animals | [189] | |||||
| rabbit anti-BDNF antibody | RVM | PSD-induced cumulative pain scores and mechanical allodynia | Rat | Incisional pain | ↓ | [190] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barde, Y.A.; Edgar, D.; Thoenen, H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982, 1, 549–553. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Mowla, S.J.; Farhadi, H.F.; Pareek, S.; Atwal, J.K.; Morris, S.J.; Seidah, N.G.; Murphy, R.A. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J. Biol. Chem. 2001, 276, 12660–12666. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V.; Bothwell, M. Neurotrophins: To cleave or not to cleave. Neuron 2002, 33, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Minichiello, L. TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009, 10, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ren, Q.; Zhang, J.C.; Chen, Q.X.; Hashimoto, K. Altered expression of BDNF, BDNF pro-peptide and their precursor proBDNF in brain and liver tissues from psychiatric disorders: Rethinking the brain-liver axis. Transl. Psychiatry 2017, 7, e1128. [Google Scholar] [CrossRef]
- Thompson Ray, M.; Weickert, C.S.; Wyatt, E.; Webster, M.J. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J. Psychiatry Neurosci. 2011, 36, 195–203. [Google Scholar] [CrossRef]
- Castren, E.; Rantamaki, T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev. Neurobiol. 2010, 70, 289–297. [Google Scholar] [CrossRef]
- Garzon, D.; Yu, G.; Fahnestock, M. A new brain-derived neurotrophic factor transcript and decrease in brain-derived neurotrophic factor transcripts 1, 2 and 3 in Alzheimer’s disease parietal cortex. J. Neurochem. 2002, 82, 1058–1064. [Google Scholar] [CrossRef]
- Phillips, H.S.; Hains, J.M.; Armanini, M.; Laramee, G.R.; Johnson, S.A.; Winslow, J.W. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron 1991, 7, 695–702. [Google Scholar] [CrossRef]
- Hock, C.; Heese, K.; Hulette, C.; Rosenberg, C.; Otten, U. Region-specific neurotrophin imbalances in Alzheimer disease: Decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 2000, 57, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Ricci, V.; Pomponi, M.; Conte, G.; Mathe, A.A.; Attilio Tonali, P.; Bria, P. Chronic heroin and cocaine abuse is associated with decreased serum concentrations of the nerve growth factor and brain-derived neurotrophic factor. J. Psychopharmacol. 2007, 21, 820–825. [Google Scholar] [CrossRef]
- Kim, D.J.; Roh, S.; Kim, Y.; Yoon, S.J.; Lee, H.K.; Han, C.S.; Kim, Y.K. High concentrations of plasma brain-derived neurotrophic factor in methamphetamine users. Neurosci. Lett. 2005, 388, 112–115. [Google Scholar] [CrossRef]
- Simao, A.P.; Mendonca, V.A.; de Oliveira Almeida, T.M.; Santos, S.A.; Gomes, W.F.; Coimbra, C.C.; Lacerda, A.C. Involvement of BDNF in knee osteoarthritis: The relationship with inflammation and clinical parameters. Rheumatol. Int. 2014, 34, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.J.; Liao, F.F.; Dang, W.H.; Ding, X.; Liu, X.D.; Cai, J.; Han, J.S.; Wan, Y.; Xing, G.G. Contribution of the spinal cord BDNF to the development of neuropathic pain by activation of the NR2B-containing NMDA receptors in rats with spinal nerve ligation. Exp. Neurol. 2010, 222, 256–266. [Google Scholar] [CrossRef]
- Hildebrand, M.E.; Xu, J.; Dedek, A.; Li, Y.; Sengar, A.S.; Beggs, S.; Lombroso, P.J.; Salter, M.W. Potentiation of Synaptic GluN2B NMDAR Currents by Fyn Kinase Is Gated through BDNF-Mediated Disinhibition in Spinal Pain Processing. Cell Rep. 2016, 17, 2753–2765. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef]
- Esvald, E.E.; Tuvikene, J.; Sirp, A.; Patil, S.; Bramham, C.R.; Timmusk, T. CREB Family Transcription Factors Are Major Mediators of BDNF Transcriptional Autoregulation in Cortical Neurons. J. Neurosci. 2020, 40, 1405–1426. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Lu, B. Diverse Functions of Multiple Bdnf Transcripts Driven by Distinct Bdnf Promoters. Biomolecules 2023, 13, 655. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J. Neurosci. 2009, 29, 12764–12767. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Begni, V.; Pariante, C.M.; Riva, M.A. The human BDNF gene: Peripheral gene expression and protein levels as biomarkers for psychiatric disorders. Transl. Psychiatry 2016, 6, e958. [Google Scholar] [CrossRef]
- Lu, B. BDNF and activity-dependent synaptic modulation. Learn. Mem. 2003, 10, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Lessmann, V.; Brigadski, T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: An update. Neurosci. Res. 2009, 65, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Brigadski, T.; Lessmann, V. The physiology of regulated BDNF release. Cell Tissue Res. 2020, 382, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Dieni, S.; Matsumoto, T.; Dekkers, M.; Rauskolb, S.; Ionescu, M.S.; Deogracias, R.; Gundelfinger, E.D.; Kojima, M.; Nestel, S.; Frotscher, M.; et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J. Cell Biol. 2012, 196, 775–788. [Google Scholar] [CrossRef]
- Arevalo, J.C.; Deogracias, R. Mechanisms Controlling the Expression and Secretion of BDNF. Biomolecules 2023, 13, 789. [Google Scholar] [CrossRef]
- Zafra, F.; Lindholm, D.; Castren, E.; Hartikka, J.; Thoenen, H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J. Neurosci. 1992, 12, 4793–4799. [Google Scholar] [CrossRef]
- Tongiorgi, E. Activity-dependent expression of brain-derived neurotrophic factor in dendrites: Facts and open questions. Neurosci. Res. 2008, 61, 335–346. [Google Scholar] [CrossRef]
- Danzer, S.C.; McNamara, J.O. Localization of brain-derived neurotrophic factor to distinct terminals of mossy fiber axons implies regulation of both excitation and feedforward inhibition of CA3 pyramidal cells. J. Neurosci. 2004, 24, 11346–11355. [Google Scholar] [CrossRef]
- Coull, J.A.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef]
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R., 3rd; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013, 155, 1596–1609. [Google Scholar] [CrossRef]
- Elkabes, S.; DiCicco-Bloom, E.M.; Black, I.B. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J. Neurosci. 1996, 16, 2508–2521. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Honda, S.; Tohyama, Y.; Imai, Y.; Kohsaka, S.; Kurihara, T. Neurotrophin secretion from cultured microglia. J. Neurosci. Res. 2001, 65, 322–331. [Google Scholar] [CrossRef]
- Yao, W.; Cao, Q.; Luo, S.; He, L.; Yang, C.; Chen, J.; Qi, Q.; Hashimoto, K.; Zhang, J.C. Microglial ERK-NRBP1-CREB-BDNF signaling in sustained antidepressant actions of (R)-ketamine. Mol. Psychiatry 2022, 27, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Yue, X.; An, J.J.; Bass, R.; Xu, H.; Xu, B. Genetic Dissection of BDNF and TrkB Expression in Glial Cells. Biomolecules 2024, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Denk, F.; Crow, M.; Didangelos, A.; Lopes, D.M.; McMahon, S.B. Persistent Alterations in Microglial Enhancers in a Model of Chronic Pain. Cell Rep. 2016, 15, 1771–1781. [Google Scholar] [CrossRef]
- Dembo, T.; Braz, J.M.; Hamel, K.A.; Kuhn, J.A.; Basbaum, A.I. Primary Afferent-Derived BDNF Contributes Minimally to the Processing of Pain and Itch. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Honey, D.; Wosnitzka, E.; Klann, E.; Weinhard, L. Analysis of microglial BDNF function and expression in the motor cortex. Front. Cell Neurosci. 2022, 16, 961276. [Google Scholar] [CrossRef]
- De Santi, L.; Annunziata, P.; Sessa, E.; Bramanti, P. Brain-derived neurotrophic factor and TrkB receptor in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Neurol. Sci. 2009, 287, 17–26. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Enkavi, G.; Girych, M.; Moliner, R.; Vattulainen, I.; Castren, E. TrkB transmembrane domain: Bridging structural understanding with therapeutic strategy. Trends Biochem. Sci. 2024, 49, 445–456. [Google Scholar] [CrossRef]
- Teng, H.K.; Teng, K.K.; Lee, R.; Wright, S.; Tevar, S.; Almeida, R.D.; Kermani, P.; Torkin, R.; Chen, Z.Y.; Lee, F.S.; et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 2005, 25, 5455–5463. [Google Scholar] [CrossRef] [PubMed]
- Schiro, G.; Iacono, S.; Ragonese, P.; Aridon, P.; Salemi, G.; Balistreri, C.R. A Brief Overview on BDNF-Trk Pathway in the Nervous System: A Potential Biomarker or Possible Target in Treatment of Multiple Sclerosis? Front. Neurol. 2022, 13, 917527. [Google Scholar] [CrossRef]
- Turkistani, A.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Elhussieny, O.; Al-Farga, A.; Aqlan, F.; Saad, H.M.; Batiha, G.E. The functional and molecular roles of p75 neurotrophin receptor (p75(NTR)) in epilepsy. J. Cent. Nerv. Syst. Dis. 2024, 16, 11795735241247810. [Google Scholar] [CrossRef] [PubMed]
- Woo, N.H.; Teng, H.K.; Siao, C.J.; Chiaruttini, C.; Pang, P.T.; Milner, T.A.; Hempstead, B.L.; Lu, B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 2005, 8, 1069–1077. [Google Scholar] [CrossRef]
- Gupta, V.K.; You, Y.; Gupta, V.B.; Klistorner, A.; Graham, S.L. TrkB receptor signalling: Implications in neurodegenerative, psychiatric and proliferative disorders. Int. J. Mol. Sci. 2013, 14, 10122–10142. [Google Scholar] [CrossRef]
- Klein, R.; Smeyne, R.J.; Wurst, W.; Long, L.K.; Auerbach, B.A.; Joyner, A.L.; Barbacid, M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell 1993, 75, 113–122. [Google Scholar] [PubMed]
- Rohrer, B.; Korenbrot, J.I.; LaVail, M.M.; Reichardt, L.F.; Xu, B. Role of neurotrophin receptor TrkB in the maturation of rod photoreceptors and establishment of synaptic transmission to the inner retina. J. Neurosci. 1999, 19, 8919–8930. [Google Scholar] [CrossRef]
- Andreska, T.; Luningschror, P.; Sendtner, M. Regulation of TrkB cell surface expression-a mechanism for modulation of neuronal responsiveness to brain-derived neurotrophic factor. Cell Tissue Res. 2020, 382, 5–14. [Google Scholar] [CrossRef]
- Zhao, L.; Sheng, A.L.; Huang, S.H.; Yin, Y.X.; Chen, B.; Li, X.Z.; Zhang, Y.; Chen, Z.Y. Mechanism underlying activity-dependent insertion of TrkB into the neuronal surface. J. Cell Sci. 2009, 122, 3123–3136. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhang, Y.; Lester, H.A.; Schuman, E.M.; Davidson, N. Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons. J. Neurosci. 1998, 18, 10231–10240. [Google Scholar] [CrossRef]
- Xu, B.; Gottschalk, W.; Chow, A.; Wilson, R.I.; Schnell, E.; Zang, K.; Wang, D.; Nicoll, R.A.; Lu, B.; Reichardt, L.F. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: Modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J. Neurosci. 2000, 20, 6888–6897. [Google Scholar] [CrossRef]
- Luongo, L.; Maione, S.; Di Marzo, V. Endocannabinoids and neuropathic pain: Focus on neuron-glia and endocannabinoid-neurotrophin interactions. Eur. J. Neurosci. 2014, 39, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Magee, J.C.; Grienberger, C. Synaptic Plasticity Forms and Functions. Annu. Rev. Neurosci. 2020, 43, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Kavalali, E.T.; Monteggia, L.M. BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell 2022, 185, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Korte, M.; Carroll, P.; Wolf, E.; Brem, G.; Thoenen, H.; Bonhoeffer, T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA 1995, 92, 8856–8860. [Google Scholar] [CrossRef]
- Patterson, S.L.; Abel, T.; Deuel, T.A.; Martin, K.C.; Rose, J.C.; Kandel, E.R. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 1996, 16, 1137–1145. [Google Scholar] [CrossRef]
- Korte, M.; Griesbeck, O.; Gravel, C.; Carroll, P.; Staiger, V.; Thoenen, H.; Bonhoeffer, T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc. Natl. Acad. Sci. USA 1996, 93, 12547–12552. [Google Scholar] [CrossRef]
- An, J.J.; Gharami, K.; Liao, G.Y.; Woo, N.H.; Lau, A.G.; Vanevski, F.; Torre, E.R.; Jones, K.R.; Feng, Y.; Lu, B.; et al. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 2008, 134, 175–187. [Google Scholar] [CrossRef]
- Gartner, A.; Polnau, D.G.; Staiger, V.; Sciarretta, C.; Minichiello, L.; Thoenen, H.; Bonhoeffer, T.; Korte, M. Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase Cgamma signaling. J. Neurosci. 2006, 26, 3496–3504. [Google Scholar] [CrossRef]
- Zakharenko, S.S.; Patterson, S.L.; Dragatsis, I.; Zeitlin, S.O.; Siegelbaum, S.A.; Kandel, E.R.; Morozov, A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron 2003, 39, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Kavalali, E.T.; Monteggia, L.M. Genetic Dissection of Presynaptic and Postsynaptic BDNF-TrkB Signaling in Synaptic Efficacy of CA3-CA1 Synapses. Cell Rep. 2018, 24, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Peng, J.; Xu, Y.N.; Zeng, W.J.; Zhang, J.; Wei, X.; Mai, C.L.; Lin, Z.J.; Liu, Y.; Murugan, M.; et al. Microglia Are Indispensable for Synaptic Plasticity in the Spinal Dorsal Horn and Chronic Pain. Cell Rep. 2019, 27, 3844–3859.e6. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhang, M.; Wang, Q.; Wu, D.Y.; Jie, W.; Hu, N.Y.; Lan, J.Z.; Zeng, K.; Li, S.J.; Li, X.W.; et al. Distinct roles of astroglia and neurons in synaptic plasticity and memory. Mol. Psychiatry 2022, 27, 873–885. [Google Scholar] [CrossRef]
- Vignoli, B.; Battistini, G.; Melani, R.; Blum, R.; Santi, S.; Berardi, N.; Canossa, M. Peri-Synaptic Glia Recycles Brain-Derived Neurotrophic Factor for LTP Stabilization and Memory Retention. Neuron 2016, 92, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, S.B.; Mogulkoc, R.; Baltaci, A.K. Molecular Mechanisms of Early and Late LTP. Neurochem. Res. 2019, 44, 281–296. [Google Scholar] [CrossRef]
- Sweatt, J.D. Toward a molecular explanation for long-term potentiation. Learn. Mem. 1999, 6, 399–416. [Google Scholar] [CrossRef]
- Edelmann, E.; Cepeda-Prado, E.; Franck, M.; Lichtenecker, P.; Brigadski, T.; Lessmann, V. Theta Burst Firing Recruits BDNF Release and Signaling in Postsynaptic CA1 Neurons in Spike-Timing-Dependent LTP. Neuron 2015, 86, 1041–1054. [Google Scholar] [CrossRef]
- Caldeira, M.V.; Melo, C.V.; Pereira, D.B.; Carvalho, R.; Correia, S.S.; Backos, D.S.; Carvalho, A.L.; Esteban, J.A.; Duarte, C.B. Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J. Biol. Chem. 2007, 282, 12619–12628. [Google Scholar] [CrossRef]
- Fortin, D.A.; Srivastava, T.; Dwarakanath, D.; Pierre, P.; Nygaard, S.; Derkach, V.A.; Soderling, T.R. Brain-derived neurotrophic factor activation of CaM-kinase kinase via transient receptor potential canonical channels induces the translation and synaptic incorporation of GluA1-containing calcium-permeable AMPA receptors. J. Neurosci. 2012, 32, 8127–8137. [Google Scholar] [CrossRef]
- Nakata, H.; Nakamura, S. Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Lett. 2007, 581, 2047–2054. [Google Scholar] [CrossRef]
- Jourdi, H.; Kabbaj, M. Acute BDNF treatment upregulates GluR1-SAP97 and GluR2-GRIP1 interactions: Implications for sustained AMPA receptor expression. PLoS ONE 2013, 8, e57124. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, Y.; Hanse, E.; Kafitz, K.W.; Konnerth, A. Postsynaptic Induction of BDNF-Mediated Long-Term Potentiation. Science 2002, 295, 1729–1734. [Google Scholar] [CrossRef]
- Caldeira, M.V.; Melo, C.V.; Pereira, D.B.; Carvalho, R.F.; Carvalho, A.L.; Duarte, C.B. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol. Cell Neurosci. 2007, 35, 208–219. [Google Scholar] [CrossRef]
- Schratt, G.M.; Nigh, E.A.; Chen, W.G.; Hu, L.; Greenberg, M.E. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J. Neurosci. 2004, 24, 7366–7377. [Google Scholar] [CrossRef] [PubMed]
- Suen, P.C.; Wu, K.; Levine, E.S.; Mount, H.T.; Xu, J.L.; Lin, S.Y.; Black, I.B. Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-D-aspartate receptor subunit 1. Proc. Natl. Acad. Sci. USA 1997, 94, 8191–8195. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Wu, K.; Levine, E.S.; Mount, H.T.; Suen, P.C.; Black, I.B. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res. Mol. Brain Res. 1998, 55, 20–27. [Google Scholar] [CrossRef]
- Patterson, S.L.; Pittenger, C.; Morozov, A.; Martin, K.C.; Scanlin, H.; Drake, C.; Kandel, E.R. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron 2001, 32, 123–140. [Google Scholar] [CrossRef]
- Hashimotodani, Y.; Nasrallah, K.; Jensen, K.R.; Chavez, A.E.; Carrera, D.; Castillo, P.E. LTP at Hilar Mossy Cell-Dentate Granule Cell Synapses Modulates Dentate Gyrus Output by Increasing Excitation/Inhibition Balance. Neuron 2017, 95, 928–943.e3. [Google Scholar] [CrossRef]
- Minichiello, L.; Calella, A.M.; Medina, D.L.; Bonhoeffer, T.; Klein, R.; Korte, M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron 2002, 36, 121–137. [Google Scholar] [CrossRef]
- Tanaka, J.; Horiike, Y.; Matsuzaki, M.; Miyazaki, T.; Ellis-Davies, G.C.; Kasai, H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 2008, 319, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Briz, V.; Zhu, G.; Wang, Y.; Liu, Y.; Avetisyan, M.; Bi, X.; Baudry, M. Activity-dependent rapid local RhoA synthesis is required for hippocampal synaptic plasticity. J. Neurosci. 2015, 35, 2269–2282. [Google Scholar] [CrossRef]
- Ding, X.; Cai, J.; Li, S.; Liu, X.D.; Wan, Y.; Xing, G.G. BDNF contributes to the development of neuropathic pain by induction of spinal long-term potentiation via SHP2 associated GluN2B-containing NMDA receptors activation in rats with spinal nerve ligation. Neurobiol. Dis. 2015, 73, 428–451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Ren, W.J.; Zhong, Y.; Yang, T.; Wei, X.H.; Xin, W.J.; Liu, C.C.; Zhou, L.H.; Li, Y.Y.; Liu, X.G. Limited BDNF contributes to the failure of injury to skin afferents to produce a neuropathic pain condition. Pain 2010, 148, 148–157. [Google Scholar] [CrossRef] [PubMed]
- White, A.O.; Kramar, E.A.; Lopez, A.J.; Kwapis, J.L.; Doan, J.; Saldana, D.; Davatolhagh, M.F.; Alaghband, Y.; Blurton-Jones, M.; Matheos, D.P.; et al. BDNF rescues BAF53b-dependent synaptic plasticity and cocaine-associated memory in the nucleus accumbens. Nat. Commun. 2016, 7, 11725. [Google Scholar] [CrossRef]
- Tanqueiro, S.R.; Mouro, F.M.; Ferreira, C.B.; Freitas, C.F.; Fonseca-Gomes, J.; Simoes do Couto, F.; Sebastiao, A.M.; Dawson, N.; Diogenes, M.J. Sustained NMDA receptor hypofunction impairs brain-derived neurotropic factor signalling in the PFC, but not in the hippocampus, and disturbs PFC-dependent cognition in mice. J. Psychopharmacol. 2021, 35, 730–743. [Google Scholar] [CrossRef]
- Miao, H.H.; Miao, Z.; Pan, J.G.; Li, X.H.; Zhuo, M. Brain-derived neurotrophic factor produced long-term synaptic enhancement in the anterior cingulate cortex of adult mice. Mol. Brain 2021, 14, 140. [Google Scholar] [CrossRef]
- Luo, C.; Kuner, T.; Kuner, R. Synaptic plasticity in pathological pain. Trends Neurosci. 2014, 37, 343–355. [Google Scholar] [CrossRef]
- Ji, R.R.; Chamessian, A.; Zhang, Y.Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage Polarization and Its Role in Liver Disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Hong, J.H.; Park, H.M.; Byun, K.H.; Lee, B.H.; Kang, W.C.; Jeong, G.B. BDNF expression of macrophages and angiogenesis after myocardial infarction. Int. J. Cardiol. 2014, 176, 1405–1408. [Google Scholar] [CrossRef]
- Asami, T.; Ito, T.; Fukumitsu, H.; Nomoto, H.; Furukawa, Y.; Furukawa, S. Autocrine activation of cultured macrophages by brain-derived neurotrophic factor. Biochem. Biophys. Res. Commun. 2006, 344, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Salzer, J.L. Schwann cell myelination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020529. [Google Scholar] [CrossRef] [PubMed]
- Su, W.F.; Wu, F.; Jin, Z.H.; Gu, Y.; Chen, Y.T.; Fei, Y.; Chen, H.; Wang, Y.X.; Xing, L.Y.; Zhao, Y.Y.; et al. Overexpression of P2X4 receptor in Schwann cells promotes motor and sensory functional recovery and remyelination via BDNF secretion after nerve injury. Glia 2019, 67, 78–90. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Wilhelm, J.C.; Xu, M.; Cucoranu, D.; Chmielewski, S.; Holmes, T.; Lau, K.S.; Bassell, G.J.; English, A.W. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J. Neurosci. 2012, 32, 5002–5009. [Google Scholar] [CrossRef]
- Luo, B.; Huang, J.; Lu, L.; Hu, X.; Luo, Z.; Li, M. Electrically induced brain-derived neurotrophic factor release from Schwann cells. J. Neurosci. Res. 2014, 92, 893–903. [Google Scholar] [CrossRef]
- Bonalume, V.; Caffino, L.; Castelnovo, L.F.; Faroni, A.; Giavarini, F.; Liu, S.; Caruso, D.; Schmelz, M.; Fumagalli, F.; Carr, R.W.; et al. Schwann Cell Autocrine and Paracrine Regulatory Mechanisms, Mediated by Allopregnanolone and BDNF, Modulate PKCepsilon in Peripheral Sensory Neurons. Cells 2020, 9, 1874. [Google Scholar] [CrossRef]
- Castelnovo, L.F.; Thomas, P. Membrane progesterone receptor alpha (mPRalpha/PAQR7) promotes migration, proliferation and BDNF release in human Schwann cell-like differentiated adipose stem cells. Mol. Cell Endocrinol. 2021, 531, 111298. [Google Scholar] [CrossRef] [PubMed]
- Bierlein De la Rosa, M.; Sharma, A.D.; Mallapragada, S.K.; Sakaguchi, D.S. Transdifferentiation of brain-derived neurotrophic factor (BDNF)-secreting mesenchymal stem cells significantly enhance BDNF secretion and Schwann cell marker proteins. J. Biosci. Bioeng. 2017, 124, 572–582. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Geng, B.; Wang, X.; Wang, S.; Yi, Q.; Tang, Y.; Xia, Y. Exercise Facilitates the M1-to-M2 Polarization of Microglia by Enhancing Autophagy via the BDNF/AKT/mTOR Pathway in Neuropathic Pain. Pain. Physician 2022, 25, E1137–E1151. [Google Scholar]
- Huang, L.; Jin, J.; Chen, K.; You, S.; Zhang, H.; Sideris, A.; Norcini, M.; Recio-Pinto, E.; Wang, J.; Gan, W.B.; et al. BDNF produced by cerebral microglia promotes cortical plasticity and pain hypersensitivity after peripheral nerve injury. PLoS Biol. 2021, 19, e3001337. [Google Scholar] [CrossRef]
- Thibault, K.; Lin, W.K.; Rancillac, A.; Fan, M.; Snollaerts, T.; Sordoillet, V.; Hamon, M.; Smith, G.M.; Lenkei, Z.; Pezet, S. BDNF-dependent plasticity induced by peripheral inflammation in the primary sensory and the cingulate cortex triggers cold allodynia and reveals a major role for endogenous BDNF as a tuner of the affective aspect of pain. J. Neurosci. 2014, 34, 14739–14751. [Google Scholar] [CrossRef]
- Lawal, O.; Ulloa Severino, F.P.; Eroglu, C. The role of astrocyte structural plasticity in regulating neural circuit function and behavior. Glia 2022, 70, 1467–1483. [Google Scholar] [CrossRef]
- Endo, F.; Kasai, A.; Soto, J.S.; Yu, X.; Qu, Z.; Hashimoto, H.; Gradinaru, V.; Kawaguchi, R.; Khakh, B.S. Molecular basis of astrocyte diversity and morphology across the CNS in health and disease. Science 2022, 378, eadc9020. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.P.; Sheng, J.G.; Mitsuo, K.; Shirabe, S.; Nishiyama, N. Trophic factor production by reactive astrocytes in injured brain. Ann. N. Y Acad. Sci. 1993, 679, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.P.; Nishiyama, N. Neurotrophic factor gene expression in astrocytes during development and following injury. Brain Res. Bull. 1994, 35, 403–407. [Google Scholar] [CrossRef]
- Elbaz, B.; Popko, B. Molecular Control of Oligodendrocyte Development. Trends Neurosci. 2019, 42, 263–277. [Google Scholar] [CrossRef]
- Butt, A.M.; Papanikolaou, M.; Rivera, A. Physiology of Oligodendroglia. Adv. Exp. Med. Biol. 2019, 1175, 117–128. [Google Scholar] [CrossRef]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef]
- Dai, X.; Lercher, L.D.; Clinton, P.M.; Du, Y.; Livingston, D.L.; Vieira, C.; Yang, L.; Shen, M.M.; Dreyfus, C.F. The trophic role of oligodendrocytes in the basal forebrain. J. Neurosci. 2003, 23, 5846–5853. [Google Scholar] [CrossRef]
- Bagayogo, I.P.; Dreyfus, C.F. Regulated release of BDNF by cortical oligodendrocytes is mediated through metabotropic glutamate receptors and the PLC pathway. ASN Neuro 2009, 1, AN20090006. [Google Scholar] [CrossRef]
- Dougherty, K.D.; Dreyfus, C.F.; Black, I.B. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol. Dis. 2000, 7, 574–585. [Google Scholar] [CrossRef]
- Bi, C.; Fu, Y.; Li, B. Brain-derived neurotrophic factor alleviates diabetes mellitus-accelerated atherosclerosis by promoting M2 polarization of macrophages through repressing the STAT3 pathway. Cell Signal 2020, 70, 109569. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.C.; Huang, H.B.; Huang Tseng, H.Y.; Lu, M.C. Brain-Derived Neurotrophic Factor Suppressed Proinflammatory Cytokines Secretion and Enhanced MicroRNA(miR)-3168 Expression in Macrophages. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Bi, C.; Fu, Y.; Zhang, Z.; Li, B. Prostaglandin E2 confers protection against diabetic coronary atherosclerosis by stimulating M2 macrophage polarization via the activation of the CREB/BDNF/TrkB signaling pathway. FASEB J. 2020, 34, 7360–7371. [Google Scholar] [CrossRef]
- Hayashi, K.; Lesnak, J.B.; Plumb, A.N.; Janowski, A.J.; Smith, A.F.; Hill, J.K.; Sluka, K.A. Brain-derived neurotrophic factor contributes to activity-induced muscle pain in male but not female mice. bioRxiv 2023, 120, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Lalisse, S.; Hua, J.; Lenoir, M.; Linck, N.; Rassendren, F.; Ulmann, L. Sensory neuronal P2RX4 receptors controls BDNF signaling in inflammatory pain. Sci. Rep. 2018, 8, 964. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, K.T.; Mohammad, H.; Sweitzer, S.M. Protein kinase C in pain: Involvement of multiple isoforms. Pharmacol. Res. 2007, 55, 578–589. [Google Scholar] [CrossRef]
- Dadkhah, M.; Baziar, M.; Rezaei, N. The regulatory role of BDNF in neuroimmune axis function and neuroinflammation induced by chronic stress: A new therapeutic strategies for neurodegenerative disorders. Cytokine 2024, 174, 156477. [Google Scholar] [CrossRef]
- Sun, C.; Deng, J.; Ma, Y.; Meng, F.; Cui, X.; Li, M.; Li, J.; Li, J.; Yin, P.; Kong, L.; et al. The dual role of microglia in neuropathic pain after spinal cord injury: Detrimental and protective effects. Exp. Neurol. 2023, 370, 114570. [Google Scholar] [CrossRef]
- Trang, T.; Beggs, S.; Wan, X.; Salter, M.W. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J. Neurosci. 2009, 29, 3518–3528. [Google Scholar] [CrossRef]
- Ulmann, L.; Hatcher, J.P.; Hughes, J.P.; Chaumont, S.; Green, P.J.; Conquet, F.; Buell, G.N.; Reeve, A.J.; Chessell, I.P.; Rassendren, F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J. Neurosci. 2008, 28, 11263–11268. [Google Scholar] [CrossRef]
- Nakajima, K.; Tohyama, Y.; Kohsaka, S.; Kurihara, T. Ceramide activates microglia to enhance the production/secretion of brain-derived neurotrophic factor (BDNF) without induction of deleterious factors in vitro. J. Neurochem. 2002, 80, 697–705. [Google Scholar] [CrossRef]
- Ferrini, F.; De Koninck, Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013, 2013, 429815. [Google Scholar] [CrossRef]
- Ding, H.; Chen, J.; Su, M.; Lin, Z.; Zhan, H.; Yang, F.; Li, W.; Xie, J.; Huang, Y.; Liu, X.; et al. BDNF promotes activation of astrocytes and microglia contributing to neuroinflammation and mechanical allodynia in cyclophosphamide-induced cystitis. J. Neuroinflamm. 2020, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, L.; Xu, D.; Zhang, W.; Zhang, Y.; Zhou, J.; Huang, W. Interaction between astrocytic colony stimulating factor and its receptor on microglia mediates central sensitization and behavioral hypersensitivity in chronic post ischemic pain model. Brain Behav. Immun. 2018, 68, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Mapplebeck, J.C.S.; Beggs, S.; Salter, M.W. Sex differences in pain: A tale of two immune cells. Pain 2016, 157 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef]
- Bergami, M.; Santi, S.; Formaggio, E.; Cagnoli, C.; Verderio, C.; Blum, R.; Berninger, B.; Matteoli, M.; Canossa, M. Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J. Cell Biol. 2008, 183, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Alderson, R.F.; Curtis, R.; Alterman, A.L.; Lindsay, R.M.; DiStefano, P.S. Truncated TrkB mediates the endocytosis and release of BDNF and neurotrophin-4/5 by rat astrocytes and schwann cells in vitro. Brain Res. 2000, 871, 210–222. [Google Scholar] [CrossRef]
- Kinboshi, M.; Mukai, T.; Nagao, Y.; Matsuba, Y.; Tsuji, Y.; Tanaka, S.; Tokudome, K.; Shimizu, S.; Ito, H.; Ikeda, A.; et al. Inhibition of Inwardly Rectifying Potassium (Kir) 4.1 Channels Facilitates Brain-Derived Neurotrophic Factor (BDNF) Expression in Astrocytes. Front. Mol. Neurosci. 2017, 10, 408. [Google Scholar] [CrossRef]
- Jean, Y.Y.; Lercher, L.D.; Dreyfus, C.F. Glutamate elicits release of BDNF from basal forebrain astrocytes in a process dependent on metabotropic receptors and the PLC pathway. Neuron Glia Biol. 2008, 4, 35–42. [Google Scholar] [CrossRef]
- Santi, S.; Cappello, S.; Riccio, M.; Bergami, M.; Aicardi, G.; Schenk, U.; Matteoli, M.; Canossa, M. Hippocampal neurons recycle BDNF for activity-dependent secretion and LTP maintenance. EMBO J. 2006, 25, 4372–4380. [Google Scholar] [CrossRef]
- Stenovec, M.; Lasic, E.; Bozic, M.; Bobnar, S.T.; Stout, R.F., Jr.; Grubisic, V.; Parpura, V.; Zorec, R. Ketamine Inhibits ATP-Evoked Exocytotic Release of Brain-Derived Neurotrophic Factor from Vesicles in Cultured Rat Astrocytes. Mol. Neurobiol. 2016, 53, 6882–6896. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Gould, E.; Xu, J.; Kim, E.J.; Kim, J.H. Oligodendrocytes regulate presynaptic properties and neurotransmission through BDNF signaling in the mouse brainstem. eLife 2019, 8, e42156. [Google Scholar] [CrossRef]
- Ji, X.C.; Dang, Y.Y.; Gao, H.Y.; Wang, Z.T.; Gao, M.; Yang, Y.; Zhang, H.T.; Xu, R.X. Local Injection of Lenti-BDNF at the Lesion Site Promotes M2 Macrophage Polarization and Inhibits Inflammatory Response After Spinal Cord Injury in Mice. Cell Mol. Neurobiol. 2015, 35, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Merighi, A.; Bardoni, R.; Salio, C.; Lossi, L.; Ferrini, F.; Prandini, M.; Zonta, M.; Gustincich, S.; Carmignoto, G. Presynaptic functional trkB receptors mediate the release of excitatory neurotransmitters from primary afferent terminals in lamina II (substantia gelatinosa) of postnatal rat spinal cord. Dev. Neurobiol. 2008, 68, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Tender, G.C.; Li, Y.Y.; Cui, J.G. Brain-derived neurotrophic factor redistribution in the dorsal root ganglia correlates with neuropathic pain inhibition after resiniferatoxin treatment. Spine J. 2010, 10, 715–720. [Google Scholar] [CrossRef]
- Lopez-Perez, A.E.; Nurgali, K.; Abalo, R. Painful neurotrophins and their role in visceral pain. Behav. Pharmacol. 2018, 29, 120–139. [Google Scholar] [CrossRef]
- Luo, X.G.; Rush, R.A.; Zhou, X.F. Ultrastructural localization of brain-derived neurotrophic factor in rat primary sensory neurons. Neurosci. Res. 2001, 39, 377–384. [Google Scholar] [CrossRef]
- Salio, C.; Ferrini, F. BDNF and GDNF expression in discrete populations of nociceptors. Ann. Anat. 2016, 207, 55–61. [Google Scholar] [CrossRef]
- Salio, C.; Lossi, L.; Ferrini, F.; Merighi, A. Ultrastructural evidence for a pre- and postsynaptic localization of full-length trkB receptors in substantia gelatinosa (lamina II) of rat and mouse spinal cord. Eur. J. Neurosci. 2005, 22, 1951–1966. [Google Scholar] [CrossRef]
- Cappoli, N.; Tabolacci, E.; Aceto, P.; Dello Russo, C. The emerging role of the BDNF-TrkB signaling pathway in the modulation of pain perception. J. Neuroimmunol. 2020, 349, 577406. [Google Scholar] [CrossRef]
- Ha, S.O.; Kim, J.K.; Hong, H.S.; Kim, D.S.; Cho, H.J. Expression of brain-derived neurotrophic factor in rat dorsal root ganglia, spinal cord and gracile nuclei in experimental models of neuropathic pain. Neuroscience 2001, 107, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shen, Z.; Xu, H.; Zhang, K.; Guo, M.; Wang, F.; Li, J. BDNF Participates in Chronic Constriction Injury-Induced Neuropathic Pain via Transcriptionally Activating P2X(7) in Primary Sensory Neurons. Mol. Neurobiol. 2021, 58, 4226–4236. [Google Scholar] [CrossRef]
- Fukuoka, T.; Kondo, E.; Dai, Y.; Hashimoto, N.; Noguchi, K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J. Neurosci. 2001, 21, 4891–4900. [Google Scholar] [CrossRef] [PubMed]
- Kerr, B.J.; Bradbury, E.J.; Bennett, D.L.; Trivedi, P.M.; Dassan, P.; French, J.; Shelton, D.B.; McMahon, S.B.; Thompson, S.W. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J. Neurosci. 1999, 19, 5138–5148. [Google Scholar] [CrossRef] [PubMed]
- Mannion, R.J.; Costigan, M.; Decosterd, I.; Amaya, F.; Ma, Q.P.; Holstege, J.C.; Ji, R.R.; Acheson, A.; Lindsay, R.M.; Wilkinson, G.A.; et al. Neurotrophins: Peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc. Natl. Acad. Sci. USA 1999, 96, 9385–9390. [Google Scholar] [CrossRef]
- Kras, J.V.; Weisshaar, C.L.; Quindlen, J.; Winkelstein, B.A. Brain-derived neurotrophic factor is upregulated in the cervical dorsal root ganglia and spinal cord and contributes to the maintenance of pain from facet joint injury in the rat. J. Neurosci. Res. 2013, 91, 1312–1321. [Google Scholar] [CrossRef]
- Ge, H.; Guan, S.; Shen, Y.; Sun, M.; Hao, Y.; He, L.; Liu, L.; Yin, C.; Huang, R.; Xiong, W.; et al. Dihydromyricetin affects BDNF levels in the nervous system in rats with comorbid diabetic neuropathic pain and depression. Sci. Rep. 2019, 9, 14619. [Google Scholar] [CrossRef]
- Li, L.; Yu, T.; Yu, L.; Li, H.; Liu, Y.; Wang, D. Exogenous brain-derived neurotrophic factor relieves pain symptoms of diabetic rats by reducing excitability of dorsal root ganglion neurons. Int. J. Neurosci. 2016, 126, 749–758. [Google Scholar] [CrossRef]
- Lin, Y.T.; Ro, L.S.; Wang, H.L.; Chen, J.C. Up-regulation of dorsal root ganglia BDNF and trkB receptor in inflammatory pain: An in vivo and in vitro study. J. Neuroinflamm. 2011, 8, 126. [Google Scholar] [CrossRef]
- Zhou, X.F.; Deng, Y.S.; Xian, C.J.; Zhong, J.H. Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur. J. Neurosci. 2000, 12, 100–105. [Google Scholar] [CrossRef]
- Obata, K.; Katsura, H.; Sakurai, J.; Kobayashi, K.; Yamanaka, H.; Dai, Y.; Fukuoka, T.; Noguchi, K. Suppression of the p75 neurotrophin receptor in uninjured sensory neurons reduces neuropathic pain after nerve injury. J. Neurosci. 2006, 26, 11974–11986. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.W.; Jin, X.H.; Wei, X.; Wang, L.N.; Yang, J.P.; Ji, F.H. Erratum: Low-affinity neurotrophin receptor p75 of brain-derived neurotrophic factor contributes to cancer-induced bone pain by upregulating mTOR signaling. Exp. Ther. Med. 2020, 19, 2804. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, Y.; Sang, R.; Yang, R.; Bao, J.; Wu, Y.; Fei, Y.; Wu, J.; Chen, G. Huntington-associated protein 1 inhibition contributes to neuropathic pain by suppressing Cav1.2 activity and attenuating inflammation. Pain 2023, 164, e286–e302. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Q.; Llinas, A.; Mendell, L.M. Effects of trkB and trkC neurotrophin receptor agonists on thermal nociception: A behavioral and electrophysiological study. Pain 1999, 80, 463–470. [Google Scholar] [CrossRef]
- Sikandar, S.; Minett, M.S.; Millet, Q.; Santana-Varela, S.; Lau, J.; Wood, J.N.; Zhao, J. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain 2018, 141, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Sanchez, T.; Kirstein, M.; Perez-Villalba, A.; Vega, J.A.; Farinas, I. BDNF is essentially required for the early postnatal survival of nociceptors. Dev. Biol. 2010, 339, 465–476. [Google Scholar] [CrossRef]
- Luo, C.; Zhong, X.L.; Zhou, F.H.; Li, J.Y.; Zhou, P.; Xu, J.M.; Song, B.; Li, C.Q.; Zhou, X.F.; Dai, R.P. Peripheral Brain Derived Neurotrophic Factor Precursor Regulates Pain as an Inflammatory Mediator. Sci. Rep. 2016, 6, 27171. [Google Scholar] [CrossRef]
- Gowler, P.R.W.; Li, L.; Woodhams, S.G.; Bennett, A.J.; Suzuki, R.; Walsh, D.A.; Chapman, V. Peripheral brain-derived neurotrophic factor contributes to chronic osteoarthritis joint pain. Pain. 2020, 161, 61–73. [Google Scholar] [CrossRef]
- Tu, Y.; Muley, M.M.; Beggs, S.; Salter, M.W. Microglia-independent peripheral neuropathic pain in male and female mice. Pain 2022, 163, e1129–e1144. [Google Scholar] [CrossRef]
- Ferrini, F.; Trang, T.; Mattioli, T.A.; Laffray, S.; Del’Guidice, T.; Lorenzo, L.E.; Castonguay, A.; Doyon, N.; Zhang, W.; Godin, A.G.; et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(-) homeostasis. Nat. Neurosci. 2013, 16, 183–192. [Google Scholar] [CrossRef]
- Ismail, C.A.N.; Suppian, R.; Ab Aziz, C.B.; Long, I. Ifenprodil Reduced Expression of Activated Microglia, BDNF and DREAM Proteins in the Spinal Cord Following Formalin Injection During the Early Stage of Painful Diabetic Neuropathy in Rats. J. Mol. Neurosci. 2021, 71, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.T.; Wu, J.R.; Chen, Z.Y.; Liu, Z.X.; Miao, B. Effects of dexmedetomidine on P2X4Rs, p38-MAPK and BDNF in spinal microglia in rats with spared nerve injury. Brain Res. 2014, 1568, 21–30. [Google Scholar] [CrossRef]
- Wong, L.; Done, J.D.; Schaeffer, A.J.; Thumbikat, P. Experimental autoimmune prostatitis induces microglial activation in the spinal cord. Prostate 2015, 75, 50–59. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Liu, Q.; Jiang, L.; Li, M.; Wang, S.; Long, T.; He, W.; Kong, X.; Qin, G.; et al. P2X4-receptor participates in EAAT3 regulation via BDNF-TrkB signaling in a model of trigeminal allodynia. Mol. Pain. 2018, 14, 1744806918795930. [Google Scholar] [CrossRef]
- Yajima, Y.; Narita, M.; Usui, A.; Kaneko, C.; Miyatake, M.; Narita, M.; Yamaguchi, T.; Tamaki, H.; Wachi, H.; Seyama, Y.; et al. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J. Neurochem. 2005, 93, 584–594. [Google Scholar] [CrossRef]
- Bao, Y.; Hou, W.; Liu, R.; Gao, Y.; Kong, X.; Yang, L.; Shi, Z.; Li, W.; Zheng, H.; Jiang, S.; et al. PAR2-mediated upregulation of BDNF contributes to central sensitization in bone cancer pain. Mol. Pain. 2014, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Gordon, R.; Woodruff, T.M.; Smith, M.T. Antiallodynic effects of alpha lipoic acid in an optimized RR-EAE mouse model of MS-neuropathic pain are accompanied by attenuation of upregulated BDNF-TrkB-ERK signaling in the dorsal horn of the spinal cord. Pharmacol. Res. Perspect. 2015, 3, e00137. [Google Scholar] [CrossRef]
- Wang, X.; Ratnam, J.; Zou, B.; England, P.M.; Basbaum, A.I. TrkB signaling is required for both the induction and maintenance of tissue and nerve injury-induced persistent pain. J. Neurosci. 2009, 29, 5508–5515. [Google Scholar] [CrossRef] [PubMed]
- Garraway, S.M.; Petruska, J.C.; Mendell, L.M. BDNF sensitizes the response of lamina II neurons to high threshold primary afferent inputs. Eur. J. Neurosci. 2003, 18, 2467–2476. [Google Scholar] [CrossRef]
- Pezet, S.; Cunningham, J.; Patel, J.; Grist, J.; Gavazzi, I.; Lever, I.J.; Malcangio, M. BDNF modulates sensory neuron synaptic activity by a facilitation of GABA transmission in the dorsal horn. Mol. Cell Neurosci. 2002, 21, 51–62. [Google Scholar] [CrossRef]
- Lever, I.; Cunningham, J.; Grist, J.; Yip, P.K.; Malcangio, M. Release of BDNF and GABA in the dorsal horn of neuropathic rats. Eur. J. Neurosci. 2003, 18, 1169–1174. [Google Scholar] [CrossRef]
- Groth, R.; Aanonsen, L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain 2002, 100, 171–181. [Google Scholar] [CrossRef]
- Yajima, Y.; Narita, M.; Narita, M.; Matsumoto, N.; Suzuki, T. Involvement of a spinal brain-derived neurotrophic factor/full-length TrkB pathway in the development of nerve injury-induced thermal hyperalgesia in mice. Brain Res. 2002, 958, 338–346. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, X.; Chen, P.; Jin, K.; Zhou, J.; Wang, G.; Yu, J.; Wu, T.; Wang, Y.; Lin, F.; et al. BDNF-TrkB signaling pathway-mediated microglial activation induces neuronal KCC2 downregulation contributing to dynamic allodynia following spared nerve injury. Mol. Pain. 2023, 19, 17448069231185439. [Google Scholar] [CrossRef]
- Phan, T.T.; Jayathilake, N.J.; Lee, K.P.; Park, J.M. BDNF/TrkB Signaling Inhibition Suppresses Astrogliosis and Alleviates Mechanical Allodynia in a Partial Crush Injury Model. Exp. Neurobiol. 2023, 32, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]
- Guo, W.; Robbins, M.T.; Wei, F.; Zou, S.; Dubner, R.; Ren, K. Supraspinal brain-derived neurotrophic factor signaling: A novel mechanism for descending pain facilitation. J. Neurosci. 2006, 26, 126–137. [Google Scholar] [CrossRef]
- Heinricher, M.M.; Tavares, I.; Leith, J.L.; Lumb, B.M. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res. Rev. 2009, 60, 214–225. [Google Scholar] [CrossRef]
- Wei, F.; Dubner, R.; Zou, S.; Ren, K.; Bai, G.; Wei, D.; Guo, W. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J. Neurosci. 2010, 30, 8624–8636. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Li, H.; Xu, Z.; Ma, D.; Guo, R.; Yang, K.; Wang, Y. Paradoxical Sleep Deprivation Aggravates and Prolongs Incision-Induced Pain Hypersensitivity via BDNF Signaling-Mediated Descending Facilitation in Rats. Neurochem. Res. 2018, 43, 2353–2361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tao, W.; Hou, Y.Y.; Wang, W.; Kenny, P.J.; Pan, Z.Z. MeCP2 repression of G9a in regulation of pain and morphine reward. J. Neurosci. 2014, 34, 9076–9087. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.C.; Kuan, Y.H.; Shyu, B.C. Targeting brain-derived neurotrophic factor in the medial thalamus for the treatment of central poststroke pain in a rodent model. Pain. 2017, 158, 1302–1313. [Google Scholar] [CrossRef]
- Sosanya, N.M.; Garza, T.H.; Stacey, W.; Crimmins, S.L.; Christy, R.J.; Cheppudira, B.P. Involvement of brain-derived neurotrophic factor (BDNF) in chronic intermittent stress-induced enhanced mechanical allodynia in a rat model of burn pain. BMC Neurosci. 2019, 20, 17. [Google Scholar] [CrossRef]
- Liu, X.; Hou, Z.; Han, M.; Chen, K.; Wang, Y.; Qing, J.; Yang, F. Salvianolic acid B alleviates comorbid pain in depression induced by chronic restraint stress through inhibiting GABAergic neuron excitation via an ERK-CREB-BDNF axis-dependent mechanism. J. Psychiatr. Res. 2022, 151, 205–216. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, Y.L.; Li, C.; Liu, D.; Wang, L.; Wang, X.Y.; Liu, M.J.; Liu, H.; Zhang, S.; Guo, X.Y.; et al. Brain-Derived Neurotrophic Factor in the Mesolimbic Reward Circuitry Mediates Nociception in Chronic Neuropathic Pain. Biol. Psychiatry 2017, 82, 608–618. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wang, H.; Wang, R.; Guo, Y.; Xu, L.; Zhang, G.; Wu, J.; Wang, G. The BDNF-TrkB signaling pathway in the rostral anterior cingulate cortex is involved in the development of pain aversion in rats with bone cancer via NR2B and ERK-CREB signaling. Brain Res. Bull. 2022, 185, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Zhan, Y.; Li, D.; Zhang, Y.; Wang, G.; Zhang, M. Contribution of BDNF/TrkB signalling in the rACC to the development of pain-related aversion via activation of ERK in rats with spared nerve injury. Brain Res. 2017, 1671, 111–120. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Ma, J.; Liu, C.; Liu, X.; Zhan, Y.; Zhang, M. Brain-derived neurotrophic factor (BDNF) in the rostral anterior cingulate cortex (rACC) contributes to neuropathic spontaneous pain-related aversion via NR2B receptors. Brain Res. Bull. 2016, 127, 56–65. [Google Scholar] [CrossRef]
- Yue, L.; Ma, L.Y.; Cui, S.; Liu, F.Y.; Yi, M.; Wan, Y. Brain-derived neurotrophic factor in the infralimbic cortex alleviates inflammatory pain. Neurosci. Lett. 2017, 655, 7–13. [Google Scholar] [CrossRef]
- Zheng, J.; Jiang, Y.Y.; Xu, L.C.; Ma, L.Y.; Liu, F.Y.; Cui, S.; Cai, J.; Liao, F.F.; Wan, Y.; Yi, M. Adult Hippocampal Neurogenesis along the Dorsoventral Axis Contributes Differentially to Environmental Enrichment Combined with Voluntary Exercise in Alleviating Chronic Inflammatory Pain in Mice. J. Neurosci. 2017, 37, 4145–4157. [Google Scholar] [CrossRef]
- Ma, L.; Yue, L.; Zhang, Y.; Wang, Y.; Han, B.; Cui, S.; Liu, F.Y.; Wan, Y.; Yi, M. Spontaneous Pain Disrupts Ventral Hippocampal CA1-Infralimbic Cortex Connectivity and Modulates Pain Progression in Rats with Peripheral Inflammation. Cell Rep. 2019, 29, 1579–1593.e6. [Google Scholar] [CrossRef]
- Infantino, R.; Schiano, C.; Luongo, L.; Paino, S.; Mansueto, G.; Boccella, S.; Guida, F.; Ricciardi, F.; Iannotta, M.; Belardo, C.; et al. MED1/BDNF/TrkB pathway is involved in thalamic hemorrhage-induced pain and depression by regulating microglia. Neurobiol. Dis. 2022, 164, 105611. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Chen, Q.; Zhou, W.; Wang, Y.; Wang, L.; Zhang, Z. Persistent inflammation-induced up-regulation of brain-derived neurotrophic factor (BDNF) promotes synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor GluA1 subunits in descending pain modulatory circuits. J. Biol. Chem. 2014, 289, 22196–22204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Wang, W.; Lu, Y.G.; Pan, Z.Z. Brain-derived neurotrophic factor-mediated downregulation of brainstem K+-Cl− cotransporter and cell-type-specific GABA impairment for activation of descending pain facilitation. Mol. Pharmacol. 2013, 84, 511–520. [Google Scholar] [CrossRef]
- Sarhan, M.; Pawlowski, S.A.; Barthas, F.; Yalcin, I.; Kaufling, J.; Dardente, H.; Zachariou, V.; Dileone, R.J.; Barrot, M.; Veinante, P. BDNF parabrachio-amygdaloid pathway in morphine-induced analgesia. Int. J. Neuropsychopharmacol. 2013, 16, 1649–1660. [Google Scholar] [CrossRef]
- Taylor, A.M.; Castonguay, A.; Taylor, A.J.; Murphy, N.P.; Ghogha, A.; Cook, C.; Xue, L.; Olmstead, M.C.; De Koninck, Y.; Evans, C.J.; et al. Microglia disrupt mesolimbic reward circuitry in chronic pain. J. Neurosci. 2015, 35, 8442–8450. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Pan, W.; Xu, N.; Zhou, Z.Q.; Zhang, G.F.; Shen, J.C. Environmental enrichment improves long-term memory impairment and aberrant synaptic plasticity by BDNF/TrkB signaling in nerve-injured mice. Neurosci. Lett. 2019, 694, 93–98. [Google Scholar] [CrossRef]
- Tao, W.; Chen, Q.; Wang, L.; Zhou, W.; Wang, Y.; Zhang, Z. Brainstem brain-derived neurotrophic factor signaling is required for histone deacetylase inhibitor-induced pain relief. Mol. Pharmacol. 2015, 87, 1035–1041. [Google Scholar] [CrossRef]
- Kuner, R.; Flor, H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2016, 18, 20–30. [Google Scholar] [CrossRef]
- Xia, S.H.; Hu, S.W.; Ge, D.G.; Liu, D.; Wang, D.; Zhang, S.; Zhang, Q.; Yuan, L.; Li, Y.Q.; Yang, J.X.; et al. Chronic Pain Impairs Memory Formation via Disruption of Neurogenesis Mediated by Mesohippocampal Brain-Derived Neurotrophic Factor Signaling. Biol. Psychiatry 2020, 88, 597–610. [Google Scholar] [CrossRef]
- Frank, L.; Wiegand, S.J.; Siuciak, J.A.; Lindsay, R.M.; Rudge, J.S. Effects of BDNF infusion on the regulation of TrkB protein and message in adult rat brain. Exp. Neurol. 1997, 145, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Siuciak, J.A.; Altar, C.A.; Wiegand, S.J.; Lindsay, R.M. Antinociceptive effect of brain-derived neurotrophic factor and neurotrophin-3. Brain Res. 1994, 633, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Siuciak, J.A.; Wong, V.; Pearsall, D.; Wiegand, S.J.; Lindsay, R.M. BDNF produces analgesia in the formalin test and modifies neuropeptide levels in rat brain and spinal cord areas associated with nociception. Eur. J. Neurosci. 1995, 7, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yan, X.; Wang, L.; Xu, J.; Li, T. Transcranial direct current stimulation attenuates chronic pain in knee osteoarthritis by modulating BDNF/TrkB signaling in the descending pain modulation system. Neurosci. Lett. 2023, 810, 137320. [Google Scholar] [CrossRef]
- Liu, D.; Tang, Q.Q.; Yin, C.; Song, Y.; Liu, Y.; Yang, J.X.; Liu, H.; Zhang, Y.M.; Wu, S.Y.; Song, Y.; et al. Brain-derived neurotrophic factor-mediated projection-specific regulation of depressive-like and nociceptive behaviors in the mesolimbic reward circuitry. Pain 2018, 159, 175. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, W.; Chen, D.; Zhou, D.; Gao, Y.; Bian, Y.; Xu, Y.; Xia, S.H.; Fang, T.; Yang, J.X.; et al. Disinhibition of Mesolimbic Dopamine Circuit by the Lateral Hypothalamus Regulates Pain Sensation. J. Neurosci. 2023, 43, 4525–4540. [Google Scholar] [CrossRef]
- Yang, C.R.; Bai, Y.Y.; Ruan, C.S.; Zhou, F.H.; Li, F.; Li, C.Q.; Zhou, X.F. Injection of Anti-proBDNF in Anterior Cingulate Cortex (ACC) Reverses Chronic Stress-Induced Adverse Mood Behaviors in Mice. Neurotox. Res. 2017, 31, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.M.; Neugebauer, V. Cortico-limbic pain mechanisms. Neurosci. Lett. 2019, 702, 15–23. [Google Scholar] [CrossRef]
- Neugebauer, V.; Kiritoshi, T. Corticolimbic plasticity in pain: Hippocampus joins the party. Pain 2023, 165, 965–967. [Google Scholar] [CrossRef]
- Moriarty, O.; McGuire, B.E.; Finn, D.P. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog. Neurobiol. 2011, 93, 385–404. [Google Scholar] [CrossRef]
- Vachon-Presseau, E.; Centeno, M.V.; Ren, W.; Berger, S.E.; Tetreault, P.; Ghantous, M.; Baria, A.; Farmer, M.; Baliki, M.N.; Schnitzer, T.J.; et al. The Emotional Brain as a Predictor and Amplifier of Chronic Pain. J. Dent. Res. 2016, 95, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Veinante, P.; Yalcin, I.; Barrot, M. The amygdala between sensation and affect: A role in pain. J. Mol. Psychiatry 2013, 1, 9. [Google Scholar] [CrossRef]
- Altar, C.A. Neurotrophins and depression. Trends Pharmacol. Sci. 1999, 20, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Shimizu, E.; Iyo, M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res. Brain Res. Rev. 2004, 45, 104–114. [Google Scholar] [CrossRef]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef]
- Rana, T.; Behl, T.; Sehgal, A.; Srivastava, P.; Bungau, S. Unfolding the Role of BDNF as a Biomarker for Treatment of Depression. J. Mol. Neurosci. 2021, 71, 2008–2021. [Google Scholar] [CrossRef] [PubMed]
- Siuciak, J.A.; Lewis, D.R.; Wiegand, S.J.; Lindsay, R.M. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol. Biochem. Behav. 1997, 56, 131–137. [Google Scholar] [CrossRef]
- Shirayama, Y.; Chen, A.C.; Nakagawa, S.; Russell, D.S.; Duman, R.S. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002, 22, 3251–3261. [Google Scholar] [CrossRef]
- Zhang, J.C.; Wu, J.; Fujita, Y.; Yao, W.; Ren, Q.; Yang, C.; Li, S.X.; Shirayama, Y.; Hashimoto, K. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int. J. Neuropsychopharmacol. 2014, 18, pyu077. [Google Scholar] [CrossRef]
- Monteggia, L.M.; Luikart, B.; Barrot, M.; Theobold, D.; Malkovska, I.; Nef, S.; Parada, L.F.; Nestler, E.J. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol. Psychiatry 2007, 61, 187–197. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, F.; Zhai, M.; He, M.; Hu, Y.; Feng, L.; Li, Y.; Yang, J.; Wu, C. Hyperactive neuronal autophagy depletes BDNF and impairs adult hippocampal neurogenesis in a corticosterone-induced mouse model of depression. Theranostics 2023, 13, 1059–1075. [Google Scholar] [CrossRef]
- Eisch, A.J.; Bolanos, C.A.; de Wit, J.; Simonak, R.D.; Pudiak, C.M.; Barrot, M.; Verhaagen, J.; Nestler, E.J. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: A role in depression. Biol. Psychiatry 2003, 54, 994–1005. [Google Scholar] [CrossRef]
- Nestler, E.J.; Carlezon, W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef]
- Berton, O.; McClung, C.A.; Dileone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef]
- Zhang, J.C.; Yao, W.; Hashimoto, K. Brain-derived Neurotrophic Factor (BDNF)-TrkB Signaling in Inflammation-related Depression and Potential Therapeutic Targets. Curr. Neuropharmacol. 2016, 14, 721–731. [Google Scholar] [CrossRef]
- Weickert, C.S.; Hyde, T.M.; Lipska, B.K.; Herman, M.M.; Weinberger, D.R.; Kleinman, J.E. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol. Psychiatry 2003, 8, 592–610. [Google Scholar] [CrossRef]
- Ashe, P.C.; Chlan-Fourney, J.; Juorio, A.V.; Li, X.M. Brain-derived neurotrophic factor (BDNF) mRNA in rats with neonatal ibotenic acid lesions of the ventral hippocampus. Brain Res. 2002, 956, 126–135. [Google Scholar] [CrossRef]
- Lipska, B.K.; Khaing, Z.Z.; Weickert, C.S.; Weinberger, D.R. BDNF mRNA expression in rat hippocampus and prefrontal cortex: Effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur. J. Neurosci. 2001, 14, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Korf, J.; Antonelli, A.; Talamini, L.; Aloe, L. Long-lasting effects of prenatal MAM treatment on water maze performance in rats: Associations with altered brain development and neurotrophin levels. Neurotoxicol Teratol. 2002, 24, 179–191. [Google Scholar] [CrossRef]
- Zorner, B.; Wolfer, D.P.; Brandis, D.; Kretz, O.; Zacher, C.; Madani, R.; Grunwald, I.; Lipp, H.P.; Klein, R.; Henn, F.A.; et al. Forebrain-specific trkB-receptor knockout mice: Behaviorally more hyperactive than “depressive”. Biol. Psychiatry 2003, 54, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Harb, M.; Jagusch, J.; Durairaja, A.; Endres, T.; Lessmann, V.; Fendt, M. BDNF haploinsufficiency induces behavioral endophenotypes of schizophrenia in male mice that are rescued by enriched environment. Transl. Psychiatry 2021, 11, 233. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Bazovkina, D.V.; Morozova, M.V.; Popova, N.K. Effects of brain-derived and glial cell line-derived neurotrophic factors on startle response and disrupted prepulse inhibition in mice of DBA/2J inbred strain. Neurosci. Lett. 2013, 550, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Li, Y.K.; Wang, W.; Wan, J.G.; Yu, B.; Wang, M.Z.; Hu, B. Small-molecule TrkB agonist 7,8-dihydroxyflavone reverses cognitive and synaptic plasticity deficits in a rat model of schizophrenia. Pharmacol. Biochem. Behav. 2014, 122, 30–36. [Google Scholar] [CrossRef]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Kang, S.; Kim, S.H.; Kim, J.C.; Yang, M.; Moon, C. Brain-derived neurotropic factor and GABAergic transmission in neurodegeneration and neuroregeneration. Neural Regen. Res. 2017, 12, 1733–1741. [Google Scholar] [CrossRef]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 2018, 361, eaan8821. [Google Scholar] [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef]
- Baquet, Z.C.; Gorski, J.A.; Jones, K.R. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J. Neurosci. 2004, 24, 4250–4258. [Google Scholar] [CrossRef] [PubMed]
- Canals, J.M.; Pineda, J.R.; Torres-Peraza, J.F.; Bosch, M.; Martin-Ibanez, R.; Munoz, M.T.; Mengod, G.; Ernfors, P.; Alberch, J. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington’s disease. J. Neurosci. 2004, 24, 7727–7739. [Google Scholar] [CrossRef]
- Gharami, K.; Xie, Y.; An, J.J.; Tonegawa, S.; Xu, B. Brain-derived neurotrophic factor over-expression in the forebrain ameliorates Huntington’s disease phenotypes in mice. J. Neurochem. 2008, 105, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hayden, M.R.; Xu, B. BDNF overexpression in the forebrain rescues Huntington’s disease phenotypes in YAC128 mice. J. Neurosci. 2010, 30, 14708–14718. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzitelli, M.; Kiritoshi, T.; Presto, P.; Hurtado, Z.; Antenucci, N.; Ji, G.; Neugebauer, V. BDNF Signaling and Pain Modulation. Cells 2025, 14, 476. https://doi.org/10.3390/cells14070476
Mazzitelli M, Kiritoshi T, Presto P, Hurtado Z, Antenucci N, Ji G, Neugebauer V. BDNF Signaling and Pain Modulation. Cells. 2025; 14(7):476. https://doi.org/10.3390/cells14070476
Chicago/Turabian StyleMazzitelli, Mariacristina, Takaki Kiritoshi, Peyton Presto, Zachary Hurtado, Nico Antenucci, Guangchen Ji, and Volker Neugebauer. 2025. "BDNF Signaling and Pain Modulation" Cells 14, no. 7: 476. https://doi.org/10.3390/cells14070476
APA StyleMazzitelli, M., Kiritoshi, T., Presto, P., Hurtado, Z., Antenucci, N., Ji, G., & Neugebauer, V. (2025). BDNF Signaling and Pain Modulation. Cells, 14(7), 476. https://doi.org/10.3390/cells14070476








