Sex Disparities in P53 Regulation and Functions: Novel Insights for Personalized Cancer Therapies
Abstract
1. Introduction
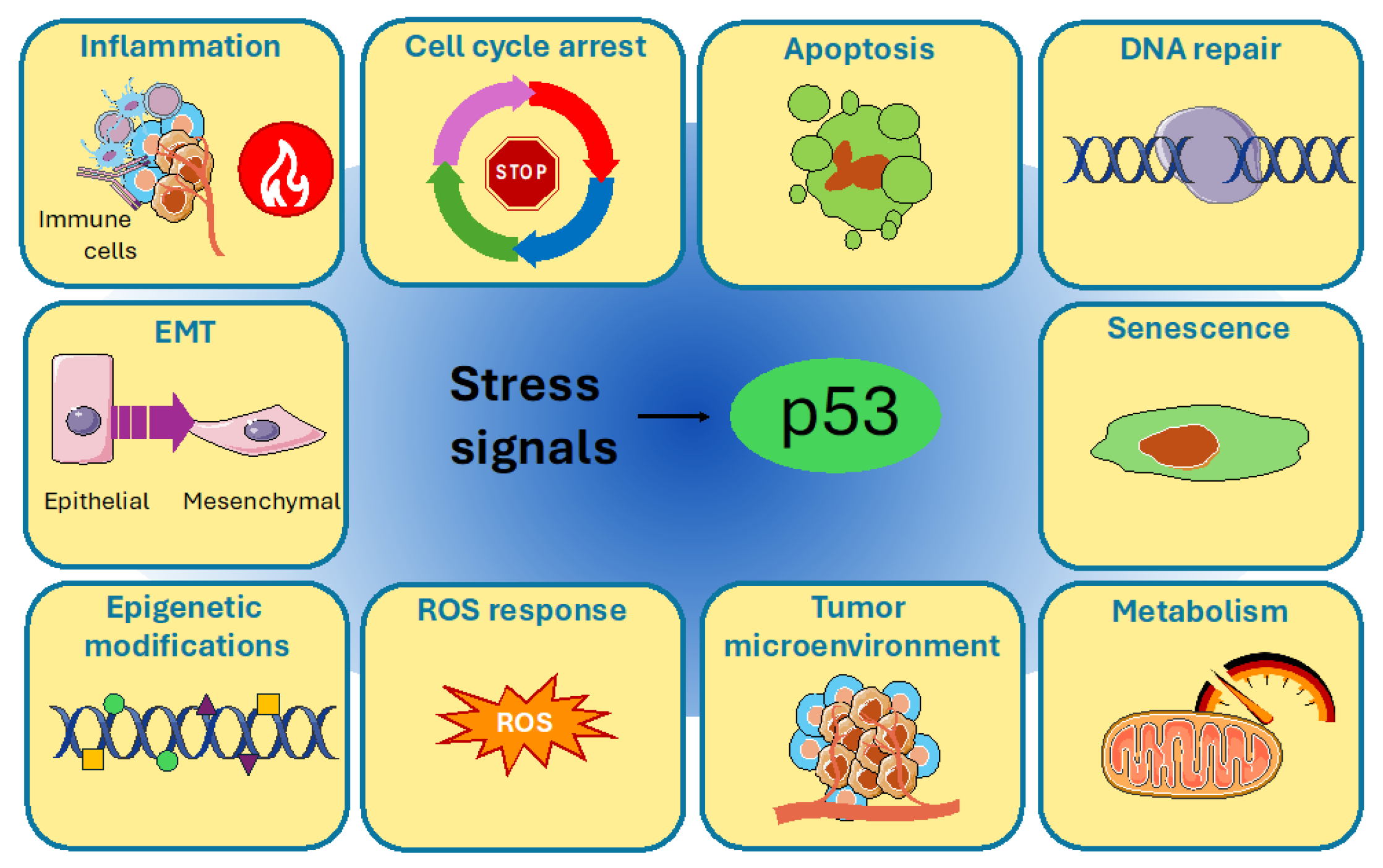
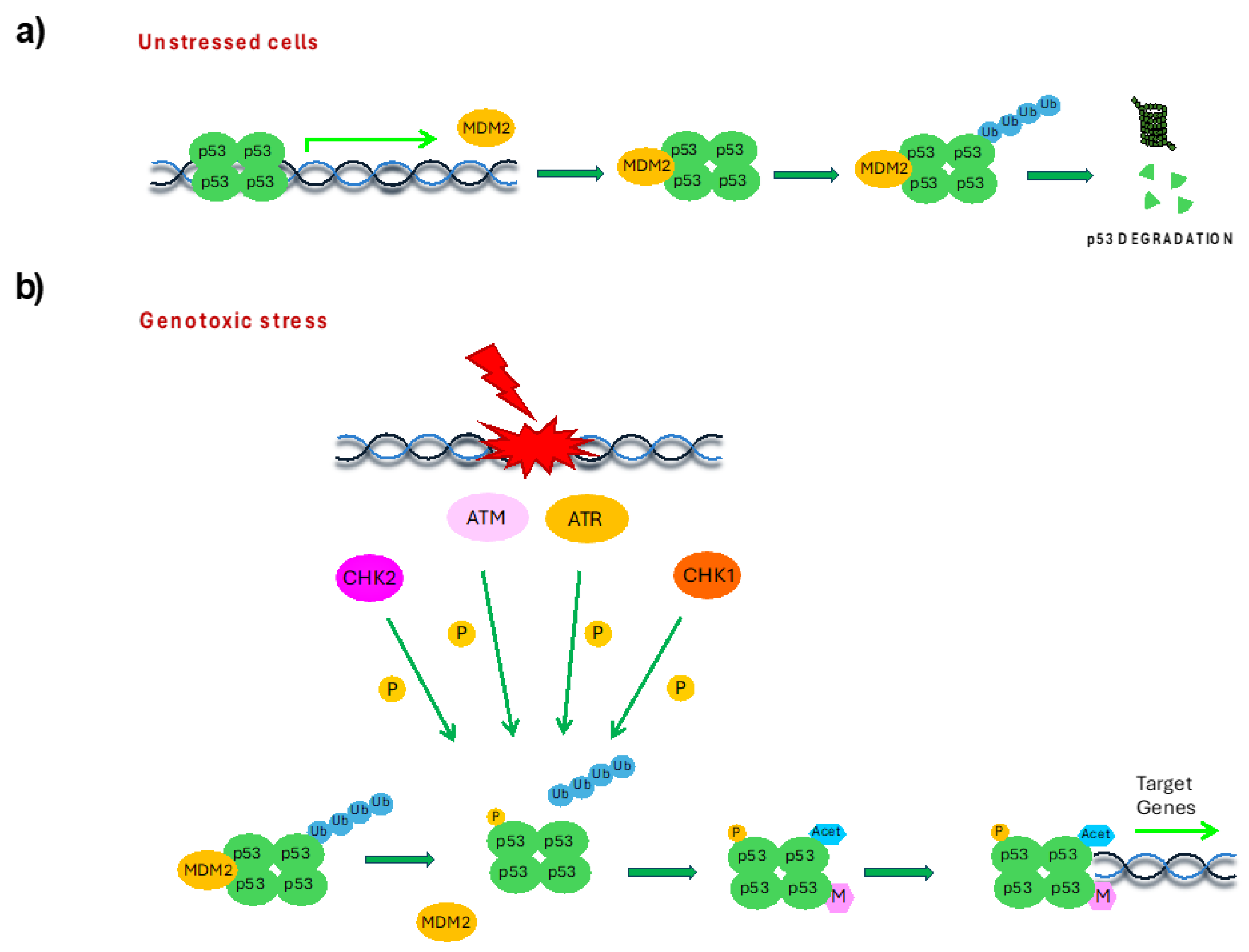
2. p53 Protein Structure and Regulation
3. Sex Disparities in p53 Regulation
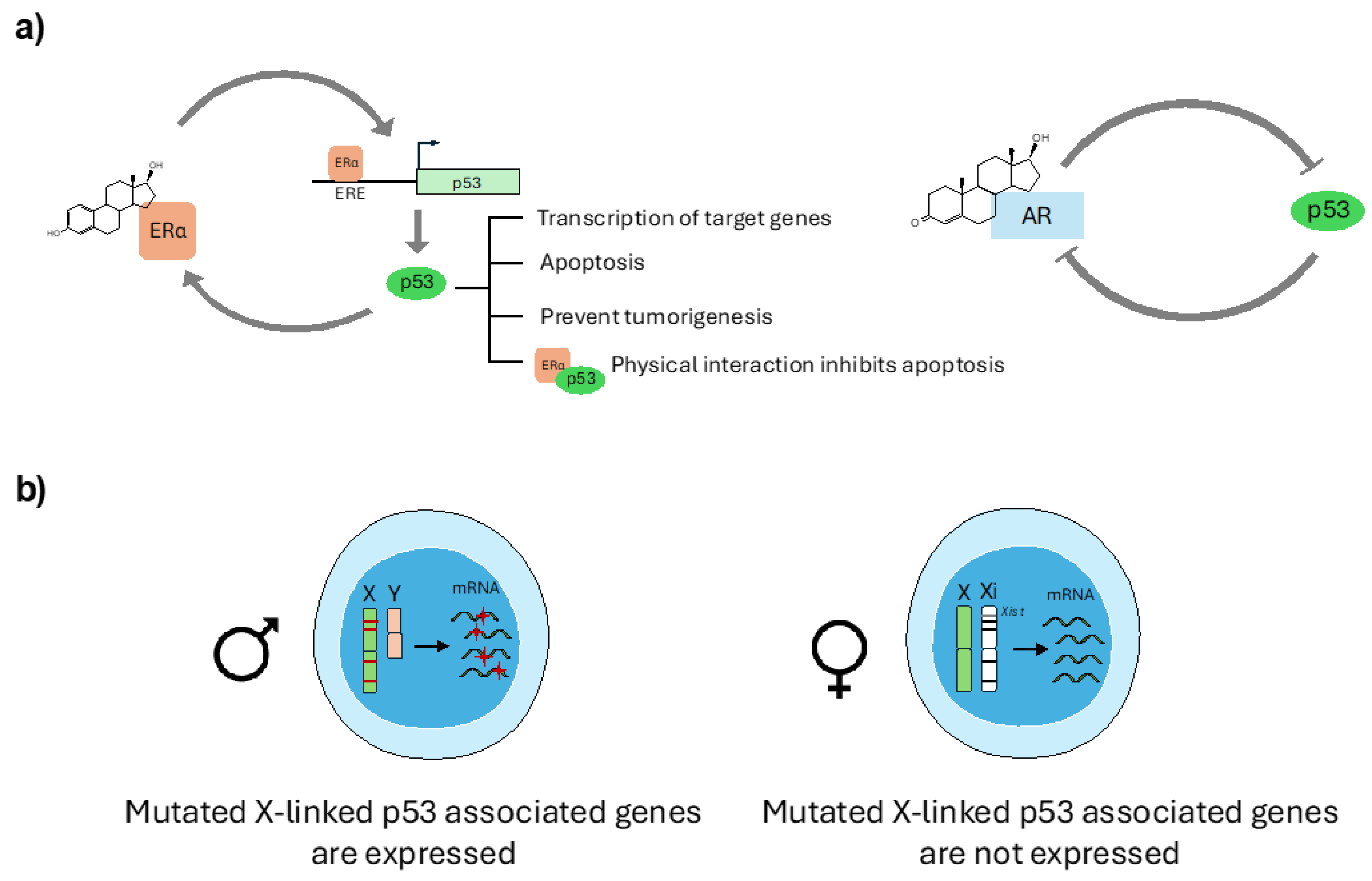
3.1. Sex Hormone Regulation of p53 Expression and Function
3.2. p53 and X-Chromosome Interplay
3.3. Sex Disparities in p53 Post-Translational Modifications
4. Sex Disparities in p53 Function
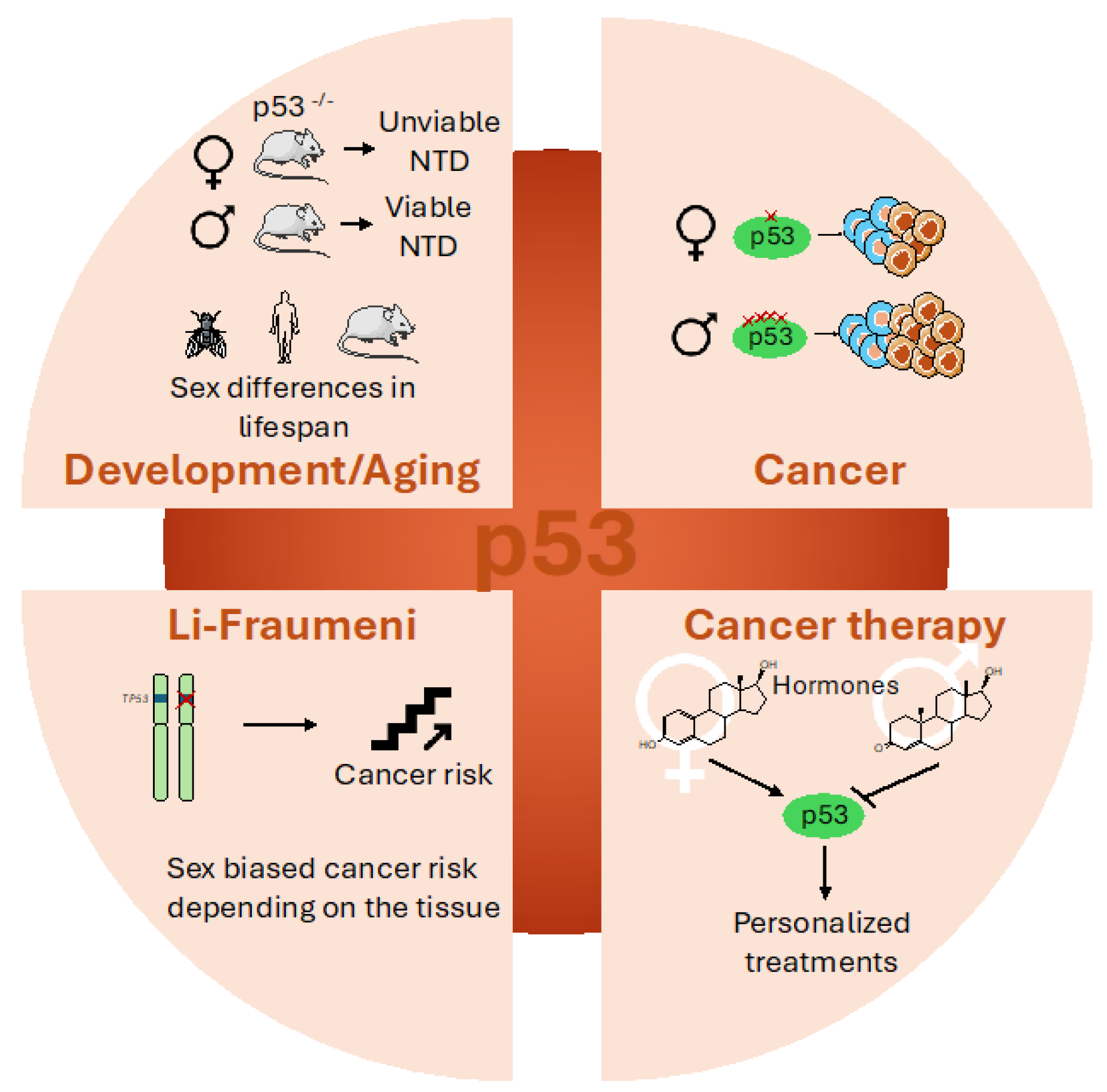
4.1. Sex Disparities in p53 During Development and Aging
4.2. Sex Disparities in p53’s Involvement in Cancer
4.3. Sex Disparities in Li-Fraumeni Patients
4.4. Sex-Dependent Effects on p53-Based Cancer Therapy
5. Conclusions and Discussion
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Haupt, S.; Caramia, F.; Klein, S.L.; Rubin, J.B.; Haupt, Y. Sex disparities matter in cancer development and therapy. Nat. Rev. Cancer 2021, 21, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Cardano, M.; Buscemi, G.; Zannini, L. Sex disparities in DNA damage response pathways: Novel determinants in cancer formation and therapy. iScience 2022, 25, 103875. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Cora, E.; Zhang, Y.; Dotto, G.P. Sexual dimorphism in cancer. Nat. Rev. Cancer 2016, 16, 330–339. [Google Scholar] [CrossRef]
- Carusillo, A.; Mussolino, C. DNA Damage: From Threat to Treatment. Cells 2020, 9, 1665. [Google Scholar] [CrossRef]
- Lanz, M.C.; Dibitetto, D.; Smolka, M.B. DNA damage kinase signaling: Checkpoint and repair at 30 years. EMBO J. 2019, 38, e101801. [Google Scholar] [CrossRef]
- Borrero, L.J.H.; El-Deiry, W.S. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef]
- Hafner, A.; Bulyk, M.L.; Jambhekar, A.; Lahav, G. The multiple mechanisms that regulate p53 activity and cell fate. Nat. Rev. Mol. Cell Biol. 2019, 20, 199–210. [Google Scholar] [CrossRef]
- Dyson, H.J.; Wright, P.E. How does p53 work? Regulation by the intrinsically disordered domains. Trends Biochem. Sci. 2024, 50, 9–17. [Google Scholar] [CrossRef]
- Grigoreva, T.A.; Romanova, A.A.; Tribulovich, V.G.; Pestov, N.B.; Oganov, R.A.; Kovaleva, D.K.; Korneenko, T.V.; Barlev, N.A. p53: The Multifaceted Roles of Covalent Modifications in Cancer. Pharmaceuticals 2024, 17, 1682. [Google Scholar] [CrossRef]
- Lane, D.P. p53, guardian of the genome. Nature 1992, 358, 15–16. [Google Scholar] [CrossRef] [PubMed]
- A Weinberg, R. How TP53 (almost) became an oncogene. J. Mol. Cell Biol. 2019, 11, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, Z.; Tavana, O.; Gu, W. Understanding the complexity of p53 in a new era of tumor suppression. Cancer Cell 2024, 42, 946–967. [Google Scholar] [CrossRef]
- Chavez-Reyes, A.; Parant, J.M.; Amelse, L.L.; Luna, R.M.D.O.; Korsmeyer, S.J.; Lozano, G. Switching mechanisms of cell death in mdm2- and mdm4-null mice by deletion of p53 downstream targets. Cancer Res. 2003, 63, 8664–8669. [Google Scholar]
- Jones, S.N.; Roe, A.E.; Donehower, L.A.; Bradley, A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 1995, 378, 206–208. [Google Scholar] [CrossRef]
- Montes de Oca Luna, R.; Wagner, D.S.; Lozano, G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 1995, 378, 203–206. [Google Scholar] [CrossRef]
- Zannini, L.; Delia, D.; Buscemi, G. CHK2 kinase in the DNA damage response and beyond. J. Mol. Cell Biol. 2014, 6, 442–457. [Google Scholar] [CrossRef]
- Joosten, H.; van Acker, F.; Dobbelsteen, D.v.D.; Horbach, G.; Krajnc, E. Genotoxicity of hormonal steroids. Toxicol. Lett. 2004, 151, 113–134. [Google Scholar] [CrossRef]
- Berger, C.; Qian, Y.; Chen, X. The p53-Estrogen Receptor Loop in Cancer. Curr. Mol. Med. 2013, 13, 1229–1240. [Google Scholar] [CrossRef]
- Berger, C.E.; Qian, Y.; Liu, G.; Chen, H.; Chen, X. p53, a Target of Estrogen Receptor (ER) α, Modulates DNA Damage-induced Growth Suppression in ER-positive Breast Cancer Cells. J. Biol. Chem. 2012, 287, 30117–30127. [Google Scholar] [CrossRef]
- Shirley, S.H.; Rundhaug, J.E.; Tian, J.; Cullinan-Ammann, N.; Lambertz, I.; Conti, C.J.; Fuchs-Young, R. Transcriptional Regulation of Estrogen Receptor-α by p53 in Human Breast Cancer Cells. Cancer Res. 2009, 69, 3405–3414. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cuesta, L.; Anaganti, S.; Hainaut, P.; Olivier, M. Estrogen levels act as a rheostat on p53 levels and modulate p53-dependent responses in breast cancer cell lines. Breast Cancer Res. Treat. 2010, 125, 35–42. [Google Scholar] [CrossRef] [PubMed]
- A Becker, K.; Lu, S.; Dickinson, E.S.; A Dunphy, K.; Mathews, L.; Schneider, S.S.; Jerry, D.J. Estrogen and progesterone regulate radiation-induced p53 activity in mammary epithelium through TGF-β-dependent pathways. Oncogene 2005, 24, 6345–6353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- A Dunphy, K.; Blackburn, A.C.; Yan, H.; O’Connell, L.R.; Jerry, D.J. Estrogen and progesterone induce persistent increases in p53-dependent apoptosis and suppress mammary tumors in BALB/c-Trp53 +/-mice. Breast Cancer Res. 2008, 10, R43. [Google Scholar] [CrossRef]
- Weige, C.C.; Allred, K.F.; Armstrong, C.M.; Allred, C.D. P53 mediates estradiol induced activation of apoptosis and DNA repair in non-malignant colonocytes. J. Steroid Biochem. Mol. Biol. 2012, 128, 113–120. [Google Scholar] [CrossRef]
- Huang, F.-Y.; Wong, D.K.-H.; Seto, W.-K.; Lai, C.-L.; Yuen, M.-F. Estradiol induces apoptosis via activation of miRNA-23a and p53: Implication for gender difference in liver cancer development. Oncotarget 2015, 6, 34941–34952. [Google Scholar] [CrossRef]
- Pok, S.; Barn, V.A.; Wong, H.J.; Blackburn, A.C.; Board, P.; Farrell, G.C.; Teoh, N.C. Testosterone regulation of cyclin E kinase: A key factor determining gender differences in hepatocarcinogenesis. J. Gastroenterol. Hepatol. 2016, 31, 1210–1219. [Google Scholar] [CrossRef]
- Wu, E.M.; Wong, L.L.; Hernandez, B.Y.; Ji, J.-F.; Jia, W.; Kwee, S.A.; Kalathil, S. Gender differences in hepatocellular cancer: Disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res. 2018, 4, 66. [Google Scholar] [CrossRef]
- Kim, J.Y.; Casaccia-Bonnefil, P. Interplay of hormones and p53 in modulating gender dimorphism of subventricular zone cell number. J. Neurosci. Res. 2008, 87, 3297–3305. [Google Scholar] [CrossRef]
- De Vitto, H.; Ryu, J.; Calderon-Aparicio, A.; Monts, J.; Dey, R.; Chakraborty, A.; Lee, M.-H.; Bode, A.M.; Dong, Z. Estrogen-related receptor alpha directly binds to p53 and cooperatively controls colon cancer growth through the regulation of mitochondrial biogenesis and function. Cancer Metab. 2020, 8, 1–19. [Google Scholar] [CrossRef]
- Liu, W.; Konduri, S.D.; Bansal, S.; Nayak, B.K.; Rajasekaran, S.A.; Karuppayil, S.M.; Rajasekaran, A.K.; Das, G.M. Estrogen Receptor-α Binds p53 Tumor Suppressor Protein Directly and Represses Its Function. J. Biol. Chem. 2006, 281, 9837–9840. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.T.; Shin, H.; Westerling, T.; Liu, X.S.; Brown, M. Estrogen receptor prevents p53-dependent apoptosis in breast cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 18060–18065. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.; Edvardsson, K.; Lindberg, K.; Zhao, C.; Williams, C.; Ström, A.; Gustafsson, J.-A. Tumor Repressive Functions of Estrogen Receptor β in SW480 Colon Cancer Cells. Cancer Res. 2009, 69, 6100–6106. [Google Scholar] [CrossRef]
- Hsu, H.-H.; Cheng, S.-F.; Wu, C.-C.; Chu, C.-H.; Weng, Y.-J.; Lin, C.-S.; Lee, S.-D.; Wu, H.-C.; Huang, C.-Y.; Kuo, W.-W. Apoptotic effects of over-expressed estrogen receptor-beta on LoVo colon cancer cell is mediated by p53 signalings in a ligand-dependent manner. Chin. J. Physiol. 2006, 49, 110–116. [Google Scholar]
- Pronsato, L.; Milanesi, L.; Vasconsuelo, A.; La Colla, A. Testosterone modulates FoxO3a and p53-related genes to protect C2C12 skeletal muscle cells against apoptosis. Steroids 2017, 124, 35–45. [Google Scholar] [CrossRef]
- Alimirah, F.; Panchanathan, R.; Chen, J.; Zhang, X.; Ho, S.-M.; Choubey, D. Expression of Androgen Receptor Is Negatively Regulated By p53. Neoplasia 2007, 9, 1152–1159. [Google Scholar] [CrossRef]
- Shenk, J.L.; Fisher, C.J.; Chen, S.-Y.; Zhou, X.-F.; Tillman, K.; Shemshedini, L. p53 Represses Androgen-induced Transactivation of Prostate-specific Antigen by Disrupting hAR Amino- to Carboxyl-terminal Interaction. J. Biol. Chem. 2001, 276, 38472–38479. [Google Scholar] [CrossRef]
- Bond, G.L.; Hu, W.; Bond, E.E.; Robins, H.; Lutzker, S.G.; Arva, N.C.; Bargonetti, J.; Bartel, F.; Taubert, H.; Wuerl, P.; et al. A Single Nucleotide Polymorphism in the MDM2 Promoter Attenuates the p53 Tumor Suppressor Pathway and Accelerates Tumor Formation in Humans. Cell 2004, 119, 591–602. [Google Scholar] [CrossRef]
- Lind, H.; Zienolddiny, S.; Ekstrøm, P.O.; Skaug, V.; Haugen, A. Association of a functional polymorphism in the promoter of the MDM2 gene with risk of nonsmall cell lung cancer. Int. J. Cancer 2006, 119, 718–721. [Google Scholar] [CrossRef]
- Bond, G.L.; Hirshfield, K.M.; Kirchhoff, T.; Alexe, G.; Bond, E.E.; Robins, H.; Bartel, F.; Taubert, H.; Wuerl, P.; Hait, W.; et al. MDM2 SNP309 Accelerates Tumor Formation in a Gender-Specific and Hormone-Dependent Manner. Cancer Res. 2006, 66, 5104–5110. [Google Scholar] [CrossRef]
- Barnoud, T.; Parris, J.L.D.; Murphy, M.E. Common genetic variants in the TP53 pathway and their impact on cancer. J. Mol. Cell Biol. 2019, 11, 578–585. [Google Scholar] [CrossRef]
- Miedl, H.; Lebhard, J.; Ehart, L.; Schreiber, M. Association of the MDM2 SNP285 and SNP309 Genetic Variants with the Risk, Age at Onset and Prognosis of Breast Cancer in Central European Women: A Hospital-Based Case-Control Study. Int. J. Mol. Sci. 2019, 20, 509. [Google Scholar] [CrossRef] [PubMed]
- Knappskog, S.; Lønning, P.E. Effects of the MDM2 promoter SNP285 and SNP309 on Sp1 transcription factor binding and cancer risk. Transcription 2011, 2, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Knappskog, S.; Trovik, J.; Marcickiewicz, J.; Tingulstad, S.; Staff, A.C.; Romundstad, P.; Hveem, K.; Vatten, L.; Salvesen, H.B.; Lønning, P.E. SNP285C modulates oestrogen receptor/Sp1 binding to the MDM2 promoter and reduces the risk of endometrial but not prostatic cancer. Eur. J. Cancer 2012, 48, 1988–1996. [Google Scholar] [CrossRef]
- Haupt, S.; Caramia, F.; Herschtal, A.; Soussi, T.; Lozano, G.; Chen, H.; Liang, H.; Speed, T.P.; Haupt, Y. Identification of cancer sex-disparity in the functional integrity of p53 and its X chromosome network. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Yang, W.H.; Kim, J.E.; Nam, H.W.; Ju, J.W.; Kim, H.S.; Kim, Y.S.; Cho, J.W. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat. Cell Biol. 2006, 8, 1074–1083. [Google Scholar] [CrossRef]
- Stichelen, S.O.-V.; Hanover, J.A. X-inactivation normalizes O-GlcNAc transferase levels and generates an O-GlcNAc-depleted Barr body. Front. Genet. 2014, 5, 256. [Google Scholar] [CrossRef]
- Kaneko, S.; Li, X. X chromosome protects against bladder cancer in females via a KDM6A -dependent epigenetic mechanism. Sci. Adv. 2018, 4, eaar5598. [Google Scholar] [CrossRef]
- Di Palo, A.; Siniscalchi, C.; Salerno, M.; Russo, A.; Gravholt, C.H.; Potenza, N. What microRNAs could tell us about the human X chromosome. Cell. Mol. Life Sci. 2020, 77, 4069–4080. [Google Scholar] [CrossRef]
- Chen, X.; Watkins, R.; Delot, E.; Reliene, R.; Schiestl, R.H.; Burgoyne, P.S.; Arnold, A.P. Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Dev. Neurobiol. 2007, 68, 265–273. [Google Scholar] [CrossRef]
- Kawamata, M.; Ochiya, T. Two distinct knockout approaches highlight a critical role for p53 in rat development. Sci. Rep. 2012, 2, srep00945. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Tian, Y.; Zhang, H.; Qu, L.; Chen, Y.; Liu, Q.; Luo, Y.; Wu, X. p53 Mutant p53N236S Induces Neural Tube Defects in Female Embryos. Int. J. Biol. Sci. 2019, 15, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Delbridge, A.R.; Kueh, A.J.; Ke, F.; Zamudio, N.M.; El-Saafin, F.; Jansz, N.; Wang, G.-Y.; Iminitoff, M.; Beck, T.; Haupt, S.; et al. Loss of p53 Causes Stochastic Aberrant X-Chromosome Inactivation and Female-Specific Neural Tube Defects. Cell Rep. 2019, 27, 442–454.e5. [Google Scholar] [CrossRef]
- Caramia, F.; Speed, T.P.; Shen, H.; Haupt, Y.; Haupt, S. Establishing the Link between X-Chromosome Aberrations and TP53 Status, with Breast Cancer Patient Outcomes. Cells 2023, 12, 2245. [Google Scholar] [CrossRef]
- Deng, X.; Berletch, J.B.; Ma, W.; Nguyen, D.K.; Hiatt, J.B.; Noble, W.S.; Shendure, J.; Disteche, C.M. Mammalian X Upregulation Is Associated with Enhanced Transcription Initiation, RNA Half-Life, and MOF-Mediated H4K16 Acetylation. Dev. Cell 2013, 25, 55–68. [Google Scholar] [CrossRef]
- Guo, M.; Fang, Z.; Chen, B.; Songyang, Z.; Xiong, Y. Distinct dosage compensations of ploidy-sensitive and -insensitive X chromosome genes during development and in diseases. iScience 2023, 26, 105997. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, W.; Teresky, A.K.; Hernando, E.; Cordon-Cardo, C.; Levine, A.J. Declining p53 function in the aging process: A possible mechanism for the increased tumor incidence in older populations. Proc. Natl. Acad. Sci. USA 2007, 104, 16633–16638. [Google Scholar] [CrossRef]
- Bahassi, E.M.; Robbins, S.B.; Yin, M.; Boivin, G.P.; Kuiper, R.; van Steeg, H.; Stambrook, P.J. Mice with the CHEK2 *1100delC SNP are predisposed to cancer with a strong gender bias. Proc. Natl. Acad. Sci. USA 2009, 106, 17111–17116. [Google Scholar] [CrossRef]
- Napoletano, F.; Gibert, B.; Yacobi-Sharon, K.; Vincent, S.; Favrot, C.; Mehlen, P.; Girard, V.; Teil, M.; Chatelain, G.; Walter, L.; et al. p53-dependent programmed necrosis controls germ cell homeostasis during spermatogenesis. PloS Genet. 2017, 13, e1007024. [Google Scholar] [CrossRef]
- Liu, C.; Moten, A.; Ma, Z.; Lin, H.-K. The foundational framework of tumors: Gametogenesis, p53, and cancer. Semin. Cancer Biol. 2021, 81, 193–205. [Google Scholar] [CrossRef]
- Hu, W. The Role of p53 Gene Family in Reproduction. Cold Spring Harb. Perspect. Biol. 2009, 1, a001073. [Google Scholar] [CrossRef] [PubMed]
- Kanduri, C.; Pant, V.; Loukinov, D.; Pugacheva, E.; Qi, C.-F.; Wolffe, A.; Ohlsson, R.; Lobanenkov, V.V. Functional association of CTCF with the insulator upstream of the H19 gene is parent of origin-specific and methylation-sensitive. Curr. Biol. 2000, 10, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Pidsley, R.; Fernandes, C.; Viana, J.; Paya-Cano, J.L.; Liu, L.; Smith, R.G.; Schalkwyk, L.C.; Mill, J. DNA methylation at the Igf2/H19 imprinting control region is associated with cerebellum mass in outbred mice. Mol. Brain 2012, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Feng, Z.; Teresky, A.K.; Levine, A.J. p53 regulates maternal reproduction through LIF. Nature 2007, 450, 721–724. [Google Scholar] [CrossRef]
- Whitehead, A.K.; Wang, Z.; Boustany, R.-J.; Vivès, R.R.; Lazartigues, E.; Liu, J.; Siggins, R.W.; Yue, X. Myeloid deficiency of heparan sulfate 6-O-endosulfatases impairs bone marrow hematopoiesis. Matrix Biol. 2024, 134, 107–118. [Google Scholar] [CrossRef]
- Austad, S.N.; Bartke, A. Sex Differences in Longevity and in Responses to Anti-Aging Interventions: A Mini-Review. Gerontology 2015, 62, 40–46. [Google Scholar] [CrossRef]
- Waskar, M.; Landis, G.N.; Shen, J.; Curtis, C.; Tozer, K.; Abdueva, D.; Skvortsov, D.; Tavaré, S.; Tower, J. Drosophila melanogaster p53 has developmental stage-specific and sex-specific effects on adult life span indicative of sexual antagonistic pleiotropy. Aging 2009, 1, 903–936. [Google Scholar] [CrossRef]
- Shen, J.; Tower, J. Drosophila foxo acts in males to cause sexual-dimorphism in tissue-specific p53 life span effects. Exp. Gerontol. 2009, 45, 97–105. [Google Scholar] [CrossRef]
- Muralidharan, A.; Sotocinal, S.G.; Yousefpour, N.; Akkurt, N.; Lima, L.V.; Tansley, S.; Parisien, M.; Wang, C.; Austin, J.-S.; Ham, B.; et al. Long-term male-specific chronic pain via telomere- and p53-mediated spinal cord cellular senescence. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef]
- Dawoud, A.A.Z.; Tapper, W.J.; Cross, N.C.P. Age-related loss of chromosome Y is associated with levels of sex hormone binding globulin and clonal hematopoiesis defined by TET2, TP53, and CBL mutations. Sci. Adv. 2023, 9, eade9746. [Google Scholar] [CrossRef]
- Matlashewski, G.J.; Tuck, S.; Pim, D.; Lamb, P.; Schneider, J.; Crawford, L.V. Primary Structure Polymorphism at Amino Acid Residue 72 of Human p53. Mol. Cell. Biol. 1987, 7, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Dumont, P.; Leu, J.I.-J.; Pietra, A.C.D., III; George, D.L.; Murphy, M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat. Genet. 2003, 33, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.K.; Leu, J.I.-J.; Zhou, Y.; Devarajan, K.; Nedelko, T.; Klein-Szanto, A.; Hollstein, M.; Murphy, M.E. The Codon 72 Polymorphism of p53 Regulates Interaction with NF-κB and Transactivation of Genes Involved in Immunity and Inflammation. Mol. Cell. Biol. 2011, 31, 1201–1213. [Google Scholar] [CrossRef]
- Whibley, C.; Pharoah, P.D.P.; Hollstein, M. p53 polymorphisms: Cancer implications. Nat. Rev. Cancer 2009, 9, 95–107. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, L.; Yue, X.; Zhang, C.; Wang, J.; Li, J.; Sun, X.; Zhu, Y.; Feng, Z.; Hu, W.; et al. A polymorphism in the tumor suppressor p53 affects aging and longevity in mouse models. eLife 2018, 7, e34701. [Google Scholar] [CrossRef]
- Bojesen, S.E.; Nordestgaard, B.G. The common germline Arg72Pro polymorphism of p53 and increased longevity in humans. Cell Cycle 2008, 7, 158–163. [Google Scholar] [CrossRef]
- Van Heemst, D.; Mooijaart, S.P.; Beekman, M.; Schreuder, J.; de Craen, A.J.; Brandt, B.W.; Slagboom, P.E.; Westendorp, R.G. Variation in the human TP53 gene affects old age survival and cancer mortality. Exp. Gerontol. 2004, 40, 11–15. [Google Scholar] [CrossRef]
- Groß, S.; Immel, U.-D.; Klintschar, M.; Bartel, F. Germline Genetics of the p53 Pathway Affect Longevity in a Gender Specific Manner. Curr. Aging Sci. 2014, 7, 91–100. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Q.; Wang, Z.; Sun, L.; Lv, Z.; Liu, J.; Xing, C.; Yuan, Y. p53 protein expression affected by TP53 polymorphism is associated with the biological behavior and prognosis of low rectal cancer. Oncol. Lett. 2019, 18, 6807–6821. [Google Scholar] [CrossRef]
- Fan, C.; Wei, J.; Yuan, C.; Wang, X.; Jiang, C.; Zhou, C.; Yang, M. The Functional TP53 rs1042522 and MDM4 rs4245739 Genetic Variants Contribute to Non-Hodgkin Lymphoma Risk. PloS ONE 2014, 9, e107047. [Google Scholar] [CrossRef]
- Ren, Y.-W.; Yin, Z.-H.; Wan, Y.; Guan, P.; Wu, W.; Li, X.-L.; Zhou, B.-S. P53 Arg72Pro and MDM2 SNP309 polymorphisms cooperate to increase lung adenocarcinoma risk in Chinese female non-smokers: A case control study. Asian Pac. J. Cancer Prev. 2013, 14, 5415–5420. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sümbül, A.T.; Akkız, H.; Bayram, S.; Bekar, A.; Akgöllü, E.; Sandıkçı, M. p53 codon 72 polymorphism is associated with susceptibility to hepatocellular carcinoma in the Turkish population: A case–control study. Mol. Biol. Rep. 2011, 39, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Pandith, A.A.; Khan, N.P.; Rashid, N.; Azad, N.; Zaroo, I.; Hafiz, A.; Siddiqi, M.A. Impact of codon 72 Arg > Pro single nucleotide polymorphism in TP53 gene in the risk of kangri cancer: A case control study in Kashmir. Tumor Biol. 2012, 33, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Fajac, A.; Simeonova, I.; Leemput, J.; Gabriel, M.; Morin, A.; Lejour, V.; Hamon, A.; Rakotopare, J.; Vaysse-Zinkhöfer, W.; Eldawra, E.; et al. Mutant mice lacking alternatively spliced p53 isoforms unveil Ackr4 as a male-specific prognostic factor in Myc-driven B-cell lymphomas. eLife 2024, 13, RP92774. [Google Scholar] [CrossRef]
- Sun, T.; Warrington, N.M.; Luo, J.; Brooks, M.D.; Dahiya, S.; Snyder, S.C.; Sengupta, R.; Rubin, J.B. Sexually dimorphic RB inactivation underlies mesenchymal glioblastoma prevalence in males. J. Clin. Investig. 2014, 124, 4123–4133. [Google Scholar] [CrossRef]
- Kfoury, N.; Sun, T.; Yu, K.; Rockwell, N.; Tinkum, K.L.; Qi, Z.; Warrington, N.M.; McDonald, P.; Roy, A.; Weir, S.J.; et al. Cooperative p16 and p21 action protects female astrocytes from transformation. Acta Neuropathol. Commun. 2018, 6, 12. [Google Scholar] [CrossRef]
- Rockwell, N.C.; Yang, W.; Warrington, N.M.; Staller, M.V.; Griffith, M.; Griffith, O.L.; Gurnett, C.A.; Cohen, B.A.; Baldridge, D.; Rubin, J.B. Sex- and Mutation-Specific p53 Gain-of-Function Activity in Gliomagenesis. Cancer Res. Commun. 2021, 1, 148–163. [Google Scholar] [CrossRef]
- Marker, D.F.; Agnihotri, S.; Amankulor, N.; Murdoch, G.H.; Pearce, T.M. The dominant TP53 hotspot mutation in IDH -mutant astrocytoma, R273C, has distinctive pathologic features and sex-specific prognostic implications. Neuro-Oncology Adv. 2021, 4, vdab182. [Google Scholar] [CrossRef]
- Li, F.P.; Fraumeni, J.F.; Mulvihill, J.J.; A Blattner, W.; Dreyfus, M.G.; A Tucker, M.; Miller, R.W. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988, 48, 5358–5362. [Google Scholar]
- Malkin, D.; Li, F.P.; Strong, L.C.; Fraumeni, J.F.; Nelson, C.E.; Kim, D.H.; Kassel, J.; Gryka, M.A.; Bischoff, F.Z.; Tainsky, M.A.; et al. Germ Line p53 Mutations in a Familial Syndrome of Breast Cancer, Sarcomas, and Other Neoplasms. Science 1990, 250, 1233–1238. [Google Scholar] [CrossRef]
- Wu, C.-C.; Shete, S.; Amos, C.I.; Strong, L.C. Joint Effects of Germ-Line p53 Mutation and Sex on Cancer Risk in Li-Fraumeni Syndrome. Cancer Res. 2006, 66, 8287–8292. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, K.D.; Noltner, K.A.; Buzin, C.H.; Gu, D.; Wen-Fong, C.Y.; Nguyen, V.Q.; Han, J.H.; Lowstuter, K.; Longmate, J.; Sommer, S.S.; et al. Beyond Li Fraumeni Syndrome: Clinical Characteristics of Families With p53 Germline Mutations. J. Clin. Oncol. 2009, 27, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Olivier, M.; E Goldgar, D.; Sodha, N.; Ohgaki, H.; Kleihues, P.; Hainaut, P.; A Eeles, R. Li-Fraumeni and related syndromes: Correlation between tumor type, family structure, and TP53 genotype. Cancer Res. 2003, 63, 6643–6650. [Google Scholar] [PubMed]
- De Andrade, K.C.; Khincha, P.P.; Hatton, J.N.; Frone, M.N.; Wegman-Ostrosky, T.; Mai, P.L.; Best, A.F.; A Savage, S. Cancer incidence, patterns, and genotype–phenotype associations in individuals with pathogenic or likely pathogenic germline TP53 variants: An observational cohort study. Lancet Oncol. 2021, 22, 1787–1798. [Google Scholar] [CrossRef]
- Mai, P.L.; Best, A.F.; Peters, J.A.; DeCastro, R.M.; Khincha, P.P.; Loud, J.T.; Bremer, R.C.; Rosenberg, P.S.; Savage, S.A. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer 2016, 122, 3673–3681. [Google Scholar] [CrossRef]
- Ribeiro, R.C.; Pinto, E.M.; Zambetti, G.P.; Rodriguez-Galindo, C. The International Pediatric Adrenocortical Tumor Registry initiative: Contributions to clinical, biological, and treatment advances in pediatric adrenocortical tumors. Mol. Cell. Endocrinol. 2011, 351, 37–43. [Google Scholar] [CrossRef]
- Geller, S.E.; Koch, A.R.; Roesch, P.; Filut, A.; Hallgren, E.; Carnes, M. The More Things Change, the More They Stay the Same: A Study to Evaluate Compliance With Inclusion and Assessment of Women and Minorities in Randomized Controlled Trials. Acad. Med. 2018, 93, 630–635. [Google Scholar] [CrossRef]
- Coates, A.S.; Millar, E.K.; A O’Toole, S.; Molloy, T.J.; Viale, G.; Goldhirsch, A.; Regan, M.M.; Gelber, R.D.; Sun, Z.; Castiglione-Gertsch, M.; et al. Prognostic interaction between expression of p53 and estrogen receptor in patients with node-negative breast cancer: Results from IBCSG Trials VIII and IX. Breast Cancer Res. 2012, 14, R143. [Google Scholar] [CrossRef]
- Fernandez-Cuesta, L.; Anaganti, S.; Hainaut, P.; Olivier, M. p53 status influences response to tamoxifen but not to fulvestrant in breast cancer cell lines. Int. J. Cancer 2011, 128, 1813–1821. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1–35. [Google Scholar] [CrossRef]
- Guo, M.; Xiong, Y. Sex-biased genome-editing effects of CRISPR-Cas9 across cancer cells dependent on p53 status. iScience 2023, 26, 107529. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardano, M.; Buscemi, G.; Zannini, L. Sex Disparities in P53 Regulation and Functions: Novel Insights for Personalized Cancer Therapies. Cells 2025, 14, 363. https://doi.org/10.3390/cells14050363
Cardano M, Buscemi G, Zannini L. Sex Disparities in P53 Regulation and Functions: Novel Insights for Personalized Cancer Therapies. Cells. 2025; 14(5):363. https://doi.org/10.3390/cells14050363
Chicago/Turabian StyleCardano, Miriana, Giacomo Buscemi, and Laura Zannini. 2025. "Sex Disparities in P53 Regulation and Functions: Novel Insights for Personalized Cancer Therapies" Cells 14, no. 5: 363. https://doi.org/10.3390/cells14050363
APA StyleCardano, M., Buscemi, G., & Zannini, L. (2025). Sex Disparities in P53 Regulation and Functions: Novel Insights for Personalized Cancer Therapies. Cells, 14(5), 363. https://doi.org/10.3390/cells14050363






