The Histomorphology to Molecular Transition: Exploring the Genomic Landscape of Poorly Differentiated Epithelial Endometrial Cancers
Abstract
1. Introduction
2. Histological Classification of PDEECs
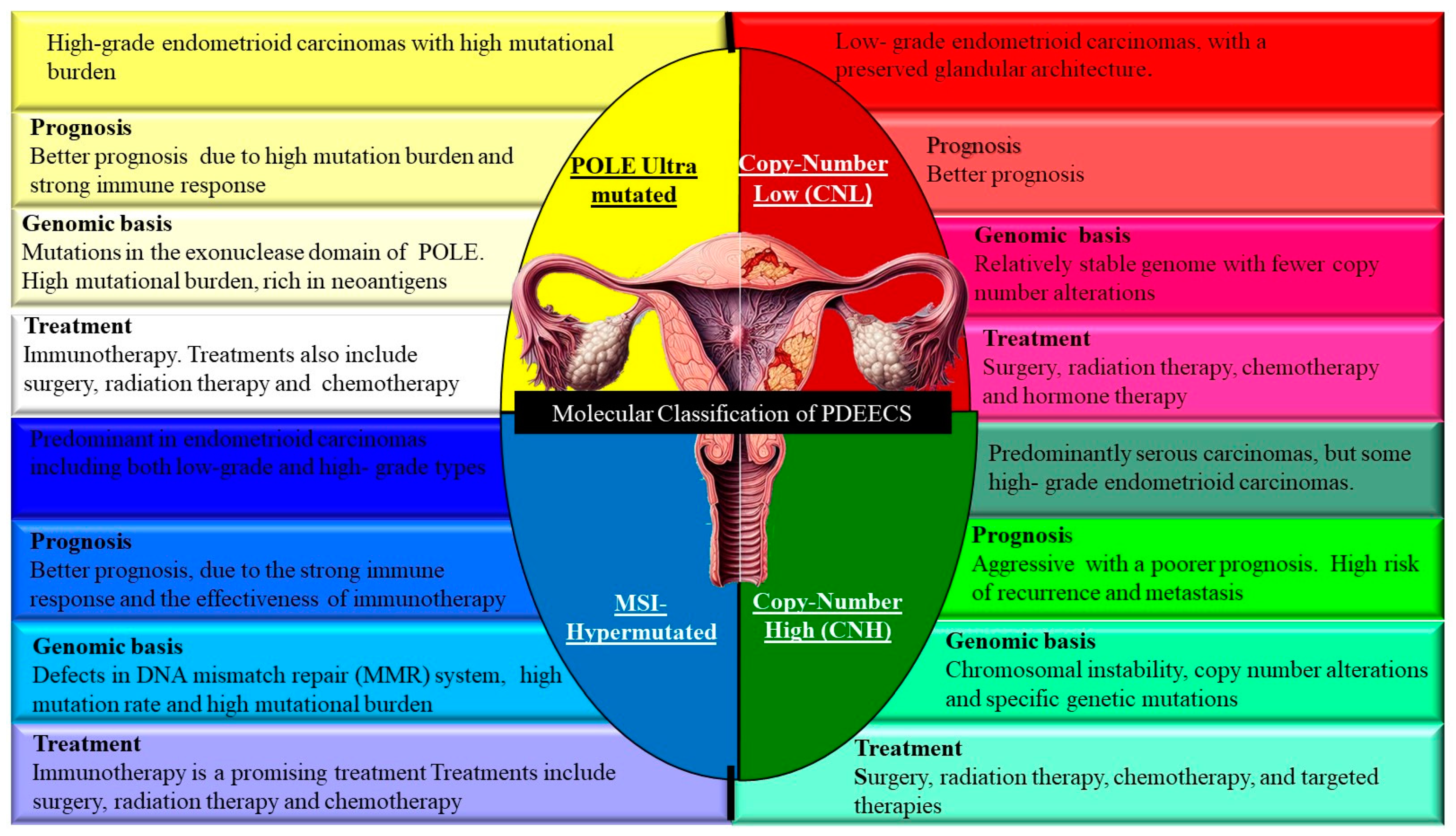
3. Molecular Classification of PDEECs
4. Genomic Landscape of PDEECs
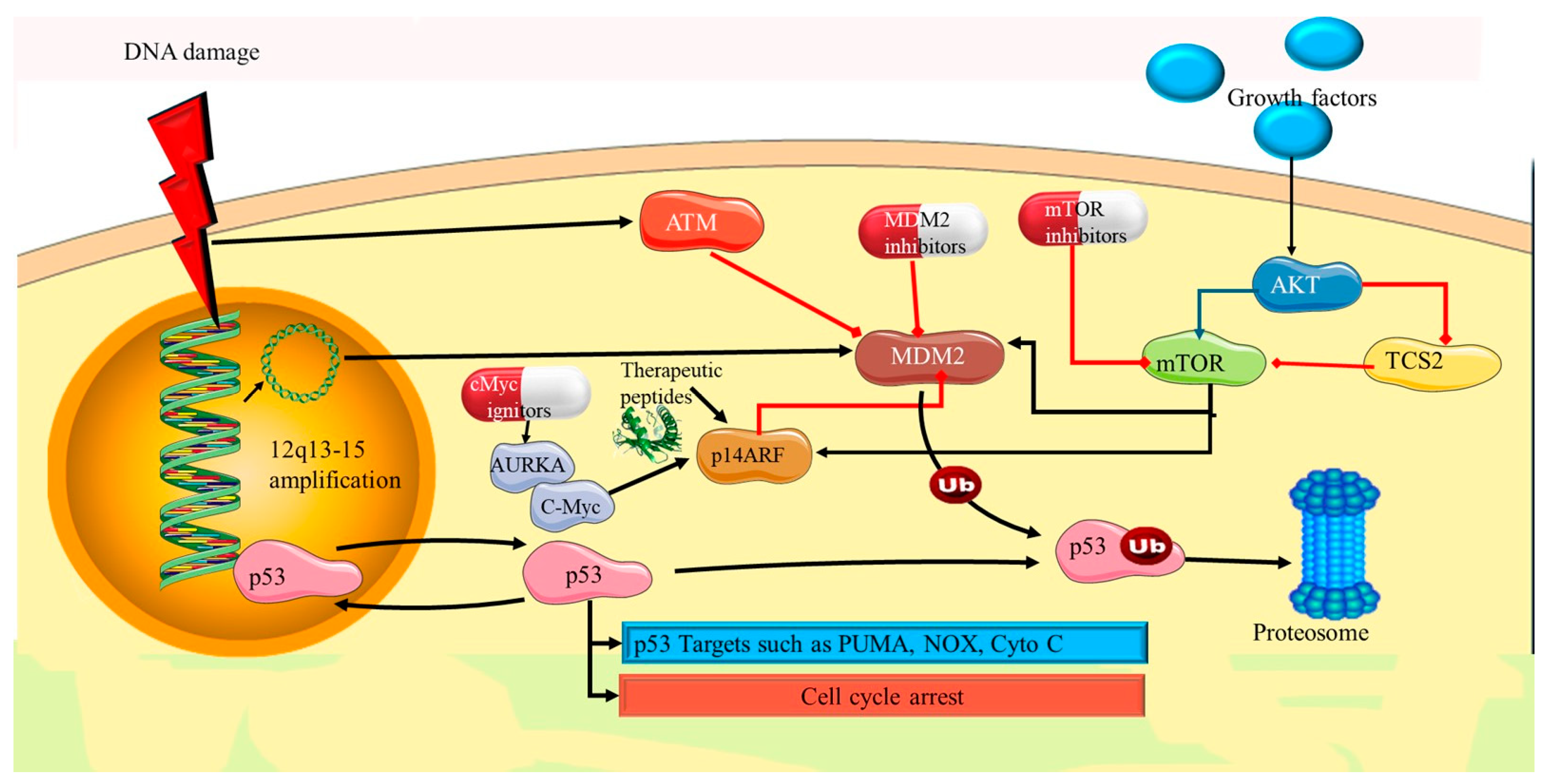
4.1. TP53 Mutations
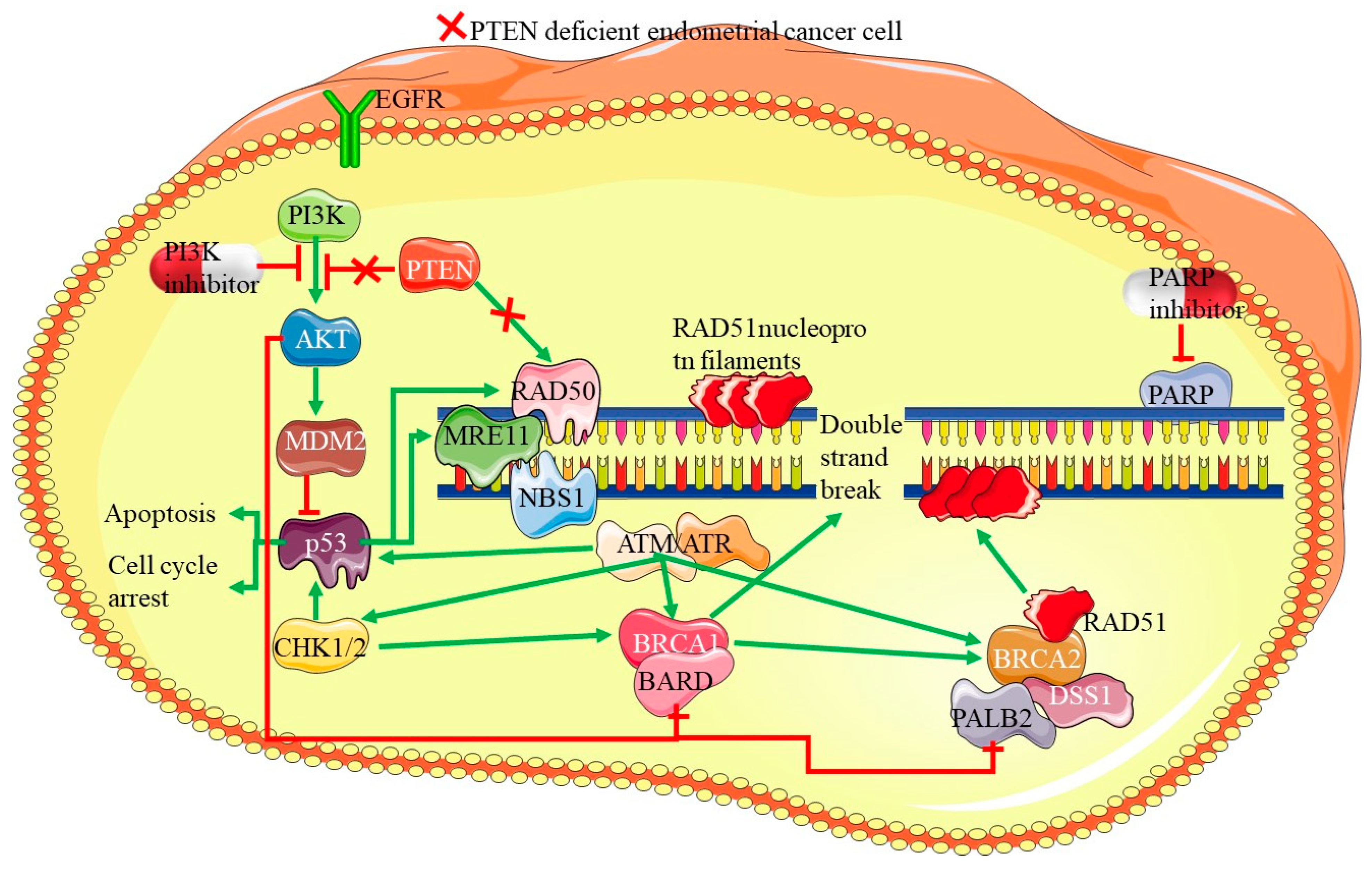
4.2. PTEN Mutations
4.3. PIK3CA Mutations
4.4. ARID1A Mutations:
4.5. CTNNB1 Mutations
4.6. POLE Mutations

4.7. Copy Number Alterations
4.8. Epigenetic Modifications
5. Molecular Pathways Implicated in PDEECs
5.1. PI3K/AKT/mTOR Pathway
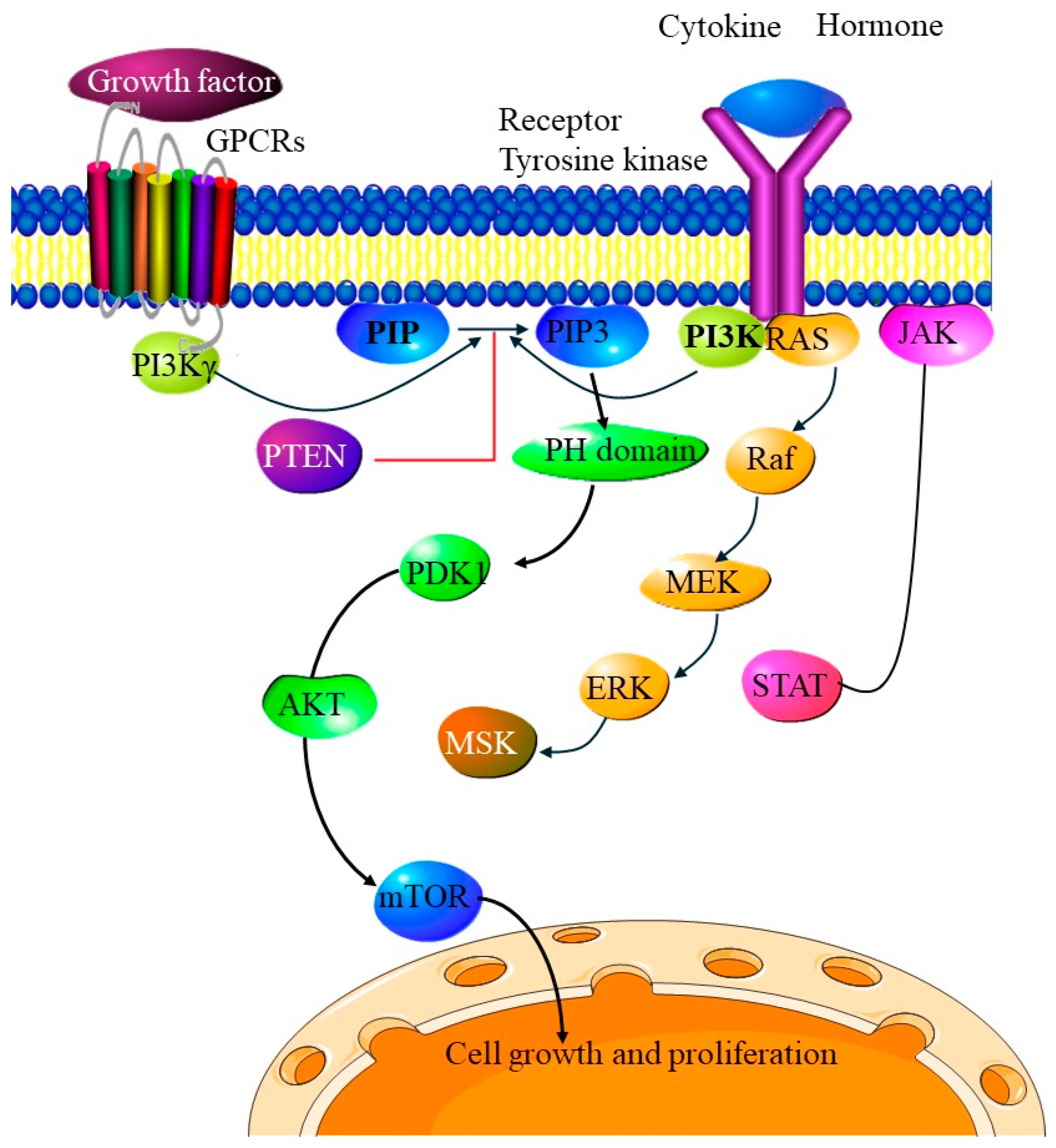
Immunosuppressive Effects of PI3K/AKT/mTOR Inhibitors
5.2. p53 Pathway Dysfunction
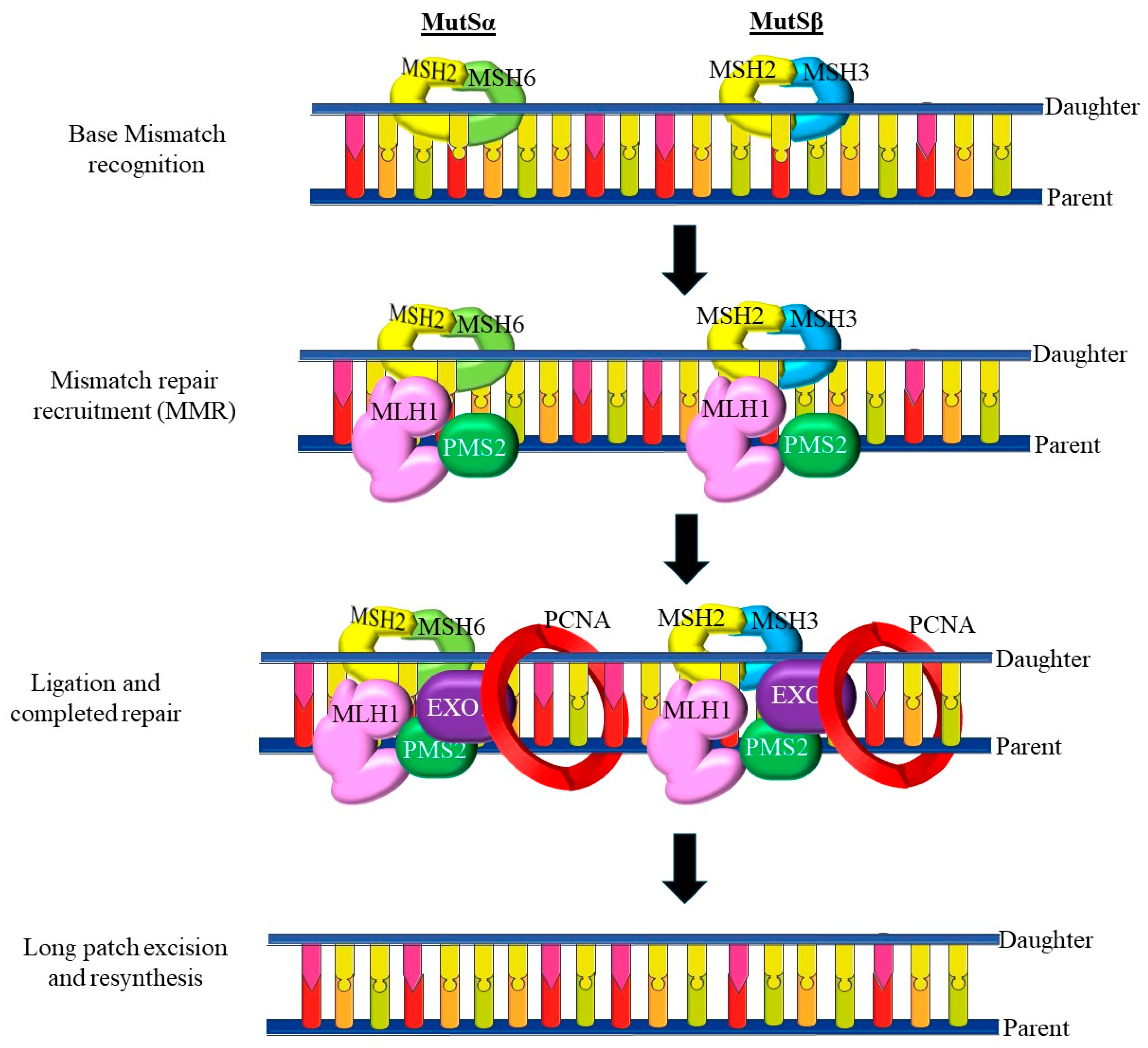
5.3. Mismatch Repair (MMR) Deficiency and Microsatellite Instability (MSI)
6. Biomarkers and Molecular Targets in PDEECs
| Genetic Alterations | Carcinoma (10–20%) | Clear Cell Carcinoma (<5%) | Carcinosarcoma (<5%) | Genetic Mutation | Epigenetic Mutation | References |
|---|---|---|---|---|---|---|
| TP53 | 57.7–92% | 29–46% | 64.3–91% | ✅ | ❌ | [182] |
| PPP2R1A | 15.4–43.2% | 15.9–36% | 0–28.1% | ✅ | ❌ | [183] |
| FBXW7 | 17.3–29% | 7.9–25% | 39% | ✅ | ❌ | [184] |
| PTEN | 2.7–22.5% | 11–21% | 19–33.3% | ✅ | ❌ | [183] |
| ARID1A | 0–10.8% | 15–21% | 12–23.8% | ✅ | ✅ | [184] |
| PIK3CA | 10–47% | 23.8–36% | 17–35% | ✅ | ❌ | [183] |
| CTNNB1 | 2.7% | 0% | 4.8% | ✅ | ❌ | [185] |
| KRAS | 2–8% | 12–16.7% | 14% | ✅ | ❌ | [173] |
| HER2 | 17–44% | 12–50% | 0–20% | ✅ | ❌ | [186] |
7. Therapeutic Targets
7.1. Targeting the PI3K/AKT/mTOR Pathway
7.2. Immune Checkpoint Inhibitors in MSI-High Tumors
7.3. PARP Inhibitors in Homologous Recombination Deficiency (HRD) Cases
8. Clinical Implications and Personalized Treatment Strategies in PDEECs
9. Model Systems for Studying PDEECs
10. Surgical Interventions for PDEECs
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, H.; Casey, M.J.; Crotzer, D. Endometrial cancer. Lancet 2018, 366, 491–505. [Google Scholar]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.; Gambini, D.; Runza, L.; Ferrero, S.; Scarfone, G.; Bulfamante, G.; Ayhan, A. Unsolved Issues in the Integrated Histo-Molecular Classification of Endometrial Carcinoma and Therapeutic Implications. Cancers 2024, 16, 2458. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Mendivil, A.; Schuler, K.M.; Gehrig, P.A. Non-endometrioid adenocarcinoma of the uterine corpus: A review of selected histological subtypes. Cancer Control 2009, 16, 46–52. [Google Scholar] [CrossRef]
- Bogani, G.; Ray-Coquard, I.; Concin, N.; Ngoi, N.Y.L.; Morice, P.; Caruso, G.; Enomoto, T.; Takehara, K.; Denys, H.; Lorusso, D.; et al. Endometrial carcinosarcoma. Int. J. Gynecol. Cancer 2023, 33, 147–174. [Google Scholar] [CrossRef] [PubMed]
- Bostan, I.S.; Mihaila, M.; Roman, V.; Radu, N.; Neagu, M.T.; Bostan, M.; Mehedintu, C. Landscape of Endometrial Cancer: Molecular Mechanisms, Biomarkers, and Target Therapy. Cancers 2024, 16, 2027. [Google Scholar] [CrossRef]
- Remmerie, M.; Janssens, V. Targeted Therapies in Type II Endometrial Cancers: Too Little, but Not Too Late. Int. J. Mol. Sci. 2018, 19, 2380. [Google Scholar] [CrossRef]
- Opławski, M.; Nowakowski, R.; Średnicka, A.; Ochnik, D.; Grabarek, B.O.; Boroń, D. Molecular Landscape of the Epithelial-Mesenchymal Transition in Endometrioid Endometrial Cancer. J. Clin. Med. 2021, 10, 1520. [Google Scholar] [CrossRef]
- Walsh, C.S.; Hacker, K.E.; Secord, A.A.; DeLair, D.F.; McCourt, C.; Urban, R. Molecular testing for endometrial cancer: An SGO clinical practice statement. Gynecol. Oncol. 2023, 168, 48–55. [Google Scholar] [CrossRef]
- Masood, M.; Singh, N. Endometrial carcinoma: Changes to classification (WHO 2020). Diagn. Histopathol. 2021, 27, 493–499. [Google Scholar] [CrossRef]
- Golia D’Augè, T.; Cuccu, I.; Santangelo, G.; Muzii, L.; Giannini, A.; Bogani, G.; Di Donato, V. Novel Insights into Molecular Mechanisms of Endometrial Diseases. Biomolecules 2023, 13, 499. [Google Scholar] [CrossRef]
- Hruda, M.; Sehnal, B.; Halaška, M.J.; Drozenová, J.; Robová, H.; Pichlík, T.; Rob, L. New staging of endometrial carcinoma—FIGO 2023. Ceska Gynekol. 2024, 89, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Vang, R.; Shih Ie, M.; Kurman, R.J. Ovarian low-grade and high-grade serous carcinoma: Pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv. Anat. Pathol. 2009, 16, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Ferioli, E.; Guareschi, D.; Tafuni, A. Dedifferentiated Endometrial Carcinoma: A Rare Aggressive Neoplasm-Clinical, Morphological and Immunohistochemical Features. Cancers 2023, 15, 5155. [Google Scholar] [CrossRef] [PubMed]
- Claudio Quiros, A.; Coudray, N.; Yeaton, A.; Yang, X.; Liu, B.; Le, H.; Chiriboga, L.; Karimkhan, A.; Narula, N.; Moore, D.A.; et al. Mapping the landscape of histomorphological cancer phenotypes using self-supervised learning on unannotated pathology slides. Nat. Commun. 2024, 15, 4596. [Google Scholar] [CrossRef]
- Bredholt, G.; Mannelqvist, M.; Stefansson, I.M.; Birkeland, E.; Bø, T.H.; Øyan, A.M.; Trovik, J.; Kalland, K.H.; Jonassen, I.; Salvesen, H.B.; et al. Tumor necrosis is an important hallmark of aggressive endometrial cancer and associates with hypoxia, angiogenesis and inflammation responses. Oncotarget 2015, 6, 39676–39691. [Google Scholar] [CrossRef]
- Fremond, S.; Koelzer, V.H.; Horeweg, N.; Bosse, T. The evolving role of morphology in endometrial cancer diagnostics: From histopathology and molecular testing towards integrative data analysis by deep learning. Front. Oncol. 2022, 12, 928977. [Google Scholar] [CrossRef]
- Turashvili, G.; Hanley, K. Practical Updates and Diagnostic Challenges in Endometrial Carcinoma. Arch. Pathol. Lab. Med. 2023, 148, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Jassar, A.; Hemali, N.; Bhatnagar, A. Assessment of endometrial carcinoma on biopsy as a predictor of final surgical pathology: Are we doing it right? A completed audit cycle and recommendations. Indian J. Pathol. Microbiol. 2024, 67, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Saglam, O. Uncommon Morphologic Types of Endometrial Cancer and Their Mimickers: How Much Does Molecular Classification Improve the Practice for Challenging Cases? Life 2024, 14, 387. [Google Scholar] [CrossRef]
- Matson, D.R.; Accola, M.A.; Henderson, L.; Shao, X.; Frater-Rubsam, L.; Horner, V.L.; Rehrauer, W.M.; Weisman, P.; Xu, J. A “Null” Pattern of p16 Immunostaining in Endometrial Serous Carcinoma: An Under-recognized and Important Aberrant Staining Pattern. Int. J. Gynecol. Pathol. 2022, 41, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Gordhandas, S.; Da Cruz Paula, A.; Kertowidjojo, E.C.; Pareja, F.; Dessources, K.; da Silva, E.M.; Derakhshan, F.; Mueller, J.J.; Abu-Rustum, N.R.; Chui, M.H.; et al. Molecular profiling of primary endometrioid endometrial cancer and matched lung metastases: CTNNB1 mutation as a potential driver. Gynecol. Oncol. Rep. 2024, 53, 101391. [Google Scholar] [CrossRef]
- Galant, N.; Krawczyk, P.; Monist, M.; Obara, A.; Gajek, Ł.; Grenda, A.; Nicoś, M.; Kalinka, E.; Milanowski, J. Molecular Classification of Endometrial Cancer and Its Impact on Therapy Selection. Int. J. Mol. Sci. 2024, 25, 5893. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Gencarelli, A.; Mollo, A.; Guida, M.; Insabato, L.; Santoro, A.; Zannoni, G.F.; Zullo, F. TCGA Classification of Endometrial Cancer: The Place of Carcinosarcoma. Pathol. Oncol. Res. POR 2020, 26, 2067–2073. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, S.F.; Bao, W. Molecular subtypes of endometrial cancer: Implications for adjuvant treatment strategies. Int. J. Gynaecol. Obstet. 2024, 164, 436–459. [Google Scholar] [CrossRef]
- Jamieson, A.; Barroilhet, L.M.; McAlpine, J.N. Molecular classification in endometrial cancer: Opportunities for precision oncology in a changing landscape. Cancer 2022, 128, 2853–2857. [Google Scholar] [CrossRef]
- Hoang, L.N.; McConechy, M.K.; Meng, B.; McIntyre, J.B.; Ewanowich, C.; Gilks, C.B.; Huntsman, D.G.; Köbel, M.; Lee, C.H. Targeted mutation analysis of endometrial clear cell carcinoma. Histopathology 2015, 66, 664–674. [Google Scholar] [CrossRef]
- Imboden, S.; Nastic, D.; Ghaderi, M.; Rydberg, F.; Rau, T.T.; Mueller, M.D.; Epstein, E.; Carlson, J.W. Phenotype of POLE-mutated endometrial cancer. PLoS ONE 2019, 14, e0214318. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, E.; Takacs, E.M.; Kaur, S.; Cheng, C.; Kurokawa, M. Predicting clinical outcomes of cancer patients with a p53 deficiency gene signature. Sci. Rep. 2022, 12, 1317. [Google Scholar] [CrossRef] [PubMed]
- Colon-Otero, G.; Zanfagnin, V.; Hou, X.; Foster, N.R.; Asmus, E.J.; Wahner Hendrickson, A.; Jatoi, A.; Block, M.S.; Langstraat, C.L.; Glaser, G.E.; et al. Phase II trial of ribociclib and letrozole in patients with relapsed oestrogen receptor-positive ovarian or endometrial cancers. ESMO Open 2020, 5, e000926. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, A.; Minaguchi, T.; Fujieda, K.; Hosokawa, Y.; Nishida, K.; Shikama, A.; Tasaka, N.; Sakurai, M.; Ochi, H.; Satoh, T. Abnormal accumulation of p53 predicts radioresistance leading to poor survival in patients with endometrial carcinoma. Oncol. Lett. 2019, 18, 5952–5958. [Google Scholar] [CrossRef]
- Presti, D.; Dall’Olio, F.G.; Besse, B.; Ribeiro, J.M.; Di Meglio, A.; Soldato, D. Tumor infiltrating lymphocytes (TILs) as a predictive biomarker of response to checkpoint blockers in solid tumors: A systematic review. Crit. Rev. Oncol. Hematol. 2022, 177, 103773. [Google Scholar] [CrossRef]
- Davidson, B.; Teien Lande, K.; Nebdal, D.; Nesbakken, A.J.; Holth, A.; Lindemann, K.; Zahl Eriksson, A.G.; Sørlie, T. Endometrial carcinomas with ambiguous histology often harbor TP53 mutations. Virchows Arch. 2024. [Google Scholar] [CrossRef]
- Whelan, K.; Dillon, M.; Strickland, K.C.; Pothuri, B.; Bae-Jump, V.; Borden, L.E.; Thaker, P.H.; Haight, P.; Arend, R.C.; Ko, E.; et al. TP53 mutation and abnormal p53 expression in endometrial cancer: Associations with race and outcomes. Gynecol. Oncol. 2023, 178, 44–53. [Google Scholar] [CrossRef]
- Capuozzo, M.; Santorsola, M.; Bocchetti, M.; Perri, F.; Cascella, M.; Granata, V.; Celotto, V.; Gualillo, O.; Cossu, A.M.; Nasti, G.; et al. p53: From Fundamental Biology to Clinical Applications in Cancer. Biology 2022, 11, 1325. [Google Scholar] [CrossRef]
- Surova, O.; Zhivotovsky, B. Various modes of cell death induced by DNA damage. Oncogene 2013, 32, 3789–3797. [Google Scholar] [CrossRef]
- Zhang, Y.; Kwok-Shing Ng, P.; Kucherlapati, M.; Chen, F.; Liu, Y.; Tsang, Y.H.; de Velasco, G.; Jeong, K.J.; Akbani, R.; Hadjipanayis, A.; et al. A Pan-Cancer Proteogenomic Atlas of PI3K/AKT/mTOR Pathway Alterations. Cancer Cell 2017, 31, 820–832.e823. [Google Scholar] [CrossRef]
- Pignata, S.; Califano, D.; Lorusso, D.; Arenare, L.; Bartoletti, M.; De Giorgi, U.; Andreetta, C.; Pisano, C.; Scambia, G.; Lombardi, D.; et al. MITO END-3: Efficacy of avelumab immunotherapy according to molecular profiling in first-line endometrial cancer therapy. Ann. Oncol. 2024, 35, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lin, P.C. Mechanisms that drive inflammatory tumor microenvironment, tumor heterogeneity, and metastatic progression. Semin. Cancer Biol. 2017, 47, 185–195. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, W.; Bi, F.; Pan, X.; Yin, L.; Zhao, C. Significance of TP53 Mutational Status-Associated Signature in the Progression and Prognosis of Endometrial Carcinoma. Oxidative Med. Cell. Longev. 2022, 2022, 1817339. [Google Scholar] [CrossRef]
- Hiraki, M.; Hwang, S.-Y.; Cao, S.; Ramadhar, T.R.; Byun, S.; Yoon, K.W.; Lee, J.H.; Chu, K.; Gurkar, A.U.; Kolev, V.; et al. Small-Molecule Reactivation of Mutant p53 to Wild-Type-like p53 through the p53-Hsp40 Regulatory Axis. Chem. Biol. 2015, 22, 1206–1216. [Google Scholar] [CrossRef]
- Schäffer, A.A.; Chung, Y.; Kammula, A.V.; Ruppin, E.; Lee, J.S. A systematic analysis of the landscape of synthetic lethality-driven precision oncology. Med 2024, 5, 73–89.e9. [Google Scholar] [CrossRef] [PubMed]
- Pitolli, C.; Wang, Y.; Candi, E.; Shi, Y.; Melino, G.; Amelio, I. p53-Mediated Tumor Suppression: DNA-Damage Response and Alternative Mechanisms. Cancers 2019, 11, 1983. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Travaglino, A.; Saccone, G.; Campanino, M.R.; Mollo, A.; De Placido, G.; Insabato, L.; Zullo, F. Loss of PTEN expression as diagnostic marker of endometrial precancer: A systematic review and meta-analysis. Acta Obstet. Et Gynecol. Scand. 2019, 98, 275–286. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Cacalano, N.; Zhu, H.; Liu, Q.; Xie, M.; Kamrava, M.; Konecny, G.; Jin, S. Oncogenic Y68 frame shift mutation of PTEN represents a mechanism of docetaxel resistance in endometrial cancer cell lines. Sci. Rep. 2019, 9, 2111. [Google Scholar] [CrossRef]
- Risinger, J.I.; Hayes, K.; Maxwell, G.L.; Carney, M.E.; Dodge, R.K.; Barrett, J.C.; Berchuck, A. PTEN mutation in endometrial cancers is associated with favorable clinical and pathologic characteristics. Clin. Cancer Res. 1998, 4, 3005–3010. [Google Scholar]
- Köbel, M.; Ronnett, B.M.; Singh, N.; Soslow, R.A.; Gilks, C.B.; McCluggage, W.G. Interpretation of P53 Immunohistochemistry in Endometrial Carcinomas: Toward Increased Reproducibility. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. S1), S123–S131. [Google Scholar] [CrossRef] [PubMed]
- Cully, M.; You, H.; Levine, A.J.; Mak, T.W. Beyond PTEN mutations: The PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer 2006, 6, 184–192. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Liu, W.; DeLair, D.; Razavian, N.; Fenyö, D. Predicting endometrial cancer subtypes and molecular features from histopathology images using multi-resolution deep learning models. Cell Rep. Med. 2021, 2, 100400. [Google Scholar] [CrossRef]
- Álvarez-Garcia, V.; Tawil, Y.; Wise, H.M.; Leslie, N.R. Mechanisms of PTEN loss in cancer: It’s all about diversity. Semin. Cancer Biol. 2019, 59, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Cantley, L.C.; Neel, B.G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 4240–4245. [Google Scholar] [CrossRef]
- Tao, Y.; Liang, B. PTEN mutation: A potential prognostic factor associated with immune infiltration in endometrial carcinoma. Pathol. —Res. Pract. 2020, 216, 152943. [Google Scholar] [CrossRef]
- Carracedo, A.; Pandolfi, P.P. The PTEN–PI3K pathway: Of feedbacks and cross-talks. Oncogene 2008, 27, 5527–5541. [Google Scholar] [CrossRef]
- Mamat Yusof, M.N.; Chew, K.T.; Hafizz, A.; Abd Azman, S.H.; Ab Razak, W.S.; Hamizan, M.R.; Kampan, N.C.; Shafiee, M.N. Efficacy and Safety of PD-1/PD-L1 Inhibitor as Single-Agent Immunotherapy in Endometrial Cancer: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 4032. [Google Scholar] [CrossRef]
- Musacchio, L.; Caruso, G.; Pisano, C.; Cecere, S.C.; Di Napoli, M.; Attademo, L.; Tambaro, R.; Russo, D.; Califano, D.; Palaia, I.; et al. PARP Inhibitors in Endometrial Cancer: Current Status and Perspectives. Cancer Manag. Res. 2020, 12, 6123–6135. [Google Scholar] [CrossRef] [PubMed]
- Sirico, M.; D’Angelo, A.; Gianni, C.; Casadei, C.; Merloni, F.; De Giorgi, U. Current State and Future Challenges for PI3K Inhibitors in Cancer Therapy. Cancers 2023, 15, 703. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Luo, T.; Shi, H. Targeting the PI3K/AKT/mTOR and RAF/MEK/ERK pathways for cancer therapy. Mol. Biomed. 2022, 3, 47. [Google Scholar] [CrossRef]
- Wilson, M.R.; Harkins, S.; Reske, J.J.; Siwicki, R.A.; Adams, M.; Bae-Jump, V.L.; Teixeira, J.M.; Chandler, R.L. PIK3CA mutation in endometriotic epithelial cells promotes viperin-dependent inflammatory response to insulin. Reprod. Biol. Endocrinol. 2023, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Davidson, B.; Fadare, O.; Carlson, J.A.; Crum, C.P.; Gilks, C.B.; Irving, J.A.; Malpica, A.; Matias-Guiu, X.; McCluggage, W.G.; et al. High-grade Endometrial Carcinomas: Morphologic and Immunohistochemical Features, Diagnostic Challenges and Recommendations. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. S1), S40–S63. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Kanto, A.; Sakai, K.; Miyagawa, C.; Takaya, H.; Nakai, H.; Kotani, Y.; Nishio, K.; Matsumura, N. Frequent PIK3CA mutations in eutopic endometrium of patients with ovarian clear cell carcinoma. Mod. Pathol. 2021, 34, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Bredin, H.K.; Krakstad, C.; Hoivik, E.A. PIK3CA mutations and their impact on survival outcomes of patients with endometrial cancer: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0283203. [Google Scholar] [CrossRef]
- Catasus, L.; Gallardo, A.; Cuatrecasas, M.; Prat, J. PIK3CA mutations in the kinase domain (exon 20) of uterine endometrial adenocarcinomas are associated with adverse prognostic parameters. Mod. Pathol. 2008, 21, 131–139. [Google Scholar] [CrossRef]
- Velasco, A.; Bussaglia, E.; Pallares, J.; Dolcet, X.; Llobet, D.; Encinas, M.; Llecha, N.; Palacios, J.; Prat, J.; Matias-Guiu, X. PIK3CA gene mutations in endometrial carcinoma: Correlation with PTEN and K-RAS alterations. Hum. Pathol. 2006, 37, 1465–1472. [Google Scholar] [CrossRef]
- Croessmann, S.; Wong, H.Y.; Zabransky, D.J.; Chu, D.; Rosen, D.M.; Cidado, J.; Cochran, R.L.; Dalton, W.B.; Erlanger, B.; Cravero, K.; et al. PIK3CA mutations and TP53 alterations cooperate to increase cancerous phenotypes and tumor heterogeneity. Breast Cancer Res. Treat. 2017, 162, 451–464. [Google Scholar] [CrossRef]
- Mazloumi Gavgani, F.; Smith Arnesen, V.; Jacobsen, R.G.; Krakstad, C.; Hoivik, E.A.; Lewis, A.E. Class I Phosphoinositide 3-Kinase PIK3CA/p110α and PIK3CB/p110β Isoforms in Endometrial Cancer. Int. J. Mol. Sci. 2018, 19, 3931. [Google Scholar] [CrossRef]
- Jenkins, M.L.; Ranga-Prasad, H.; Parson, M.A.H.; Harris, N.J.; Rathinaswamy, M.K.; Burke, J.E. Oncogenic mutations of PIK3CA lead to increased membrane recruitment driven by reorientation of the ABD, p85 and C-terminus. Nat. Commun. 2023, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, X.; Ren, Y.; Fan, Z.; Zhang, J.; He, G.; Fu, L. Targeting PI3K family with small-molecule inhibitors in cancer therapy: Current clinical status and future directions. Mol. Cancer 2024, 23, 164. [Google Scholar] [CrossRef]
- Kato, M.K.; Yoshida, H.; Tanase, Y.; Uno, M.; Ishikawa, M.; Kato, T. Loss of ARID1A Expression as a Favorable Prognostic Factor in Early-Stage Grade 3 Endometrioid Endometrial Carcinoma Patients. Pathol. Oncol. Res. 2021, 27, 598550. [Google Scholar] [CrossRef]
- De Leo, A.; Ravegnini, G.; Musiani, F.; Maloberti, T.; Visani, M.; Sanza, V.; Angelini, S.; Perrone, A.M.; De Iaco, P.; Corradini, A.G.; et al. Relevance of ARID1A Mutations in Endometrial Carcinomas. Diagnostics 2022, 12, 592. [Google Scholar] [CrossRef]
- He, Z.; Chen, K.; Ye, Y.; Chen, Z. Structure of the SWI/SNF complex bound to the nucleosome and insights into the functional modularity. Cell Discov. 2021, 7, 28. [Google Scholar] [CrossRef]
- Chandler, R.L.; Damrauer, J.S.; Raab, J.R.; Schisler, J.C.; Wilkerson, M.D.; Didion, J.P.; Starmer, J.; Serber, D.; Yee, D.; Xiong, J.; et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat. Commun. 2015, 6, 6118. [Google Scholar] [CrossRef] [PubMed]
- Blümli, S.; Wiechens, N.; Wu, M.Y.; Singh, V.; Gierlinski, M.; Schweikert, G.; Gilbert, N.; Naughton, C.; Sundaramoorthy, R.; Varghese, J.; et al. Acute depletion of the ARID1A subunit of SWI/SNF complexes reveals distinct pathways for activation and repression of transcription. Cell Rep. 2021, 37, 109943. [Google Scholar] [CrossRef] [PubMed]
- Bakr, A.; Della Corte, G.; Veselinov, O.; Kelekçi, S.; Chen, M.M.; Lin, Y.Y.; Sigismondo, G.; Iacovone, M.; Cross, A.; Syed, R.; et al. ARID1A regulates DNA repair through chromatin organization and its deficiency triggers DNA damage-mediated anti-tumor immune response. Nucleic Acids Res. 2024, 52, 5698–5719. [Google Scholar] [CrossRef]
- Bosse, T.; ter Haar, N.T.; Seeber, L.M.; v Diest, P.J.; Hes, F.J.; Vasen, H.F.; Nout, R.A.; Creutzberg, C.L.; Morreau, H.; Smit, V.T. Loss of ARID1A expression and its relationship with PI3K-Akt pathway alterations, TP53 and microsatellite instability in endometrial cancer. Mod. Pathol. 2013, 26, 1525–1535. [Google Scholar] [CrossRef]
- Fujiwara, K.; Shintani, D.; Nishikawa, T. Clear-cell carcinoma of the ovary. Ann. Oncol. 2016, 27 (Suppl. S1), i50–i52. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, J.; Zhang, L.; Li, J.; Zhang, Y.; Zhao, C.; Wang, H. Comprehensive analysis of multiple parameters associated with tumor immune microenvironment in ARID1A mutant cancers. Future Oncol. 2020, 16, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Mao, T.L.; Panuganti, P.K.; Kuhn, E.; Kurman, R.J.; Maeda, D.; Chen, E.; Jeng, Y.M.; Wang, T.L.; Shih Ie, M. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am. J. Surg. Pathol. 2011, 35, 625–632. [Google Scholar] [CrossRef]
- Mandal, J.; Mandal, P.; Wang, T.-L.; Shih, I.-M. Treating ARID1A mutated cancers by harnessing synthetic lethality and DNA damage response. J. Biomed. Sci. 2022, 29, 71. [Google Scholar] [CrossRef]
- Shen, J.; Peng, Y.; Wei, L.; Zhang, W.; Yang, L.; Lan, L.; Kapoor, P.; Ju, Z.; Mo, Q.; Shih Ie, M.; et al. ARID1A Deficiency Impairs the DNA Damage Checkpoint and Sensitizes Cells to PARP Inhibitors. Cancer Discov. 2015, 5, 752–767. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kurnit, K.C.; Djordjevic, B.; Singh, C.; Munsell, M.F.; Wang, W.-L.; Lazar, A.J.; Zhang, W.; Broaddus, R. Nuclear β-catenin localization and mutation of the CTNNB1 gene: A context-dependent association. Mod. Pathol. 2018, 31, 1553–1559. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Saccone, G.; Mascolo, M.; D’Alessandro, P.; Arduino, B.; Mollo, A.; Insabato, L.; Zullo, F. Nuclear expression of β-catenin in endometrial hyperplasia as marker of premalignancy. Acta Pathol. Microbiol. Immunol. Scand. 2019, 127, 699–709. [Google Scholar] [CrossRef]
- Ledinek, Ž.; Sobočan, M.; Knez, J. The Role of CTNNB1 in Endometrial Cancer. Dis. Markers 2022, 2022, 1442441. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Z.; Dang, Q.; Xu, H.; Lv, J.; Li, H.; Han, X. Immunosuppression in tumor immune microenvironment and its optimization from CAR-T cell therapy. Theranostics 2022, 12, 6273–6290. [Google Scholar] [CrossRef]
- Bugter, J.M.; Fenderico, N.; Maurice, M.M. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat. Rev. Cancer 2021, 21, 5–21. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Raimondo, D.; Reppuccia, S.; Ruggiero, A.; Arena, A.; Casadio, P.; Zullo, F.; Insabato, L.; Seracchioli, R.; et al. Prognostic significance of CTNNB1 mutation in early stage endometrial carcinoma: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2022, 306, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Li, V.S.; Ng, S.S.; Boersema, P.J.; Low, T.Y.; Karthaus, W.R.; Gerlach, J.P.; Mohammed, S.; Heck, A.J.; Maurice, M.M.; Mahmoudi, T.; et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell 2012, 149, 1245–1256. [Google Scholar] [CrossRef]
- Michalczyk, K.; Cymbaluk-Płoska, A. Metalloproteinases in Endometrial Cancer-Are They Worth Measuring? Int. J. Mol. Sci. 2021, 22, 2472. [Google Scholar] [CrossRef] [PubMed]
- Jeanes, A.; Gottardi, C.J.; Yap, A.S. Cadherins and cancer: How does cadherin dysfunction promote tumor progression? Oncogene 2008, 27, 6920–6929. [Google Scholar] [CrossRef]
- Kurnit, K.C.; Kim, G.N.; Fellman, B.M.; Urbauer, D.L.; Mills, G.B.; Zhang, W.; Broaddus, R.R. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod. Pathol. 2017, 30, 1032–1041. [Google Scholar] [CrossRef]
- Kobayashi Kato, M.; Asami, Y.; Takayanagi, D.; Matsuda, M.; Shimada, Y.; Hiranuma, K.; Kuno, I.; Komatsu, M.; Hamamoto, R.; Matsumoto, K.; et al. Clinical impact of genetic alterations of CTNNB1 in patients with grade 3 endometrial endometrioid carcinoma. Cancer Sci. 2022, 113, 1712–1721. [Google Scholar] [CrossRef]
- Haddadi, N.; Lin, Y.; Travis, G.; Simpson, A.M.; Nassif, N.T.; McGowan, E.M. PTEN/PTENP1: ‘Regulating the regulator of RTK-dependent PI3K/Akt signalling’, new targets for cancer therapy. Mol. Cancer 2018, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Patel, L.; Mills, G.B.; Lu, K.H.; Sood, A.K.; Ding, L.; Kucherlapati, R.; Mardis, E.R.; Levine, D.A.; Shmulevich, I.; et al. Clinical significance of CTNNB1 mutation and Wnt pathway activation in endometrioid endometrial carcinoma. J. Natl. Cancer Inst. 2014, 106, dju245. [Google Scholar] [CrossRef]
- Roncolato, F.; Lindemann, K.; Willson, M.L.; Martyn, J.; Mileshkin, L. PI3K/AKT/mTOR inhibitors for advanced or recurrent endometrial cancer. Cochrane Database Syst. Rev. 2019, 10, Cd012160. [Google Scholar] [CrossRef]
- Yao, X.; Feng, M.; Wang, W. The Clinical and Pathological Characteristics of POLE-Mutated Endometrial Cancer: A Comprehensive Review. Cancer Manag. Res. 2024, 16, 117–125. [Google Scholar] [CrossRef]
- Casanova, J.; Duarte, G.S.; da Costa, A.G.; Catarino, A.; Nave, M.; Antunes, T.; Serra, S.S.; Dias, S.S.; Abu-Rustum, N.; Lima, J. Prognosis of polymerase epsilon (POLE) mutation in high-grade endometrioid endometrial cancer: Systematic review and meta-analysis. Gynecol. Oncol. 2024, 182, 99–107. [Google Scholar] [CrossRef]
- Selves, J.; de Castro, E.G.H.; Brunac, A.C.; Saffi, J.; Guimbaud, R.; Brousset, P.; Hoffmann, J.S. Exploring the basis of heterogeneity of cancer aggressiveness among the mutated POLE variants. Life Sci. Alliance 2024, 7, e202302290. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, I.C.; Rayner, E.; Osse, E.M.; Nout, R.A.; Creutzberg, C.L.; Tomlinson, I.P.M.; Church, D.N.; Smit, V.; de Wind, N.; Bosse, T.; et al. Adjuvant Treatment for POLE Proofreading Domain-Mutant Cancers: Sensitivity to Radiotherapy, Chemotherapy, and Nucleoside Analogues. Clin. Cancer Res. 2018, 24, 3197–3203. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, J.M.; Panda, A.; Zhong, H.; Hirshfield, K.; Damare, S.; Lane, K.; Sokol, L.; Stein, M.N.; Rodriguez-Rodriquez, L.; Kaufman, H.L.; et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J. Clin. Investig. 2016, 126, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Stenzinger, A.; Pfarr, N.; Endris, V.; Penzel, R.; Jansen, L.; Wolf, T.; Herpel, E.; Warth, A.; Klauschen, F.; Kloor, M.; et al. Mutations in POLE and survival of colorectal cancer patients--link to disease stage and treatment. Cancer Med. 2014, 3, 1527–1538. [Google Scholar] [CrossRef]
- McAlpine, J.N.; Chiu, D.S.; Nout, R.A.; Church, D.N.; Schmidt, P.; Lam, S.; Leung, S.; Bellone, S.; Wong, A.; Brucker, S.Y.; et al. Evaluation of treatment effects in patients with endometrial cancer and POLE mutations: An individual patient data meta-analysis. Cancer 2021, 127, 2409–2422. [Google Scholar] [CrossRef]
- Bell, D.W.; Ellenson, L.H. Molecular Genetics of Endometrial Carcinoma. Annu. Rev. Pathol. 2019, 14, 339–367. [Google Scholar] [CrossRef]
- Li, Y.; Bian, Y.; Wang, K.; Wan, X.-P. POLE mutations improve the prognosis of endometrial cancer via regulating cellular metabolism through AMF/AMFR signal transduction. BMC Med. Genet. 2019, 20, 202. [Google Scholar] [CrossRef]
- Sorbe, B.; Andersson, H.; Boman, K.; Rosenberg, P.; Kalling, M. Treatment of primary advanced and recurrent endometrial carcinoma with a combination of carboplatin and paclitaxel-long-term follow-up. Int. J. Gynecol. Cancer 2008, 18, 803–808. [Google Scholar] [CrossRef]
- Dugo, E.; Piva, F.; Giulietti, M.; Giannella, L.; Ciavattini, A. Copy number variations in endometrial cancer: From biological significance to clinical utility. Int. J. Gynecol. Cancer 2024, 34, 1089–1097. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, P.; Lu, Y.; Dou, H. Correlation of MACC1/c-Myc Expression in Endometrial Carcinoma with Clinical/Pathological Features or Prognosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 4738–4744. [Google Scholar] [CrossRef]
- Kang, J.U. Chromosome 8q as the most frequent target for amplification in early gastric carcinoma. Oncol. Lett. 2014, 7, 1139–1143. [Google Scholar] [CrossRef]
- Dumbrava, E.E.I.; Balaji, K.; Raghav, K.; Hess, K.; Javle, M.; Blum-Murphy, M.; Ajani, J.; Kopetz, S.; Broaddus, R.; Routbort, M.; et al. Targeting ERBB2 (HER2) Amplification Identified by Next-Generation Sequencing in Patients With Advanced or Metastatic Solid Tumors Beyond Conventional Indications. JCO Precis. Oncol. 2019, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Beinse, G.; Rance, B.; Just, P.A.; Izac, B.; Letourneur, F.; Saidu, N.E.B.; Chouzenoux, S.; Nicco, C.; Goldwasser, F.; Batteux, F.; et al. Identification of TP53 mutated group using a molecular and immunohistochemical classification of endometrial carcinoma to improve prognostic evaluation for adjuvant treatments. Int. J. Gynecol. Cancer 2020, 30, 640–647. [Google Scholar] [CrossRef]
- Hernández Borrero, L.J.; El-Deiry, W.S. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim. Et Biophys. Acta. Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, X.; Liu, X.; Ma, K.; Meng, Y.-T.; Yin, H.-F.; Wen, J.; Yang, J.-H.; Zhen, Z.; Feng, Z.-H.; et al. Clinical characteristics and prognostic characterization of endometrial carcinoma: A comparative analysis of molecular typing protocols. BMC Cancer 2023, 23, 243. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Carey-Smith, S.L.; Kotecha, R.S.; Cheung, L.C.; Malinge, S. Insights into the Clinical, Biological and Therapeutic Impact of Copy Number Alteration in Cancer. Int. J. Mol. Sci. 2024, 25, 6815. [Google Scholar] [CrossRef]
- Kato, M.K.; Fujii, E.; Asami, Y.; Momozawa, Y.; Hiranuma, K.; Komatsu, M.; Hamamoto, R.; Ebata, T.; Matsumoto, K.; Ishikawa, M.; et al. Clinical features and impact of p53 status on sporadic mismatch repair deficiency and Lynch syndrome in uterine cancer. Cancer Sci. 2024, 115, 1646–1655. [Google Scholar] [CrossRef]
- Steele, C.D.; Abbasi, A.; Islam, S.M.A.; Bowes, A.L.; Khandekar, A.; Haase, K.; Hames-Fathi, S.; Ajayi, D.; Verfaillie, A.; Dhami, P.; et al. Signatures of copy number alterations in human cancer. Nature 2022, 606, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef]
- Inoue, F.; Sone, K.; Toyohara, Y.; Takahashi, Y.; Kukita, A.; Hara, A.; Taguchi, A.; Tanikawa, M.; Tsuruga, T.; Osuga, Y. Targeting Epigenetic Regulators for Endometrial Cancer Therapy: Its Molecular Biology and Potential Clinical Applications. Int. J. Mol. Sci. 2021, 22, 2305. [Google Scholar] [CrossRef]
- Gotoh, O.; Sugiyama, Y.; Tonooka, A.; Kosugi, M.; Kitaura, S.; Minegishi, R.; Sano, M.; Amino, S.; Furuya, R.; Tanaka, N.; et al. Genetic and epigenetic alterations in precursor lesions of endometrial endometrioid carcinoma. J. Pathol. 2024, 263, 275–287. [Google Scholar] [CrossRef]
- Manning-Geist, B.L.; Liu, Y.L.; Devereaux, K.A.; Paula, A.D.C.; Zhou, Q.C.; Ma, W.; Selenica, P.; Ceyhan-Birsoy, O.; Moukarzel, L.A.; Hoang, T.; et al. Microsatellite Instability-High Endometrial Cancers with MLH1 Promoter Hypermethylation Have Distinct Molecular and Clinical Profiles. Clin. Cancer Res. 2022, 28, 4302–4311. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.W.; Li, J.; Podratz, K.; Dowdy, S. Application of DNA methylation biomarkers for endometrial cancer management. Expert Rev. Mol. Diagn. 2008, 8, 607–616. [Google Scholar] [CrossRef]
- Arif, K.M.T.; Elliott, E.K.; Haupt, L.M.; Griffiths, L.R. Regulatory Mechanisms of Epigenetic miRNA Relationships in Human Cancer and Potential as Therapeutic Targets. Cancers 2020, 12, 2922. [Google Scholar] [CrossRef]
- Psilopatis, I.; Pergaris, A.; Giaginis, C.; Theocharis, S. Histone Deacetylase Inhibitors: A Promising Therapeutic Alternative for Endometrial Carcinoma. Dis. Markers 2021, 2021, 7850688. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, G.; Rakshit, S.; Sarkar, K. HDAC inhibitors: Targets for tumor therapy, immune modulation and lung diseases. Transl. Oncol. 2022, 16, 101312. [Google Scholar] [CrossRef]
- Alam, H.; Gu, B.; Lee, M.G. Histone methylation modifiers in cellular signaling pathways. Cell. Mol. Life Sci. 2015, 72, 4577–4592. [Google Scholar] [CrossRef]
- Kazmi, I.; Afzal, M.; Almalki, W.H.; Alzarea, S.I.; Kumar, A.; Sinha, A.; Kukreti, N.; Ali, H. From oncogenes to tumor suppressors: The dual role of ncRNAs in fibrosarcoma. Pathol. Res. Pract. 2024, 258, 155329. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L.; Wan, X.P. The role of lncRNAs in the development of endometrial carcinoma. Oncol. Lett. 2018, 16, 3424–3429. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, X.; Zeng, Y.; Liu, J.; Wu, F. DNA methyltransferase inhibitors combination therapy for the treatment of solid tumor: Mechanism and clinical application. Clin. Epigenetics 2021, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Cornel, K.M.C.; Wouters, K.; Van de Vijver, K.K.; van der Wurff, A.A.M.; van Engeland, M.; Kruitwagen, R.; Pijnenborg, J.M.A. Gene Promoter Methylation in Endometrial Carcinogenesis. Pathol. Oncol. Res. 2019, 25, 659–667. [Google Scholar] [CrossRef]
- Glaser, K.B. HDAC inhibitors: Clinical update and mechanism-based potential. Biochem. Pharmacol. 2007, 74, 659–671. [Google Scholar] [CrossRef]
- Kaneko, E.; Sato, N.; Sugawara, T.; Noto, A.; Takahashi, K.; Makino, K.; Terada, Y. MLH1 promoter hypermethylation predicts poorer prognosis in mismatch repair deficiency endometrial carcinomas. J. Gynecol. Oncol. 2021, 32, e79. [Google Scholar] [CrossRef]
- Sadida, H.Q.; Abdulla, A.; Marzooqi, S.A.; Hashem, S.; Macha, M.A.; Akil, A.S.A.-S.; Bhat, A.A. Epigenetic modifications: Key players in cancer heterogeneity and drug resistance. Transl. Oncol. 2024, 39, 101821. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Philip, C.A.; Laskov, I.; Beauchamp, M.C.; Marques, M.; Amin, O.; Bitharas, J.; Kessous, R.; Kogan, L.; Baloch, T.; Gotlieb, W.H.; et al. Inhibition of PI3K-AKT-mTOR pathway sensitizes endometrial cancer cell lines to PARP inhibitors. BMC Cancer 2017, 17, 638. [Google Scholar] [CrossRef]
- Hassan, B.; Akcakanat, A.; Holder, A.M.; Meric-Bernstam, F. Targeting the PI3-kinase/Akt/mTOR signaling pathway. Surg. Oncol. Clin. N. Am. 2013, 22, 641–664. [Google Scholar] [CrossRef]
- Huo, X.; Sun, H.; Liu, Q.; Ma, X.; Peng, P.; Yu, M.; Zhang, Y.; Cao, D.; Shen, K. Clinical and Expression Significance of AKT1 by Co-expression Network Analysis in Endometrial Cancer. Front. Oncol. 2019, 9, 1147. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef]
- Slomovitz, B.M.; Coleman, R.L. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin. Cancer Res. 2012, 18, 5856–5864. [Google Scholar] [CrossRef] [PubMed]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef]
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated cell death (RCD) in cancer: Key pathways and targeted therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sahra, I.; Manning, B.D. mTORC1 signaling and the metabolic control of cell growth. Curr. Opin. Cell Biol. 2017, 45, 72–82. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Meng, D.; He, W.; Zhang, Y.; Liang, Z.; Zheng, J.; Zhang, X.; Zheng, X.; Zhan, P.; Chen, H.; Li, W.; et al. Development of PI3K inhibitors: Advances in clinical trials and new strategies (Review). Pharmacol. Res. 2021, 173, 105900. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Curigliano, G.; Delord, J.P.; Harb, W.; Azaro, A.; Han, Y.; Wilke, C.; Donnet, V.; Sellami, D.; Beck, T. A Phase Ib, open-label, dose-finding study of alpelisib in combination with paclitaxel in patients with advanced solid tumors. Oncotarget 2018, 9, 31709–31718. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial cancer. Nat. Rev. Dis. Primers 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Zheng, X.F.S. Toward rapamycin analog (rapalog)-based precision cancer therapy. Acta Pharmacol. Sin. 2015, 36, 1163–1169. [Google Scholar] [CrossRef]
- Wright, S.C.E.; Vasilevski, N.; Serra, V.; Rodon, J.; Eichhorn, P.J.A. Mechanisms of Resistance to PI3K Inhibitors in Cancer: Adaptive Responses, Drug Tolerance and Cellular Plasticity. Cancers 2021, 13, 1538. [Google Scholar] [CrossRef] [PubMed]
- Slomovitz, B.M.; Filiaci, V.L.; Walker, J.L.; Taub, M.C.; Finkelstein, K.A.; Moroney, J.W.; Fleury, A.C.; Muller, C.Y.; Holman, L.L.; Copeland, L.J.; et al. A randomized phase II trial of everolimus and letrozole or hormonal therapy in women with advanced, persistent or recurrent endometrial carcinoma: A GOG Foundation study. Gynecol. Oncol. 2022, 164, 481–491. [Google Scholar] [CrossRef]
- Herrero-Sánchez, M.C.; Rodríguez-Serrano, C.; Almeida, J.; San Segundo, L.; Inogés, S.; Santos-Briz, Á.; García-Briñón, J.; Corchete, L.A.; San Miguel, J.F.; Del Cañizo, C.; et al. Targeting of PI3K/AKT/mTOR pathway to inhibit T cell activation and prevent graft-versus-host disease development. J. Hematol. Oncol. 2016, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Massi, D.; Teng, M.W.L.; Mandala, M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin. Cancer Biol. 2018, 48, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef]
- Jamieson, A.; Thompson, E.F.; Huvila, J.; Gilks, C.B.; McAlpine, J.N. p53abn Endometrial Cancer: Understanding the most aggressive endometrial cancers in the era of molecular classification. Int. J. Gynecol. Cancer 2021, 31, 907–913. [Google Scholar] [CrossRef]
- Hientz, K.; Mohr, A.; Bhakta-Guha, D.; Efferth, T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2017, 8, 8921–8946. [Google Scholar] [CrossRef]
- Traweek, R.S.; Cope, B.M.; Roland, C.L.; Keung, E.Z.; Nassif, E.F.; Erstad, D.J. Targeting the MDM2-p53 pathway in dedifferentiated liposarcoma. Front. Oncol. 2022, 12, 1006959. [Google Scholar] [CrossRef]
- Riedinger, C.J.; Esnakula, A.; Haight, P.J.; Suarez, A.A.; Chen, W.; Gillespie, J.; Villacres, A.; Chassen, A.; Cohn, D.E.; Goodfellow, P.J.; et al. Characterization of mismatch-repair/microsatellite instability-discordant endometrial cancers. Cancer 2024, 130, 385–399. [Google Scholar] [CrossRef]
- Addante, F.; d’Amati, A.; Santoro, A.; Angelico, G.; Inzani, F.; Arciuolo, D.; Travaglino, A.; Raffone, A.; D’Alessandris, N.; Scaglione, G.; et al. Mismatch Repair Deficiency as a Predictive and Prognostic Biomarker in Endometrial Cancer: A Review on Immunohistochemistry Staining Patterns and Clinical Implications. Int. J. Mol. Sci. 2024, 25, 1056. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008, 18, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Curtius, K.; Gupta, S.; Boland, C.R. Review article: Lynch Syndrome-a mechanistic and clinical management update. Aliment. Pharmacol. Ther. 2022, 55, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Bhamidipati, D.; Subbiah, V. Tumor-agnostic drug development in dMMR/MSI-H solid tumors. Trends Cancer 2023, 9, 828–839. [Google Scholar] [CrossRef]
- Di Tucci, C.; Schiavi, M.C.; Faiano, P.; D’Oria, O.; Prata, G.; Sciuga, V.; Giannini, A.; Palaia, I.; Muzii, L.; Benedetti Panici, P. Therapeutic vaccines and immune checkpoints inhibition options for gynecological cancers. Crit. Rev. Oncol. Hematol. 2018, 128, 30–42. [Google Scholar] [CrossRef]
- Vafaei, S.; Zekiy, A.O.; Khanamir, R.A.; Zaman, B.A.; Ghayourvahdat, A.; Azimizonuzi, H.; Zamani, M. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 2022, 22, 2. [Google Scholar] [CrossRef]
- Stelloo, E.; Jansen, A.M.L.; Osse, E.M.; Nout, R.A.; Creutzberg, C.L.; Ruano, D.; Church, D.N.; Morreau, H.; Smit, V.; van Wezel, T.; et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann. Oncol. 2017, 28, 96–102. [Google Scholar] [CrossRef]
- Sahin, I.H.; Akce, M.; Alese, O.; Shaib, W.; Lesinski, G.B.; El-Rayes, B.; Wu, C. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br. J. Cancer 2019, 121, 809–818. [Google Scholar] [CrossRef]
- Niu, S.; Molberg, K.; Castrillon, D.H.; Lucas, E.; Chen, H. Biomarkers in the Diagnosis of Endometrial Precancers. Molecular Characteristics, Candidate Immunohistochemical Markers, and Promising Results of Three-Marker Panel: Current Status and Future Directions. Cancers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Yip, H.Y.K.; Papa, A. Signaling Pathways in Cancer: Therapeutic Targets, Combinatorial Treatments, and New Developments. Cells 2021, 10, 659. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Etemadmoghadam, D.; Temple, J.; Lynch, A.G.; Riad, M.; Sharma, R.; Stewart, C.; Fereday, S.; Caldas, C.; Defazio, A.; et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J. Pathol. 2010, 221, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Haesen, D.; Abbasi Asbagh, L.; Derua, R.; Hubert, A.; Schrauwen, S.; Hoorne, Y.; Amant, F.; Waelkens, E.; Sablina, A.; Janssens, V. Recurrent PPP2R1A Mutations in Uterine Cancer Act through a Dominant-Negative Mechanism to Promote Malignant Cell Growth. Cancer Res. 2016, 76, 5719–5731. [Google Scholar] [CrossRef]
- McConechy, M.K.; Ding, J.; Cheang, M.C.; Wiegand, K.; Senz, J.; Tone, A.; Yang, W.; Prentice, L.; Tse, K.; Zeng, T.; et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J. Pathol. 2012, 228, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Urick, M.E.; Yu, E.J.; Bell, D.W. High-risk endometrial cancer proteomic profiling reveals that FBXW7 mutation alters L1CAM and TGM2 protein levels. Cancer 2021, 127, 2905–2915. [Google Scholar] [CrossRef]
- Davis, H.; Lewis, A.; Spencer-Dene, B.; Tateossian, H.; Stamp, G.; Behrens, A.; Tomlinson, I. FBXW7 mutations typically found in human cancers are distinct from null alleles and disrupt lung development. J. Pathol. 2011, 224, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Bellon, M.; Ju, M.; Zhao, L.; Wei, M.; Fu, L.; Nicot, C. Clinical significance of FBXW7 loss of function in human cancers. Mol. Cancer 2022, 21, 87. [Google Scholar] [CrossRef]
- Liu, X.; Mei, W.; Zhang, P.; Zeng, C. PIK3CA mutation as an acquired resistance driver to EGFR-TKIs in non-small cell lung cancer: Clinical challenges and opportunities. Pharmacol. Res. 2024, 202, 107123. [Google Scholar] [CrossRef]
- Cheung, L.W.; Hennessy, B.T.; Li, J.; Yu, S.; Myers, A.P.; Djordjevic, B.; Lu, Y.; Stemke-Hale, K.; Dyer, M.D.; Zhang, F.; et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011, 1, 170–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Ke, X.P.; Liu, P. The identification of a PTEN-associated gene signature for the prediction of prognosis and planning of therapeutic strategy in endometrial cancer. Transl. Cancer Res. 2023, 12, 3409–3424. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Wang, W.; Wang, Z.; Zhang, Y.; Pan, X.; Wen, X.; Leng, H.; Guo, J.; Ma, X.X. WTAP/IGF2BP3 mediated m6A modification of the EGR1/PTEN axis regulates the malignant phenotypes of endometrial cancer stem cells. J. Exp. Clin. Cancer Res. 2024, 43, 204. [Google Scholar] [CrossRef] [PubMed]
- Bazzichetto, C.; Conciatori, F.; Pallocca, M.; Falcone, I.; Fanciulli, M.; Cognetti, F.; Milella, M.; Ciuffreda, L. PTEN as a Prognostic/Predictive Biomarker in Cancer: An Unfulfilled Promise? Cancers 2019, 11, 435. [Google Scholar] [CrossRef]
- Forbes, S.A.; Beare, D.; Boutselakis, H.; Bamford, S.; Bindal, N.; Tate, J.; Cole, C.G.; Ward, S.; Dawson, E.; Ponting, L.; et al. COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Res. 2017, 45, D777–D783. [Google Scholar] [CrossRef] [PubMed]
- Kogan, L.; Octeau, D.; Amajoud, Z.; Abitbol, J.; Laskov, I.; Ferenczy, A.; Pelmus, M.; Eisenberg, N.; Kessous, R.; Lau, S.; et al. Impact of lower uterine segment involvement in type II endometrial cancer and the unique mutational profile of serous tumors. Gynecol. Oncol. Rep. 2018, 24, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, A.D.; Shen, H.; Walter, V.; Stewart, C.; Murray, B.A.; Bowlby, R.; Hu, X.; Ling, S.; Soslow, R.A.; Broaddus, R.R.; et al. Integrated Molecular Characterization of Uterine Carcinosarcoma. Cancer Cell 2017, 31, 411–423. [Google Scholar] [CrossRef] [PubMed]
- DeLair, D.F.; Burke, K.A.; Selenica, P.; Lim, R.S.; Scott, S.N.; Middha, S.; Mohanty, A.S.; Cheng, D.T.; Berger, M.F.; Soslow, R.A.; et al. The genetic landscape of endometrial clear cell carcinomas. J. Pathol. 2017, 243, 230–241. [Google Scholar] [CrossRef]
- Jones, N.L.; Xiu, J.; Chatterjee-Paer, S.; Buckley de Meritens, A.; Burke, W.M.; Tergas, A.I.; Wright, J.D.; Hou, J.Y. Distinct molecular landscapes between endometrioid and nonendometrioid uterine carcinomas. Int. J. Cancer 2017, 140, 1396–1404. [Google Scholar] [CrossRef]
- Onoprienko, A.; Hofstetter, G.; Muellauer, L.; Dorittke, T.; Polterauer, S.; Grimm, C.; Bartl, T. Prognostic role of transcription factor ARID1A in patients with endometrial cancer of no specific molecular profile (NSMP) subtype. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2024, 34, 840–846. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, H.B.; Wang, J.; Cui, S.J.; Wang, X.Q.; Jiang, Y.H.; Feng, L.; Yang, P.Y.; Liu, F. Chromatin remodeling gene AT-rich interactive domain-containing protein 1A suppresses gastric cancer cell proliferation by targeting PIK3CA and PDK1. Oncotarget 2016, 7, 46127–46141. [Google Scholar] [CrossRef]
- Castel, P.; Toska, E.; Engelman, J.A.; Scaltriti, M. The present and future of PI3K inhibitors for cancer therapy. Nat. Cancer 2021, 2, 587–597. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Park, J.-I. Wnt signaling in cancer: Therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp. Mol. Med. 2020, 52, 183–191. [Google Scholar] [CrossRef]
- Luke, J.J.; Bao, R.; Sweis, R.F.; Spranger, S.; Gajewski, T.F. WNT/β-catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin. Cancer Res. 2019, 25, 3074–3083. [Google Scholar] [CrossRef]
- Buza, N. HER2 Testing in Endometrial Serous Carcinoma: Time for Standardized Pathology Practice to Meet the Clinical Demand. Arch. Pathol. Lab. Med. 2021, 145, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Balestra, A.; Larsimont, D.; Noël, J.C. HER2 Amplification in p53-Mutated Endometrial Carcinomas. Cancers 2023, 15, 1435. [Google Scholar] [CrossRef] [PubMed]
- Bruce, S.F.; Wu, S.; Ribeiro, J.R.; Farrell, A.; Oberley, M.J.; Winer, I.; Erickson, B.K.; Klc, T.; Jones, N.L.; Thaker, P.H.; et al. HER2+ endometrioid endometrial cancer possesses distinct molecular and immunologic features associated with a more active immune microenvironment and worse prognosis. Gynecol. Oncol. 2023, 172, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhuang, G.; Sun, X.; Shen, Y.; Zhao, A.; Di, W. Risk prediction model for epithelial ovarian cancer using molecular markers and clinical characteristics. J. Ovarian Res. 2015, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Bang, Y.J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Levine, D.A.; Getz, G.; Gabriel, S.B.; Cibulskis, K.; Lander, E.; Sivachenko, A.; Sougnez, C.; Lawrence, M.; Kandoth, C.; Dooling, D.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef]
- Kilowski, K.A.; Dietrich, M.F.; Xiu, J.; Baca, Y.; Hinton, A.; Ahmad, S.; Herzog, T.J.; Thaker, P.; Holloway, R.W. KRAS mutations in endometrial cancers: Possible prognostic and treatment implications. Gynecol. Oncol. 2024, 191, 299–306. [Google Scholar] [CrossRef]
- Ma, X.; Ma, C.X.; Wang, J. Endometrial carcinogenesis and molecular signaling pathways. Am. J. Mol. Biol. 2014, 4, 134–149. [Google Scholar] [CrossRef]
- Ring, K.L.; Yates, M.S.; Schmandt, R.; Onstad, M.; Zhang, Q.; Celestino, J.; Kwan, S.Y.; Lu, K.H. Endometrial Cancers With Activating KRas Mutations Have Activated Estrogen Signaling and Paradoxical Response to MEK Inhibition. Int. J. Gynecol. Cancer 2017, 27, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Michalová, K.; Presl, J.; Straková-Peteříková, A.; Ondič, O.; Vaneček, T.; Hejhalová, N.; Holub, P.; Slavík, P.; Hluchý, A.; Gettse, P.; et al. Advantages of next-generation sequencing (NGS) in the molecular classifi cation of endometrial carcinomas—Our experience with 270 cases. Ceska Gynekol. 2024, 89, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Peng, H.; Qi, X.; Wu, M.; Zhao, X. Targeted therapies in gynecological cancers: A comprehensive review of clinical evidence. Signal Transduct. Target. Ther. 2020, 5, 137. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, J.; Meng, L.H. PI3K isoform-selective inhibitors: Next-generation targeted cancer therapies. Acta Pharmacol. Sin. 2015, 36, 1170–1176. [Google Scholar] [CrossRef]
- Garrett, J.T.; Chakrabarty, A.; Arteaga, C.L. Will PI3K pathway inhibitors be effective as single agents in patients with cancer? Oncotarget 2011, 2, 1314–1321. [Google Scholar] [CrossRef][Green Version]
- Xu, T.; Sun, D.; Chen, Y.; Ouyang, L. Targeting mTOR for fighting diseases: A revisited review of mTOR inhibitors. Eur. J. Med. Chem. 2020, 199, 112391. [Google Scholar] [CrossRef] [PubMed]
- Formisano, L.; Napolitano, F.; Rosa, R.; D’Amato, V.; Servetto, A.; Marciano, R.; De Placido, P.; Bianco, C.; Bianco, R. Mechanisms of resistance to mTOR inhibitors. Crit. Rev. Oncol. Hematol. 2020, 147, 102886. [Google Scholar] [CrossRef]
- Wu, X.; Xu, Y.; Liang, Q.; Yang, X.; Huang, J.; Wang, J.; Zhang, H.; Shi, J. Recent Advances in Dual PI3K/mTOR Inhibitors for Tumour Treatment. Front. Pharmacol. 2022, 13, 875372. [Google Scholar] [CrossRef]
- De Wispelaere, W.; Annibali, D.; Tuyaerts, S.; Messiaen, J.; Antoranz, A.; Shankar, G.; Dubroja, N.; Herreros-Pomares, A.; Baiden-Amissah, R.E.M.; Orban, M.P.; et al. PI3K/mTOR inhibition induces tumour microenvironment remodelling and sensitises pS6(high) uterine leiomyosarcoma to PD-1 blockade. Clin. Transl. Med. 2024, 14, e1655. [Google Scholar] [CrossRef]
- Butterfield, L.H.; Najjar, Y.G. Immunotherapy combination approaches: Mechanisms, biomarkers and clinical observations. Nat. Rev. Immunol. 2024, 24, 399–416. [Google Scholar] [CrossRef]
- Javed, S.A.; Najmi, A.; Ahsan, W.; Zoghebi, K. Targeting PD-1/PD-L-1 immune checkpoint inhibition for cancer immunotherapy: Success and challenges. Front. Immunol. 2024, 15, 1383456. [Google Scholar] [CrossRef] [PubMed]
- Eso, Y.; Shimizu, T.; Takeda, H.; Takai, A.; Marusawa, H. Microsatellite instability and immune checkpoint inhibitors: Toward precision medicine against gastrointestinal and hepatobiliary cancers. J. Gastroenterol. 2020, 55, 15–26. [Google Scholar] [CrossRef]
- Guo, Y.-E.; Liu, Y.; Zhang, W.; Luo, H.; Shu, P.; Chen, G.; Li, Y. The clinicopathological characteristics, prognosis and immune microenvironment mapping in MSI-H/MMR-D endometrial carcinomas. Discov. Oncol. 2022, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-J.; Zhao, J.-W.; Zhang, D.-H.; Zheng, A.-H.; Wu, G.-Q. Immunotherapy of Cancer by Targeting Regulatory T cells. Int. Immunopharmacol. 2022, 104, 108469. [Google Scholar] [CrossRef]
- Akagi, K.; Oki, E.; Taniguchi, H.; Nakatani, K.; Aoki, D.; Kuwata, T.; Yoshino, T. Real-world data on microsatellite instability status in various unresectable or metastatic solid tumors. Cancer Sci. 2021, 112, 1105–1113. [Google Scholar] [CrossRef]
- Van Gorp, T.; Mirza, M.R.; Lortholary, A.; Cibula, D.; Walther, A.; Savarese, A.; Barretina-Ginesta, M.P.; Ortaç, F.; Papadimitriou, C.; Bodnar, L. ENGOT-en11/GOG-3053/KEYNOTE-B21: Phase 3 study of pembrolizumab or placebo in combination with adjuvant chemotherapy with/without radiotherapy in patients with newly diagnosed high-risk endometrial cancer. J. Clin. Oncol. 2021, 39, TPS5608. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, L.; Lei, Y.; Li, T.; Li, H.; Cheng, X. Integrated analysis of tumor mutation burden and immune infiltrates in endometrial cancer. Curr. Probl. Cancer 2021, 45, 100660. [Google Scholar] [CrossRef]
- Meng, L.; Wu, H.; Wu, J.; Ding, P.a.; He, J.; Sang, M.; Liu, L. Mechanisms of immune checkpoint inhibitors: Insights into the regulation of circular RNAS involved in cancer hallmarks. Cell Death Dis. 2024, 15, 3. [Google Scholar] [CrossRef]
- Yan, X.; Hui, Y.; Hua, Y.; Huang, L.; Wang, L.; Peng, F.; Tang, C.; Liu, D.; Song, J.; Wang, F. EG-VEGF silencing inhibits cell proliferation and promotes cell apoptosis in pancreatic carcinoma via PI3K/AKT/mTOR signaling pathway. Biomed. Pharmacother. 2019, 109, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, H.A.; Villar, R.C. Radiotherapy and immune response: The systemic effects of a local treatment. Clinics 2018, 73, e557s. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Hellmann, M.D. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020, 37, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Sarmento-Ribeiro, A.B.; Scorilas, A.; Gonçalves, A.C.; Efferth, T.; Trougakos, I.P. The emergence of drug resistance to targeted cancer therapies: Clinical evidence. Drug Resist. Updates 2019, 47, 100646. [Google Scholar] [CrossRef]
- McGrail, D.J.; Li, Y.; Smith, R.S.; Feng, B.; Dai, H.; Hu, L.; Dennehey, B.; Awasthi, S.; Mendillo, M.L.; Sood, A.K.; et al. Widespread BRCA1/2-independent homologous recombination defects are caused by alterations in RNA-binding proteins. Cell Rep. Med. 2023, 4, 101255. [Google Scholar] [CrossRef]
- Hampel, H.; Pearlman, R.; de la Chapelle, A.; Pritchard, C.C.; Zhao, W.; Jones, D.; Yilmaz, A.; Chen, W.; Frankel, W.L.; Suarez, A.A.; et al. Double somatic mismatch repair gene pathogenic variants as common as Lynch syndrome among endometrial cancer patients. Gynecol. Oncol. 2021, 160, 161–168. [Google Scholar] [CrossRef]
- Kolesnichenko, M.; Scheidereit, C. Synthetic lethality by PARP inhibitors: New mechanism uncovered based on unresolved transcription-replication conflicts. Signal Transduct. Target. Ther. 2024, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Franzese, E.; Centonze, S.; Diana, A.; Carlino, F.; Guerrera, L.P.; Di Napoli, M.; De Vita, F.; Pignata, S.; Ciardiello, F.; Orditura, M. PARP inhibitors in ovarian cancer. Cancer Treat. Rev. 2019, 73, 1–9. [Google Scholar] [CrossRef]
- Lorusso, D.; Mouret-Reynier, M.A.; Harter, P.; Cropet, C.; Caballero, C.; Wolfrum-Ristau, P.; Satoh, T.; Vergote, I.; Parma, G.; Nøttrup, T.J.; et al. Updated progression-free survival and final overall survival with maintenance olaparib plus bevacizumab according to clinical risk in patients with newly diagnosed advanced ovarian cancer in the phase III PAOLA-1/ENGOT-ov25 trial. Int. J. Gynecol. Cancer 2024, 34, 550–558. [Google Scholar] [CrossRef]
- Kristeleit, R.; Leary, A.; Oaknin, A.; Redondo, A.; George, A.; Chui, S.; Seiller, A.; Liste-Hermoso, M.; Willis, J.; Shemesh, C.S.; et al. PARP inhibition with rucaparib alone followed by combination with atezolizumab: Phase Ib COUPLET clinical study in advanced gynaecological and triple-negative breast cancers. Br. J. Cancer 2024, 131, 820–831. [Google Scholar] [CrossRef]
- Lee, E.K.; Liu, J.F. Rational Combinations of PARP Inhibitors with HRD-Inducing Molecularly Targeted Agents. Cancer Treat. Res. 2023, 186, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Helleday, T. The underlying mechanism for the PARP and BRCA synthetic lethality: Clearing up the misunderstandings. Mol. Oncol. 2011, 5, 387–393. [Google Scholar] [CrossRef]
- Post, C.C.B.; Westermann, A.M.; Bosse, T.; Creutzberg, C.L.; Kroep, J.R. PARP and PD-1/PD-L1 checkpoint inhibition in recurrent or metastatic endometrial cancer. Crit. Rev. Oncol. Hematol. 2020, 152, 102973. [Google Scholar] [CrossRef]
- Bhamidipati, D.; Haro-Silerio, J.I.; Yap, T.A.; Ngoi, N. PARP inhibitors: Enhancing efficacy through rational combinations. Br. J. Cancer 2023, 129, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, T.; Cai, X.; Dong, J.; Xia, C.; Zhou, Y.; Ding, R.; Yang, R.; Tan, J.; Zhang, L.; et al. Neoadjuvant Immunotherapy for MSI-H/dMMR Locally Advanced Colorectal Cancer: New Strategies and Unveiled Opportunities. Front. Immunol. 2022, 13, 795972. [Google Scholar] [CrossRef] [PubMed]
- How, J.A.; Jazaeri, A.A.; Westin, S.N.; Lawson, B.C.; Klopp, A.H.; Soliman, P.T.; Lu, K.H. Translating biological insights into improved management of endometrial cancer. Nat. Rev. Clin. Oncol. 2024, 21, 781–800. [Google Scholar] [CrossRef] [PubMed]
- Liwei, L.; He, L.; Yibo, D.; Luyang, Z.; Zhihui, S.; Nan, K.; Danhua, S.; Junzhu, W.; Zhiqi, W.; Jianliu, W. Re-stratification of patients with copy-number low endometrial cancer by clinicopathological characteristics. World J. Surg. Oncol. 2023, 21, 332. [Google Scholar] [CrossRef]
- Bonazzoli, E.; Cocco, E.; Lopez, S.; Bellone, S.; Zammataro, L.; Bianchi, A.; Manzano, A.; Yadav, G.; Manara, P.; Perrone, E.; et al. PI3K oncogenic mutations mediate resistance to afatinib in HER2/neu overexpressing gynecological cancers. Gynecol. Oncol. 2019, 153, 158–164. [Google Scholar] [CrossRef]
- Hoang, L.N.; Lee, Y.S.; Karnezis, A.N.; Tessier-Cloutier, B.; Almandani, N.; Coatham, M.; Gilks, C.B.; Soslow, R.A.; Stewart, C.J.; Köbel, M.; et al. Immunophenotypic features of dedifferentiated endometrial carcinoma—Insights from BRG1/INI1-deficient tumours. Histopathology 2016, 69, 560–569. [Google Scholar] [CrossRef]
- Groenendijk, F.H.; Bernards, R. Drug resistance to targeted therapies: Déjà vu all over again. Mol. Oncol. 2014, 8, 1067–1083. [Google Scholar] [CrossRef]
- Sharma, S.; George, P.; Waddell, N. Precision diagnostics: Integration of tissue pathology and genomics in cancer. Pathology 2021, 53, 809–817. [Google Scholar] [CrossRef]
- Murchan, P.; Ó’Brien, C.; O’Connell, S.; McNevin, C.S.; Baird, A.M.; Sheils, O.; Pilib, Ó.B.; Finn, S.P. Deep Learning of Histopathological Features for the Prediction of Tumour Molecular Genetics. Diagnostics 2021, 11, 1406. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Jooya, H.; Ghorbanian, K.; Gohari, S.; Dadashpour, M. Potentials and future perspectives of multi-target drugs in cancer treatment: The next generation anti-cancer agents. Cell Commun. Signal. 2024, 22, 228. [Google Scholar] [CrossRef] [PubMed]
- Noor, J.; Chaudhry, A.; Noor, R.; Batool, S. Advancements and Applications of Liquid Biopsies in Oncology: A Narrative Review. Cureus 2023, 15, e42731. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Abedalthagafi, M. Cancer diagnostics: The journey from histomorphology to molecular profiling. Oncotarget 2016, 7, 58696–58708. [Google Scholar] [CrossRef] [PubMed]
- Gaia, G.; Holloway, R.W.; Santoro, L.; Ahmad, S.; Di Silverio, E.; Spinillo, A. Robotic-assisted hysterectomy for endometrial cancer compared with traditional laparoscopic and laparotomy approaches: A systematic review. Obstet. Gynecol. 2010, 116, 1422–1431. [Google Scholar] [CrossRef]
- Galaal, K.; Donkers, H.; Bryant, A.; Lopes, A.D. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst. Rev. 2018, 10, Cd006655. [Google Scholar] [CrossRef]
- Janda, M.; Gebski, V.; Brand, A.; Hogg, R.; Jobling, T.W.; Land, R.; Manolitsas, T.; McCartney, A.; Nascimento, M.; Neesham, D.; et al. Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): A randomised trial. Lancet. Oncol. 2010, 11, 772–780. [Google Scholar] [CrossRef]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schlaerth, J.B.; Mannel, R.S.; Spiegel, G.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J. Clin. Oncol. 2009, 27, 5331–5336. [Google Scholar] [CrossRef]
- Creasman, W.T.; Morrow, C.P.; Bundy, B.N.; Homesley, H.D.; Graham, J.E.; Heller, P.B. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987, 60, 2035–2041. [Google Scholar] [CrossRef]
- Todo, Y.; Kato, H.; Kaneuchi, M.; Watari, H.; Takeda, M.; Sakuragi, N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): A retrospective cohort analysis. Lancet 2010, 375, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.R.; Chi, D.S.; Leitao, M.; Oke, E.A.; Hensley, M.L.; Alektiar, K.M.; Barakat, R.R. What is the incidence of isolated paraaortic nodal recurrence in grade 1 endometrial carcinoma? Gynecol. Oncol. 2008, 111, 46–48. [Google Scholar] [CrossRef]
- Kitchener, H.; Swart, A.M.; Qian, Q.; Amos, C.; Parmar, M.K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar] [CrossRef]
- Benedetti Panici, P.; Basile, S.; Maneschi, F.; Alberto Lissoni, A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J. Natl. Cancer Inst. 2008, 100, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Redondo, A.; Guerra, E.; Manso, L.; Martin-Lorente, C.; Martinez-Garcia, J.; Perez-Fidalgo, J.A.; Varela, M.Q.; Rubio, M.J.; Barretina-Ginesta, M.P.; Gonzalez-Martin, A. SEOM clinical guideline in ovarian cancer (2020). Clin. Transl. Oncol. 2021, 23, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Iwagoi, Y.; Motohara, T.; Hwang, S.; Fujimoto, K.; Ikeda, T.; Katabuchi, H. Omental metastasis as a predictive risk factor for unfavorable prognosis in patients with stage III-IV epithelial ovarian cancer. Int. J. Clin. Oncol. 2021, 26, 995–1004. [Google Scholar] [CrossRef]
- Ben Arie, A.; McNally, L.; Kapp, D.S.; Teng, N.N. The omentum and omentectomy in epithelial ovarian cancer: A reappraisal. Part I--Omental function and history of omentectomy. Gynecol. Oncol. 2013, 131, 780–783. [Google Scholar] [CrossRef]
- Bilbao, M.; Aikins, J.K.; Ostrovsky, O. Is routine omentectomy of grossly normal omentum helpful in surgery for ovarian cancer? A look at the tumor microenvironment and its clinical implications. Gynecol. Oncol. 2021, 161, 78–82. [Google Scholar] [CrossRef]
- Albright, B.B.; Monuszko, K.A.; Kaplan, S.J.; Davidson, B.A.; Moss, H.A.; Huang, A.B.; Melamed, A.; Wright, J.D.; Havrilesky, L.J.; Previs, R.A. Primary cytoreductive surgery for advanced stage endometrial cancer: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021, 225, e231–e237. [Google Scholar] [CrossRef]
- Elattar, A.; Bryant, A.; Winter-Roach, B.A.; Hatem, M.; Naik, R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst. Rev. 2011, 2011, Cd007565. [Google Scholar] [CrossRef]
- Rajkumar, S.; Nath, R.; Lane, G.; Mehra, G.; Begum, S.; Sayasneh, A. Advanced stage (IIIC/IV) endometrial cancer: Role of cytoreduction and determinants of survival. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Lambrou, N.C.; Gómez-Marín, O.; Mirhashemi, R.; Beach, H.; Salom, E.; Almeida-Parra, Z.; Peñalver, M. Optimal surgical cytoreduction in patients with Stage III and Stage IV endometrial carcinoma: A study of morbidity and survival. Gynecol. Oncol. 2004, 93, 653–658. [Google Scholar] [CrossRef]
- Argenta, P.A.; Mattson, J.; Rivard, C.L.; Luther, E.; Schefter, A.; Vogel, R.I. Robot-assisted versus laparoscopic minimally invasive surgery for the treatment of stage I endometrial cancer. Gynecol. Oncol. 2022, 165, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, A. Minimally invasive surgery for endometrial cancer. Curr. Opin. Obstet. Gynecol. 2015, 27, 302–307. [Google Scholar] [CrossRef]
- Uwins, C.; Patel, H.; Prakash Bhandoria, G.; Butler-Manuel, S.; Tailor, A.; Ellis, P.; Chatterjee, J. Laparoscopic and Robotic Surgery for Endometrial and Cervical Cancer. Clin. Oncol. 2021, 33, e372–e382. [Google Scholar] [CrossRef]
- Kim, N.R.; Lee, A.J.; Yang, E.J.; So, K.A.; Lee, S.J.; Kim, T.J.; Shim, S.H. Minimally invasive surgery versus open surgery in high-risk histologic endometrial cancer patients: A meta-analysis. Gynecol. Oncol. 2022, 166, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Rodolakis, A.; Scambia, G.; Planchamp, F.; Acien, M.; Di Spiezio Sardo, A.; Farrugia, M.; Grynberg, M.; Pakiz, M.; Pavlakis, K.; Vermeulen, N. ESGO/ESHRE/ESGE Guidelines for the fertility-sparing treatment of patients with endometrial carcinoma. Hum. Reprod. Open 2023, 2023, hoac057. [Google Scholar] [CrossRef] [PubMed]
- Terzic, M.; Norton, M.; Terzic, S.; Bapayeva, G.; Aimagambetova, G. Fertility preservation in endometrial cancer patients: Options, challenges and perspectives. Ecancermedicalscience 2020, 14, 1030. [Google Scholar] [CrossRef]
- de Bree, E.; Michelakis, D.; Anagnostopoulou, E. The current role of secondary cytoreductive surgery for recurrent ovarian cancer. Front. Oncol. 2022, 12, 1029976. [Google Scholar] [CrossRef]


| PDEEC Subtypes | Description | Reference |
|---|---|---|
| High-grade endometrioid carcinoma | Displays solid growth with focal glandular differentiation. Tumor cells exhibit marked nuclear pleomorphism, frequent mitotic figures, and areas of necrosis. Distinguished from low-grade endometrioid carcinoma by the absence of well-formed glands and the presence of diffuse atypia. | [2] |
| Serous carcinoma | Composed of highly pleomorphic cells arranged in papillary, micropapillary, or solid patterns. Characterized by prominent nucleoli, high mitotic index, and frequent psammoma bodies. Often associated with TP53 mutations and extensive lymphovascular invasion. | [15] |
| Clear cell carcinoma | Features polygonal or hobnail cells with clear or eosinophilic cytoplasm. Growth patterns include solid, papillary, and tubulocystic structures. Tumor cells frequently express Napsin A and HNF1β. | [12] |
| Undifferentiated carcinoma | Composed of sheets of highly atypical cells lacking glandular differentiation. Frequent loss of epithelial markers such as E-cadherin, leading to growth as a result of the loss of cohesion. Often diagnosed in association with dedifferentiated carcinoma. | [7] |
| Dedifferentiated carcinoma | Contains a biphasic pattern with a high-grade endometrioid component adjacent to an undifferentiated carcinoma. Demonstrates loss of clonal differentiation markers, such as SWI/SNF complex proteins (ARID1A, SMARCA4). | [9] |
| Mutation Type | Examples | Mechanism | Functional Impact |
|---|---|---|---|
| Missense mutations (hotspot mutations) | R130G/Q, C124S, H123Y, G129E | Disrupts the phosphatase domain, impairing enzymatic activity | Partial loss of function, protein may still be detectable on IHC |
| Frameshift/Nonsense mutations (truncating mutations) | R233*, Y68fs, R335* | Introduces premature stop codons leading to nonsense-mediated decay | Complete loss of PTEN expression, strong PI3K/AKT activation |
| Large deletions/Copy number loss | LOH at 10q23 | Entire gene or large regions deleted | Total absence of PTEN protein, highly aggressive tumor phenotype |
| Component | Activated by PI3K Mutation | Pro-Tumorigenic Effects | References |
|---|---|---|---|
| AKT (protein kinase B) | Phosphorylated at T308 (by PDK1) and S473 (by mTORC2) | Enhances cell survival, proliferation, metabolism | [65,67] |
| mTOR (mechanistic target of rapamycin) | Activated downstream of AKT | Promotes protein synthesis, cell growth, metabolic reprogramming | [10,63] |
| FOXO transcription factors | Inhibited by AKT phosphorylation | Prevents apoptosis, supports immune evasion | [7,65] |
| BAD (pro-apoptotic protein) | Inactivated by AKT phosphorylation | Suppresses apoptosis, enhances chemoresistance | [64,67] |
| GSK3β (glycogen synthase kinase 3 beta) | Inhibited by AKT phosphorylation | Deregulates β-catenin signaling, promotes metastasis | [64,69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molefi, T.; Mabonga, L.; Hull, R.; Mwazha, A.; Sebitloane, M.; Dlamini, Z. The Histomorphology to Molecular Transition: Exploring the Genomic Landscape of Poorly Differentiated Epithelial Endometrial Cancers. Cells 2025, 14, 382. https://doi.org/10.3390/cells14050382
Molefi T, Mabonga L, Hull R, Mwazha A, Sebitloane M, Dlamini Z. The Histomorphology to Molecular Transition: Exploring the Genomic Landscape of Poorly Differentiated Epithelial Endometrial Cancers. Cells. 2025; 14(5):382. https://doi.org/10.3390/cells14050382
Chicago/Turabian StyleMolefi, Thulo, Lloyd Mabonga, Rodney Hull, Absalom Mwazha, Motshedisi Sebitloane, and Zodwa Dlamini. 2025. "The Histomorphology to Molecular Transition: Exploring the Genomic Landscape of Poorly Differentiated Epithelial Endometrial Cancers" Cells 14, no. 5: 382. https://doi.org/10.3390/cells14050382
APA StyleMolefi, T., Mabonga, L., Hull, R., Mwazha, A., Sebitloane, M., & Dlamini, Z. (2025). The Histomorphology to Molecular Transition: Exploring the Genomic Landscape of Poorly Differentiated Epithelial Endometrial Cancers. Cells, 14(5), 382. https://doi.org/10.3390/cells14050382







