Spheroids Composed of Reaggregated Neonatal Porcine Islets and Human Endothelial Cells Accelerate Development of Normoglycemia in Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation and Validation of Cord Blood-Derived Blood Outgrowth Endothelial Cells (BOECs)
2.3. Islet Isolation and Generation of Spheroids Consisted of REPI-BOEC Cells
2.4. Cluster Composition and Cell Viability
2.5. Static Glucose-Stimulated Insulin Secretion
2.6. Quantitative Reverse Transcription Polymerase Chain Reaction
2.7. Transplantation
2.8. Immunohistochemistry
2.9. Flow Cytometry
2.10. Statistical Analysis
3. Results
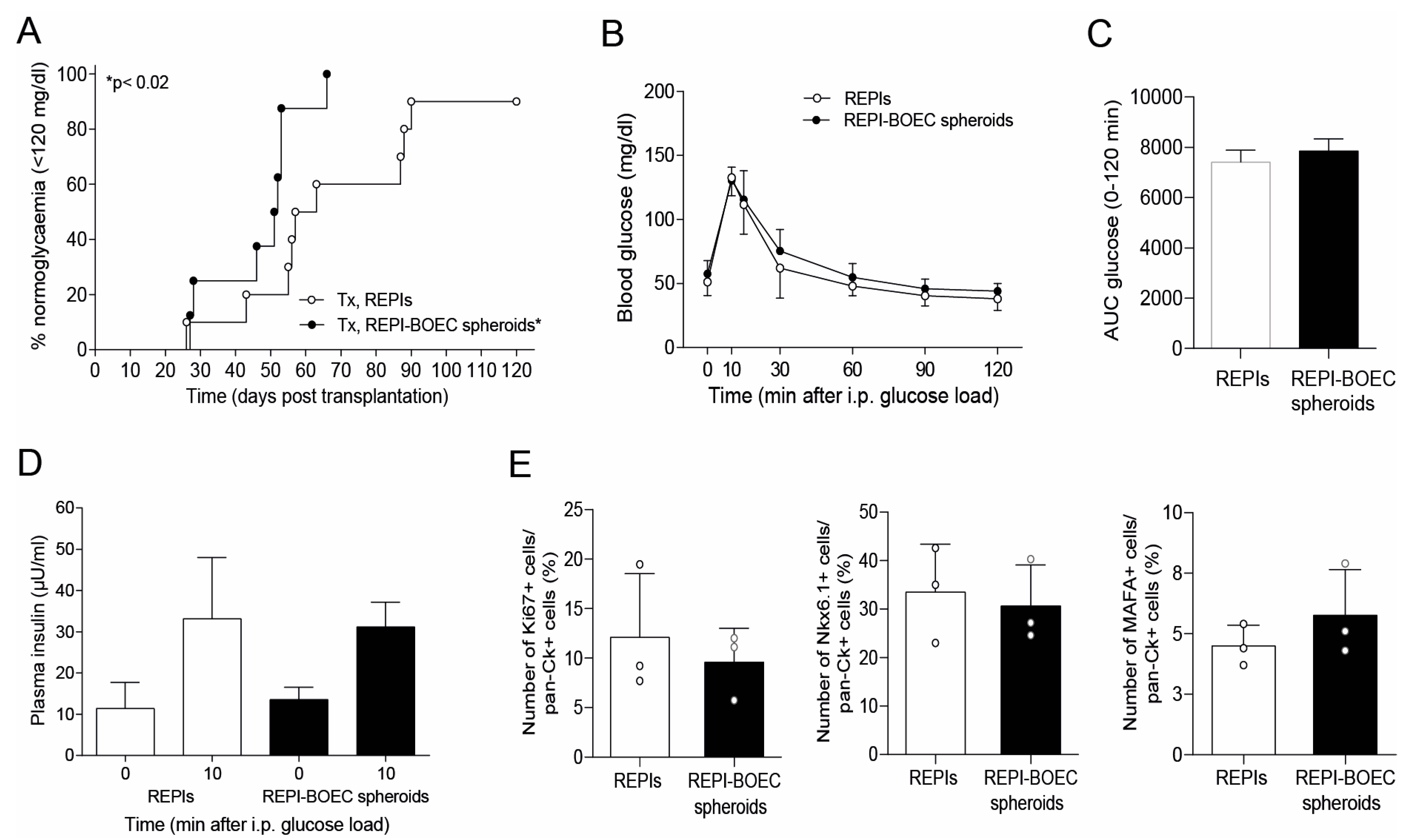
3.1. Formation and Characterization of REPI-BOEC Spheroids Before and After Transplantation
3.2. BOECs Accelerate the Achievement of Full Graft Function
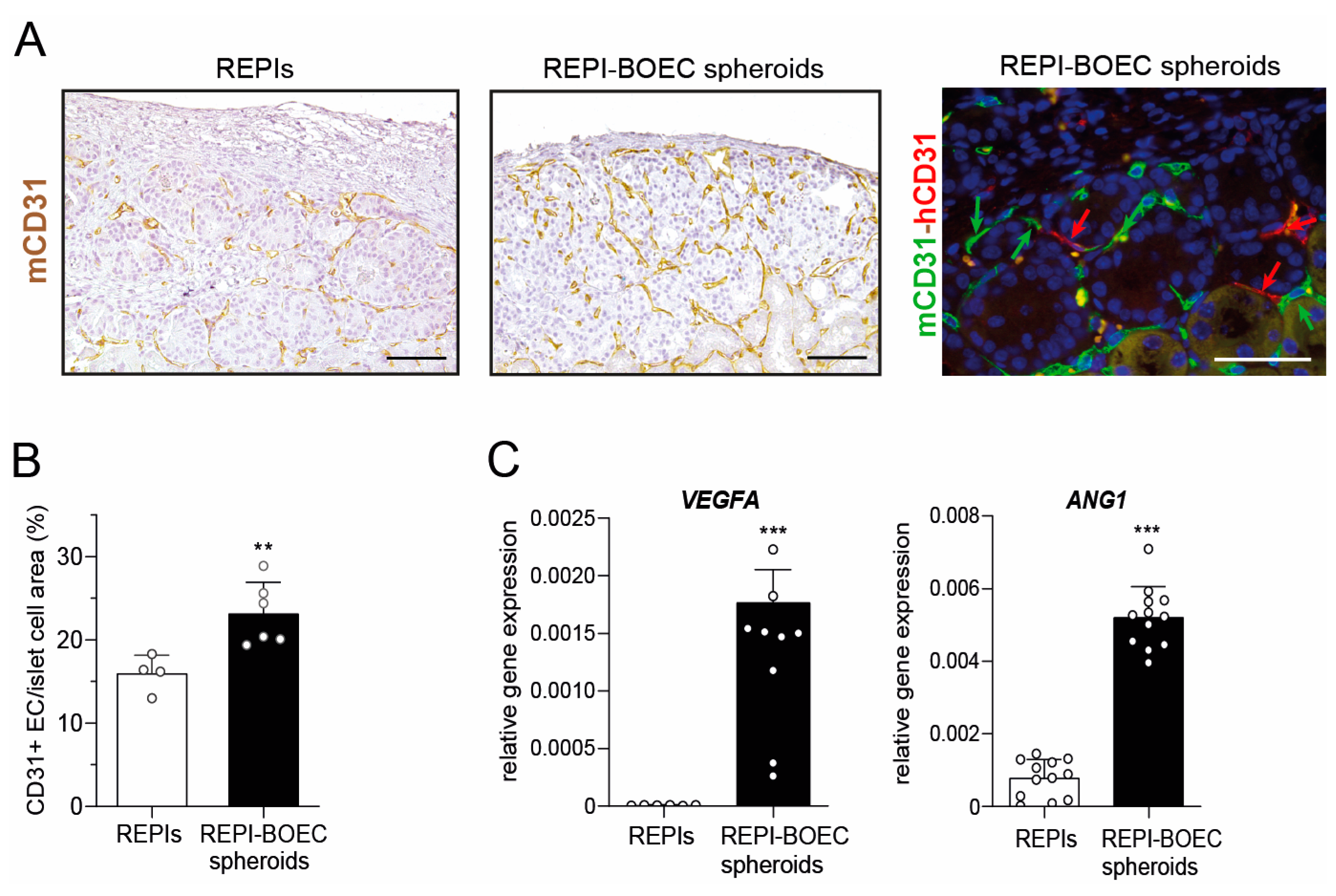
3.3. BOECs Increase Intragraft Vascular Density After Transplantation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Ekaterine Berishvili, Laura Mar Fonseca, Fanny Lebreton, Kevin Bellofatto, Juliette Bignard, University of Geneva, Department of Surgery, Geneva, Switzerland
- Antonia Follenzi, Cristina Olgasi, Alessia Cucci, Chiara Borsotti, Simone Assanelli, Department of Health Sciences, University of Piemonte Orientale, Novara, Italy
- Lorenzo Piemonti, Antonio Citro, Francesco Campo, Cataldo Pignatelli, IRCCS Ospedale San Raffaele, Diabetes Research Institute, Milano, Italy
- Lorenzo Piemonti, Francesco Campo, Università Vita-Salute San Raffaele, Milan, Italy.
- Olivier Thaunat, Lyon Claude Bernard University, Department Transplantation, Nephrology and Clinical Immunology, Lyon, France
- Emma Massey, Dide de Jongh, Erasmus MC Transplant Institute, Department of Internal Medicine, University Medical Centre Rotterdam, The Netherlands
- Eline Bunnik, Department of Medical Ethics, Philosophy and History of Medicine, University Medical Centre Rotterdam, The Netherlands
- Antonia J. Cronin, King’s College, London, UK
- Devi Mey, Chiara Parisotto, Michelle De Guia, European Society for Organ Transplantation, Padova, Italy
- Patrick Kugelmeier, Petra Wolint, Markus Mühlemann, Karolina Pal-Kutas, Kugelmeiers AG, Erlenbach, Switzerland
References
- Chetboun, M.; Drumez, E.; Ballou, C.; Maanaoui, M.; Payne, E.; Barton, F.; Kerr-Conte, J.; Vantyghem, M.C.; Piemonti, L.; Rickels, M.R.; et al. Association between primary graft function and 5-year outcomes of islet allogeneic transplantation in type 1 diabetes: A retrospective, multicentre, observational cohort study in 1210 patients from the Collaborative Islet Transplant Registry. Lancet Diabetes Endocrinol. 2023, 11, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Vantyghem, M.C.; de Koning, E.J.P.; Pattou, F.; Rickels, M.R. Advances in β-cell replacement therapy for the treatment of type 1 diabetes. Lancet 2019, 394, 1274–1285. [Google Scholar] [CrossRef]
- Lehmann, R.; Zuellig, R.A.; Kugelmeier, P.; Baenninger, P.B.; Moritz, W.; Perren, A.; Clavien, P.A.; Weber, M.; Spinas, G.A. Superiority of small islets in human islet transplantation. Diabetes 2007, 56, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Zuellig, R.A.; Cavallari, G.; Gerber, P.; Tschopp, O.; Spinas, G.A.; Moritz, W.; Lehmann, R. Improved physiological properties of gravity-enforced reassembled rat and human pancreatic pseudo-islets. J. Tissue Eng. Reg. Med. 2017, 11, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Cook, C.; Wang, C.H.; Medrano, L.; Lin, H.; Kandeel, F.; Tai, Y.C.; Mullen, Y. Oxygen environment and islet size are the primary limiting factors of isolated pancreatic islet survival. PLoS ONE 2017, 12, e0183780. [Google Scholar] [CrossRef]
- Honarpisheh, M.; Lei, Y.; Zhang, Y.; Pehl, M.; Kemter, E.; Kraetzl, M.; Lange, A.; Wolf, E.; Wolf-van Buerck, L.; Seissler, J. Formation of re-aggregated neonatal porcine islet clusters improves in vitro function and transplantation outcome. Transpl. Int. 2022, 35, 10697. [Google Scholar] [CrossRef]
- Chen, C.C.; Pouliquen, E.; Broisat, A.; Andreata, F.; Racapé, M.; Bruneval, P.; Kessler, L.; Ahmadi, M.; Bacot, S.; Saison-Delaplace, C.; et al. Endothelial chimerism and vascular sequestration protect pancreatic islet grafts from antibody-mediated rejection. J. Clin. Investig. 2018, 128, 219–232. [Google Scholar] [CrossRef]
- Jia, J.; Ma, B.; Wang, S.; Feng, L. Therapeutic potential of endothelial colony forming cells derived from human umbilical cord blood. Curr. Stem Cell Res. Ther. 2019, 14, 460–465. [Google Scholar] [CrossRef]
- Olgasi, C.; Borsotti, C.; Merlin, S.; Bergmann, T.; Bittorf, P.; Adewoye, A.B.; Wragg, N.; Patterson, K.; Calabria, A.; Benedicenti, F.; et al. Efficient and safe correction of hemophilia A by lentiviral vector-transduced BOECs in an implantable device. Mol. Ther. Meth. Clin. Dev. 2021, 23, 551–566. [Google Scholar] [CrossRef]
- Grapensparr, L.; Christoffersson, G.; Carlsson, P.O. Bioengineering with endothelial progenitor cells improves the vascular engraftment of transplanted human islets. Cell Transplant. 2018, 27, 948–956. [Google Scholar] [CrossRef]
- Coppens, V.; Heremans, Y.; Leuckx, G.; Suenens, K.; Jacobs-Tulleneers-Thevissen, D.; Verdonck, K.; Luttun, A.; Heimberg, H.; De Leu, N. Reversal of hyperglycemia in diabetic mice by a marginal islet mass together with human blood outgrowth endothelial cells is independent of the delivery technique and blood clot-induced processes. Islets 2013, 5, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.J.; Jin, S.M.; Choi, J.M.; Oh, S.H.; Shim, W.; Lee, M.S.; Lee, M.K.; Kim, J.H. Improved revascularization of islet grafts using an angiogenic monocyte subpopulation derived from spheroid culture of bone marrow mononuclear cells. Am. J. Transplant. 2015, 15, 1543–1554. [Google Scholar] [CrossRef]
- Quaranta, P.; Antonini, S.; Spiga, S.; Mazzanti, B.; Curcio, M.; Mulas, G.; Diana, M.; Marzola, P.; Mosca, F.; Longoni, B. Co-transplantation of endothelial progenitor cells and pancreatic islets to induce long-lasting normoglycemia in streptozotocin-treated diabetic rats. PLoS ONE 2014, 9, e94783. [Google Scholar] [CrossRef] [PubMed]
- Rambøl, M.H.; Han, E.; Niklason, L.E. Microvessel network formation and interactions with pancreatic islets in three-dimensional chip cultures. Tissue Eng. Part A 2020, 26, 556–568. [Google Scholar] [CrossRef]
- Wassmer, C.H.; Lebreton, F.; Bellofatto, K.; Perez, L.; Cottet-Dumoulin, D.; Andres, A.; Bosco, D.; Berney, T.; Othenin-Girard, V.; Martinez De Tejada, B.; et al. Bio-engineering of pre-vascularized islet organoids for the treatment of Type 1 diabetes. Transpl. Int. 2021, 35, 10214. [Google Scholar] [CrossRef] [PubMed]
- Coppens, V.; Heremans, Y.; Leuckx, G.; Suenens, K.; Jacobs-Tulleneers-Thevissen, D.; Verdonck, K.; Lahoutte, T.; Luttun, A.; Heimberg, H.; De Leu, N. Human blood outgrowth endothelial cells improve islet survival and function when co-transplanted in a mouse model of diabetes. Diabetologia 2013, 56, 382–390. [Google Scholar] [CrossRef]
- Kang, S.; Park, H.S.; Jo, A.; Hong, S.H.; Lee, H.N.; Lee, Y.Y.; Park, J.S.; Jung, H.S.; Chung, S.S.; Park, K.S. Endothelial progenitor cell cotransplantation enhances islet engraftment by rapid revascularization. Diabetes 2012, 61, 866–876. [Google Scholar] [CrossRef]
- Emamaullee, J.A.; Shapiro, A.M.; Rajotte, R.V.; Korbutt, G.; Elliott, J.F. Neonatal porcine islets exhibit natural resistance to hypoxia-induced apoptosis. Transplantation 2006, 82, 945–952. [Google Scholar] [CrossRef]
- Wolf-van Buerck, L.; Schuster, M.; Baehr, A.; Mayr, T.; Guethoff, S.; Abicht, J.; Reichart, B.; Nam-Apostolopoulos, Y.C.; Klymiuk, N.; Wolf, E.; et al. Engraftment and reversal of diabetes after intramuscular transplantation of neonatal porcine islet-like clusters. Xenotransplantation 2015, 22, 443–450. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.; Honarpisheh, M.; Kemter, E.; Wolf, E.; Seissler, J. Butyrate and class I histone deacetylase inhibitors promote differentiation of neonatal porcine islet cells into beta cells. Cells 2021, 10, 3249. [Google Scholar] [CrossRef]
- Öllinger, R.; Pratschke, J. Role of heme oxygenase-1 in transplantation. Transpl. Int. 2010, 23, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Vivot, K.; Langlois, A.; Bietiger, W.; Dal, S.; Seyfritz, E.; Pinget, M.; Jeandidier, N.; Maillard, E.; Gies, J.P.; Sigrist, S. Pro-inflammatory and pro-oxidant status of pancreatic islet in vitro is controlled by TLR-4 and HO-1 pathways. PLoS ONE 2014, 9, e107656. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, W.; Atkinson, S.D.; Kelly, C. A20 controls expression of beta-cell regulatory genes and transcription factors. J. Mol. Endocrinol. 2021, 67, 189–201. [Google Scholar] [CrossRef]
- Zammit, N.W.; Walters, S.N.; Seeberger, K.L.; O’Connell, P.J.; Korbutt, G.S.; Grey, S.T. A20 as an immune tolerance factor can determine islet transplant outcomes. JCI Insight 2019, 4, e131028. [Google Scholar] [CrossRef] [PubMed]
- Vaithilingam, V.; Sundaram, G.; Tuch, B.E. Islet cell transplantation. Curr. Opin. Organ Transpl. 2008, 13, 633–638. [Google Scholar] [CrossRef]
- Brissova, M.; Shostak, A.; Shiota, M.; Wiebe, P.O.; Poffenberger, G.; Kantz, J.; Chen, Z.; Carr, C.; Jerome, W.G.; Chen, J.; et al. Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function. Diabetes 2006, 55, 2974–2985. [Google Scholar] [CrossRef]
- Xiong, Y.; Scerbo, M.J.; Seelig, A.; Volta, F.; O’Brien, N.; Dicker, A.; Padula, D.; Lickert, H.; Gerdes, J.M.; Berggren, P.O. Islet vascularization is regulated by primary endothelial cilia via VEGF-A-dependent signaling. eLife 2020, 9, e56914. [Google Scholar] [CrossRef] [PubMed]
- Brissova, M.; Aamodt, K.; Brahmachary, P.; Prasad, N.; Hong, J.Y.; Dai, C.; Mellati, M.; Shostak, A.; Poffenberger, G.; Aramandla, R.; et al. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration. Cell Metab. 2014, 19, 498–511. [Google Scholar] [CrossRef]
- Schuh, A.; Liehn, E.A.; Sasse, A.; Hristov, M.; Sobota, R.; Kelm, M.; Merx, M.W.; Weber, C. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic Res. Cardiol. 2008, 103, 69–77. [Google Scholar] [CrossRef]
- Finney, M.R.; Greco, N.J.; Haynesworth, S.E.; Martin, J.M.; Hedrick, D.P.; Swan, J.Z.; Winter, D.G.; Kadereit, S.; Joseph, M.E.; Fu, P.; et al. Direct comparison of umbilical cord blood versus bone marrow-derived endothelial precursor cells in mediating neovascularization in response to vascular ischemia. Biol. Blood Marrow Transpl. 2006, 12, 585–593. [Google Scholar] [CrossRef]
- Cantley, J.; Grey, S.T.; Maxwell, P.H.; Withers, D.J. The hypoxia response pathway and β-cell function. Diabetes Obes. Metabol. 2010, 12 (Suppl. 2), 159–167. [Google Scholar] [CrossRef] [PubMed]
- Nalbach, L.; Roma, L.P.; Schmitt, B.M.; Becker, V.; Körbel, C.; Wrublewsky, S.; Pack, M.; Später, T.; Metzger, W.; Menger, M.M.; et al. Improvement of islet transplantation by the fusion of islet cells with functional blood vessels. EMBO Mol. Med. 2021, 13, e12616. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, K.; Barrera, Y.B.; Groth, T.; Stamatialis, D. Endothelial and beta cell composite aggregates for improved function of a bioartificial pancreas encapsulation device. Int. J. Artif. Organs 2018, 41, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.H.; Hur, J.; Park, K.W.; Kim, J.H.; Lee, C.S.; Oh, I.Y.; Kim, T.Y.; Cho, H.J.; Kang, H.J.; Chae, I.H.; et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation 2005, 112, 1618–1627. [Google Scholar] [CrossRef]
- Su, D.; Zhang, N.; He, J.; Qu, S.; Slusher, S.; Bottino, R.; Bertera, S.; Bromberg, J.; Dong, H.H. Angiopoietin-1 production in islets improves islet engraftment and protects islets from cytokine-induced apoptosis. Diabetes 2007, 56, 2274–2283. [Google Scholar] [CrossRef]
- Lammert, E.; Gu, G.; McLaughlin, M.; Brown, D.; Brekken, R.; Murtaugh, L.C.; Gerber, H.P.; Ferrara, N.; Melton, D.A. Role of VEGF-A in vascularization of pancreatic islets. Curr. Biol. 2003, 13, 1070–1074. [Google Scholar] [CrossRef]
- Hajizadeh-Saffar, E.; Tahamtani, Y.; Aghdami, N.; Azadmanesh, K.; Habibi-Anbouhi, M.; Heremans, Y.; De Leu, N.; Heimberg, H.; Ravassard, P.; Shokrgozar, M.A.; et al. Inducible VEGF expression by human embryonic stem cell-derived mesenchymal stromal cells reduces the minimal islet mass required to reverse diabetes. Sci. Rep. 2015, 5, 9322. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honarpisheh, M.; Lei, Y.; Follenzi, A.; Cucci, A.; Olgasi, C.; Berishvili, E.; Lebreton, F.; Bellofatto, K.; Piemonti, L.; Citro, A.; et al. Spheroids Composed of Reaggregated Neonatal Porcine Islets and Human Endothelial Cells Accelerate Development of Normoglycemia in Diabetic Mice. Cells 2025, 14, 366. https://doi.org/10.3390/cells14050366
Honarpisheh M, Lei Y, Follenzi A, Cucci A, Olgasi C, Berishvili E, Lebreton F, Bellofatto K, Piemonti L, Citro A, et al. Spheroids Composed of Reaggregated Neonatal Porcine Islets and Human Endothelial Cells Accelerate Development of Normoglycemia in Diabetic Mice. Cells. 2025; 14(5):366. https://doi.org/10.3390/cells14050366
Chicago/Turabian StyleHonarpisheh, Mohsen, Yutian Lei, Antonia Follenzi, Alessia Cucci, Cristina Olgasi, Ekaterine Berishvili, Fanny Lebreton, Kevin Bellofatto, Lorenzo Piemonti, Antonio Citro, and et al. 2025. "Spheroids Composed of Reaggregated Neonatal Porcine Islets and Human Endothelial Cells Accelerate Development of Normoglycemia in Diabetic Mice" Cells 14, no. 5: 366. https://doi.org/10.3390/cells14050366
APA StyleHonarpisheh, M., Lei, Y., Follenzi, A., Cucci, A., Olgasi, C., Berishvili, E., Lebreton, F., Bellofatto, K., Piemonti, L., Citro, A., Campo, F., Pignatelli, C., Thaunat, O., Kemter, E., Kraetzl, M., Wolf, E., Seissler, J., Wolf-van Buerck, L., & VANGUARD Consortium. (2025). Spheroids Composed of Reaggregated Neonatal Porcine Islets and Human Endothelial Cells Accelerate Development of Normoglycemia in Diabetic Mice. Cells, 14(5), 366. https://doi.org/10.3390/cells14050366







